Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Tamotsu Tsukahara and Version 2 by Rita Xu.
Osteoarthritis (OA), a chronic degenerative joint disease, is the most common form of arthritis. OA occurs when the protective cartilage that cushions the ends of bones gradually breaks down. This leads to the rubbing of bones against each other, resulting in pain and stiffness. Cyclic phosphatidic acid (cPA) shows promise as a treatment for OA.
- cyclic phosphatidic acid
- inflammation
- osteoarthritis
1. Overview
According to the World Health Organization (WHO), osteoarthritis (OA) is one of the most common musculoskeletal disorders, affecting millions of people worldwide [1].
OA is a comprehensive joint disease that affects various joint tissues, manifesting as subchondral bone remodeling [2], meniscal degeneration [3], inflammation [4], fibrosis of the infrapatellar fat pad [5], and synovial membrane inflammation [6]. OA is a chronic joint condition characterized by the degeneration of the joint cartilage and underlying bone, leading to symptoms such as pain, stiffness, and reduced mobility. OA is more prevalent in older age groups than in younger age groups, and it is estimated that a significant percentage of people aged 60 years and older have OA to some degree [7]. While OA can affect both men and women, it may be more common in women, especially in certain joints such as the hands and knees [8]. The WHO emphasizes the importance of early diagnosis and appropriate management of OA. This includes a combination of non-pharmacological interventions (e.g., exercise, weight management) and pharmacological treatments (e.g., pain relievers, anti-inflammatory drugs) to control symptoms and improve quality of life for individuals with OA. Various drugs and medications are used to manage the symptoms and improve the quality of life of patients with OA.
2. Medications for OA
Currently, treatment standards for OA are primarily limited to pain management, steroids, other anti-inflammatory drugs, physical therapy, and eventual joint replacement [9][10][11][11,12,13]. The most common symptoms of OA include joint pain, stiffness, and a decreased range of motion. These symptoms tend to worsen over time, particularly after periods of inactivity or excessive use of the affected joint. Several risk factors are associated with OA, including aging, genetics, joint injury or trauma, obesity, and certain joint abnormalities [12][13][14][14,15,16]. Repetitive stress on a joint, such as from certain occupations or sports activities, can also increase one’s risk of OA. Although there is no cure for OA, several treatment options are available to manage its symptoms and improve joint function. Corticosteroid and hyaluronic acid injections are two different types of treatments commonly used for various medical conditions, particularly in the fields of orthopedics and rheumatology [15][17]. Corticosteroids are potent anti-inflammatory drugs. Injection of such drugs into a joint or soft tissue can help reduce inflammation and alleviate pain. Hyaluronic acid is a natural substance found in joint fluids and cartilage. Viscosupplementation involves the injection of hyaluronic acid into the joint to improve lubrication and reduce pain [16][18]. However, recent research has found that viscosupplementation is not effective in significantly reducing pain or improving function [17][19]. Biological therapies are newer medications used for severe OA and include drugs such as DMOADs, PRP, and stem cell injections. DMOADs are a class of medications that aim to modify the underlying processes and progression of OA, rather than simply managing the symptoms; several potential DMOADs have been investigated in clinical trials and research studies. Potential DMOADs being explored include sprifermin, a growth factor that stimulates cartilage growth, and various agents targeting specific inflammatory pathways involved in OA [18][19][20][20,21,22]. PRP is a component of the blood that contains a high concentration of platelets. Platelets are rich in growth factors and bioactive proteins that play crucial roles in tissue repair and regeneration [21][22][23][23,24,25]. Additionally, injected stem cells are believed to have several potential mechanisms of action, including anti-inflammatory effects, cartilage repair, and pain reduction [24][26]. These treatments promote tissue repair and reduce inflammation. Although some patients report pain relief after the procedure, others are not helped by the injections [25][27]. The choice between corticosteroids and hyaluronic acid injections is made depending on the specific condition being treated, the patient’s medical history, and the physician’s recommendation [26][28]. Both corticosteroids and hyaluronic acid injections have their own unique mechanisms of action and are used for different purposes. Corticosteroids are powerful anti-inflammatory medications that work by suppressing the immune response and reducing inflammation in the body. They are commonly used for conditions involving acute or chronic inflammation. Hyaluronic acid is a natural component found in joint fluid and cartilage, which helps lubricate and cushion the joints. Hyaluronic acid injections are primarily used for conditions with characteristics of joint pain and reduced joint lubrication, particularly in OA [27][29]. It is important to note that the choice between corticosteroid and hyaluronic acid injections should be made by a qualified healthcare provider after a thorough evaluation of the patient’s medical history, the specific condition being treated, as well as a consideration of the potential benefits and risks associated with each treatment option. These drugs target various aspects of the disease, including cartilage protection, inflammation reduction, and joint function improvement, highlighting the existence of alternative and complementary therapies for OA that may have different profiles regarding treatment resistance, side effects, and costs, as well as acknowledging that not all patients respond equally to OA treatments.3. Inflammation and OA
OA is characterized by low-grade chronic inflammation of the affected joint [28][30]. Inflammation primarily involves synovium and joint tissues. Although not as severe or systemic as rheumatoid arthritis (RA), inflammation contributes to the symptoms and progression of OA. Inflammatory molecules, such as cytokines and chemokines, are produced in inflamed joint tissues [29][31]. As shown in Figure 1, these molecules promote cartilage degradation, inhibit cartilage repair, and contribute to joint pain and stiffness. Levels of several pro-inflammatory cytokines, e.g., interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), are increased in the synovial fluid and joint tissues of individuals with OA [30][31][32][32,33,34]. In healthy joints, there is a balance between proinflammatory and anti-inflammatory cytokines. In OA, this balance is disrupted by an increase in proinflammatory cytokines and a decrease in anti-inflammatory cytokines. This imbalance contributes to the ongoing inflammation and tissue destruction [33][35]. Cytokines, especially IL-1 and TNF-α, can sensitize pain receptors in the joint tissues, leading to increased pain perception in individuals with OA [34][36]. This heightened pain sensation can further reduce joint function and quality of life. These mediators in OA can also stimulate chondrocytes to produce enzymes, such as matrix metalloproteinases (MMPs) and aggrecanases [35][37]. MMPs are a family of enzymes that play crucial roles in tissue remodeling, particularly in the breakdown of the extracellular matrix (ECM). These enzymes, particularly MMP-1, MMP-3, and MMP-13, break down important ECM components such as collagen and proteoglycans, which are essential for cartilage integrity [36][37][38,39]. This degradation leads to the thinning and erosion of the cartilage in OA-affected joints. MMPs are produced by various cell types, including chondrocytes, synovial cells, and immune cells [38][40]. Inflammation in OA joints can stimulate the release of MMPs, further accelerating cartilage breakdown and contributing to joint damage [39][41]. MMPs are essential for normal tissue remodeling and repair. However, in OA, there is often an imbalance between MMP production and tissue repair mechanisms, leading to excessive matrix degradation and inadequate tissue regeneration [40][42]. The body contains natural MMP inhibitors, such as tissue inhibitors of metalloproteinases (TIMPs). In individuals with OA, the balance between MMPs and TIMPs may be disrupted, favoring MMP activity and cartilage breakdown [41][43]. This imbalance may contribute to the disease progression. Targeting MMPs is a potential therapeutic strategy for OA [42][44]. Developing drugs or interventions that can inhibit the activity of specific MMPs may help slow cartilage degradation and mitigate OA symptoms [43][45]. However, identifying effective and safe MMP inhibitors for clinical use remains a challenge. While MMPs are involved in the degradation of cartilage in OA and have been explored as therapeutic targets, developing effective MMP inhibitors has proven to be a complex task [43][45]. MMPs play various roles in tissue remodeling and homeostasis. Developing inhibitors that selectively target the MMPs involved in OA without affecting beneficial MMPs can be challenging. Many MMP inhibitors developed in the past have shown off-target effects, impacting other biological processes. The lack of specificity can lead to unwanted side effects and safety concerns [44][46]. MMP inhibitors may need to be used in combination with other OA treatments, such as non-steroidal anti-inflammatory drugs (NSAIDs) or disease-modifying OA drugs (DMOADs), to achieve optimal results. The interactions and safety of combination therapies need careful evaluation [45][47]. In some cases of OA, the synovial membrane becomes inflamed, leading to increased production of synovial fluid and joint swelling [6]. Synovial inflammation can exacerbate joint pain and discomfort. Additionally, inflammatory processes within the joint can lead to pain, which is a hallmark of OA. Inflammation contributes to pain perception in patients with OA, and it accelerates the progression of OA by promoting cartilage damage and joint deterioration [46][48].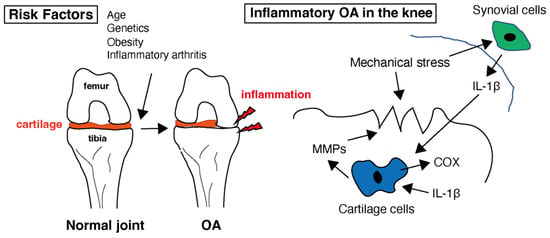
Figure 1. Healthy cartilage provides a smooth and lubricated surface within the joint, allowing for pain-free movement. Several risk factors that can increase the likelihood of developing osteoarthritis (OA) have been identified. While OA is primarily considered a non-inflammatory arthritis, low-level inflammation does play a role in the disease. In the context of OA, interleukin-1 beta (IL-1β) is of particular interest because it can contribute to the inflammation and tissue damage seen in affected joints. Inflammation in the joint can lead to the release of enzymes that further break down cartilage. Matrix metalloproteinases (MMPs) are involved in the breakdown of the extracellular matrix, which is the structural framework of tissues in the body, including cartilage in joints. Cyclooxygenase (COX) is an enzyme involved in the production of prostaglandins within the cartilage tissue.
4. Overview of Cyclic Phosphatidic Acid
Lipids are versatile molecules with different chemical structures and properties, and researchers have harnessed the properties of lipids to create drugs and drug delivery systems for different therapeutic purposes. Cyclic phosphatidic acid (cPA) is a unique phospholipid with a cyclic structure. This cyclic ring differentiates it from typical linear phosphatidic acids including lysophosphatidylcholine (LPC) and lysophosphatidic acid (LPA) [47][48][68,69]. The first cPA family member was originally isolated from slime mold and designated as physarum lysophosphatidic acid [49][70]. Several cPA activities have been attributed to albumin-associated lipid factors. Autotaxin (ATX) was initially identified as an enzyme secreted by cancer cells and found to stimulate cell motility, i.e., “taxis” [50][71]. cPA generation by autotaxin (ATX) under nonphysiological conditions was first reported in 2006 [51][72]. ATX is a lysophospholipase D and converts LPC into LPA [52][53][54][73,74,75]. LPA is a bioactive lipid that plays a role in inflammation [55][76]. ATX and the LPA it generates are involved in several physiological and pathological processes, including inflammation, angiogenesis, fibrosis, and cancer progression [56][57][58][59][77,78,79,80]. LPA is a potent chemoattractant that can attract immune cells, such as neutrophils and monocytes, to sites of inflammation [60][81]. This is important for the immune response against infections or tissue damage. Thus, ATX in LPA production can contribute to the recruitment of immune cells to inflamed tissues [61][82]. LPA can also stimulate the production of pro-inflammatory cytokines, such as IL-6 and TNF-α, by immune cells. These cytokines further amplify the inflammatory response. Excessive LPA signaling can lead to fibrosis, which is the formation of excess fibrous connective tissue [62][83]. Fibrosis is characterized by excessive accumulation of ECM proteins, e.g., collagen, in tissues, leading to tissue scarring and dysfunction [63][64][84,85]. This can occur in various organs and impair their function. LPA can induce fibrosis-associated inflammation [65][86]. Inflammatory cells, such as macrophages, can release LPA, and LPA, in turn, can recruit and activate immune cells, perpetuating the inflammatory response and contributing to fibrosis [66][87]. The signaling pathways triggered by LPA are complex and can have both pro-inflammatory and pro-survival effects [67][88]. Because of their involvement in various diseases, particularly cancer and inflammatory conditions, ATX and LPA have been investigated as potential therapeutic targets for OA. By inhibiting ATX, these inhibitors decrease LPA levels in the body, which can have therapeutic effects in certain medical conditions. ATX inhibitors have been explored as potential treatments for conditions such as fibrosis, cancer, and autoimmune diseases involving dysregulated LPA signaling [68][89]. The development of ATX inhibitors is an active area of research. Several compounds with inhibitory activity against ATX have been investigated in preclinical and clinical studies [69][90]. These inhibitors can be small molecules or biologics designed to disrupt the enzymatic activity of ATX or its interactions with its substrates [70][91]. cPA is also a physiological constituent of human serum [71][92]. Its stability allows cPA to function as a bioactive lipid mediator in various physiological processes [72][93]. cPA shows several unique actions compared with those of LPA. cPA inhibits cell proliferation, whereas LPA stimulates cell proliferation, migration, and differentiation [73][94]. cPA suppresses cancer cell invasion and metastasis by inhibiting ATX and transient activation of low-molecular-weight GTPases and RhoA [74][95]. Additionally, cPA can modulate ATX activity, affecting LPA levels [75][96] and, consequently, inflammation [76][77][97,98]. However, it is important to note that the metabolic stability of cPA can vary depending on factors such as the specific tissue or cell type, presence of enzymes or other molecules that may degrade it, and the local microenvironment. cPA has been implicated in various cellular processes and has important roles in cell signaling, cell growth, and differentiation [78][79][80][81][82][83][99,100,101,102,103,104]. It acts as a potent signaling molecule in various physiological contexts. The synthesis of cPA is tightly regulated, and its levels can be influenced by various cellular signals and stimuli. For example, certain growth factors, hormones, and neurotransmitters can modulate the activity of glycerophosphodiesterase 7 (GDE7) and thus affect cPA levels in the cell [84][105]. GDE7 suppresses the peroxisome proliferator-activated receptor gamma (PPARγ) pathway, suggesting that cPA functions as an intracellular lipid mediator. PPARγ is a type of nuclear receptor protein that plays a crucial role in regulating gene expression and is primarily involved in the control of lipid metabolism and glucose homeostasis [85][106].
Moreover, 2carba-cyclic phosphatidic acid (2carba-cPA) is a modified form of cPA [86][107], in which the phosphate group in cPA is replaced with a carba linkage, a carbon-carbon bond. This modification eliminates the negative charge typically associated with the phosphate group in cPA and can considerably affect the properties of a molecule and its biological activities [75][87][88][89][90][96,108,109,110,111]. One of the key features of 2carba-cPA is its enhanced stability compared to natural cPA. This stability allows it to persist longer in biological systems, making it a valuable tool for research and potential therapeutic applications. Like natural cPA, 2carba-cPA can interact with specific receptors, including G protein-coupled receptors (GPCRs), and initiate intracellular signaling cascades [91][112]. It may modulate various cellular processes, including cell proliferation, migration, and calcium signaling, depending on the cell type and receptor subtype involved. Research suggests that 2carba-cPA may have anti-cancer properties [91][112]. Preclinical studies have shown that 2carba-cPA inhibits the growth and metastasis of cancer cells. The stability and ability of 2carba-cPA to interfere with cancer cell signaling pathways make it a potential candidate for cancer therapy research. Some studies indicate that 2carba-cPA may have neuroprotective effects [88][109]. Therefore, its ability to protect neurons and potentially mitigate neurodegenerative diseases could be further explored. Some studies have shown the anti-inflammatory properties of 2carba-cPA [92][113], indicating its potential to modulate immune responses and inflammation-related diseases [93][114]. Research suggests that 2carba-cPA may play a role in metabolic regulation, including the control of lipid metabolism and glucose homeostasis, which could have implications for the treatment of metabolic disorders like diabetes and obesity. Due to its stability and promising biological activities, 2carba-cPA is a candidate for therapeutic development that may be used in drug discovery and development for conditions such as cancer, neurodegenerative diseases, inflammation-related disorders, and metabolic disorders.
