Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Hao Zhang.
Nitrogen (N) is a crucial nutrient that plays a significant role in enhancing crop yield. Its availability, including both supply and deficiency, serves as a crucial signal for plant development. WThe discuss recent advances in understanding how epigenetic modifications, including DNA methylation, histone modification, and small RNA, participate in the regulation of N response and LN adaptation were discussed here. Decoding the epigenome at various levels could accelerate the functional study of how plants respond to N availability. Understanding the epigenetic control of N signaling and adaptation can lead to new strategies to improve NUE and enhance crop productivity sustainably
- nitrogen signaling
- low-nitrogen adaptation
- epigenetic regulation
1. Epigenetic Regulation of N Uptake
N metabolism in plants encompasses processes such as uptake, transportation, and assimilation, all of which are subject to precise and dynamic regulation by NMGs [9,10][1][2]. Notably, the expression of N transporters, such as NRT2.1, can be rapidly induced within just one hour in Arabidopsis [30][3]. Altering the expression levels of genes related to N transport, assimilation, and signaling using transgenic approaches can significantly enhance crop yield or NUE [31,32,33,34,35][4][5][6][7][8]. Recent studies have highlighted the impact of chromatin regulation, including histone modification and chromatin structure, in modulating the activity of N transporters [17,23,36][9][10][11].
In dicots, histone modification, such as tri-methylation of lysine 36 or lysine 27 on histone H3 (H3K36me3, H3K27me3) are implicated in the regulation of N uptake process. In Arabidopsis, the H3K36me3 ‘writer’ SET DOMAIN GROUP8 (SDG8) plays a significant role in regulating genes related to energy metabolism, including NMGs and photosynthesis, through H3K36me3 modification [21][12]. SDG8 is induced by N treatment in roots, particularly in the lateral root cap and pericycle [37][13]. Recent studies have shown that SDG8 mediates N-triggered gene-expression reprogramming, including NRT2.1, NRT1.5, and GLUTAMINE SYNTHETASE 1;4 (GLN1;4) [38][14] (Figure 1). sdg8 mutants have shown lower nitrate acquisition than wild-type plants under high N conditions [38][14]. In tomatoes (Solanum lycopersicum), the homologs of SDG8, namely SlSDG33 and SlSDG34, regulate SlNRT1.1 in an N-dependent manner that is associated with the root response to N treatments [36][11] (Figure 1). Moreover, polycomb repressive complex 2 (PRC2), responsible for the addition of methyl groups to H3K27, directly targets and downregulates AtNRT2.1 expression in Arabidopsis [6][15]. In high N conditions, HIGH NITROGEN INSENSITIVE 9/ interacts with SUPT6H, and CTD Assembly Factor 1 (HNI9/AtIWS1) recruits PRC2 to the AtNRT2.1 locus to repress its transcription [23][10] (Figure 1). Moreover, AtHNI9 control reactive oxygen species (ROS) homeostasis under conditions of excess N. The mutation of AtHNI9 leads to elevated ROS levels and ROS-dependent upregulation of AtNRT2.1, indicating the role of HNI9 in balancing N uptake and ROS levels in response to N supply [39][16].
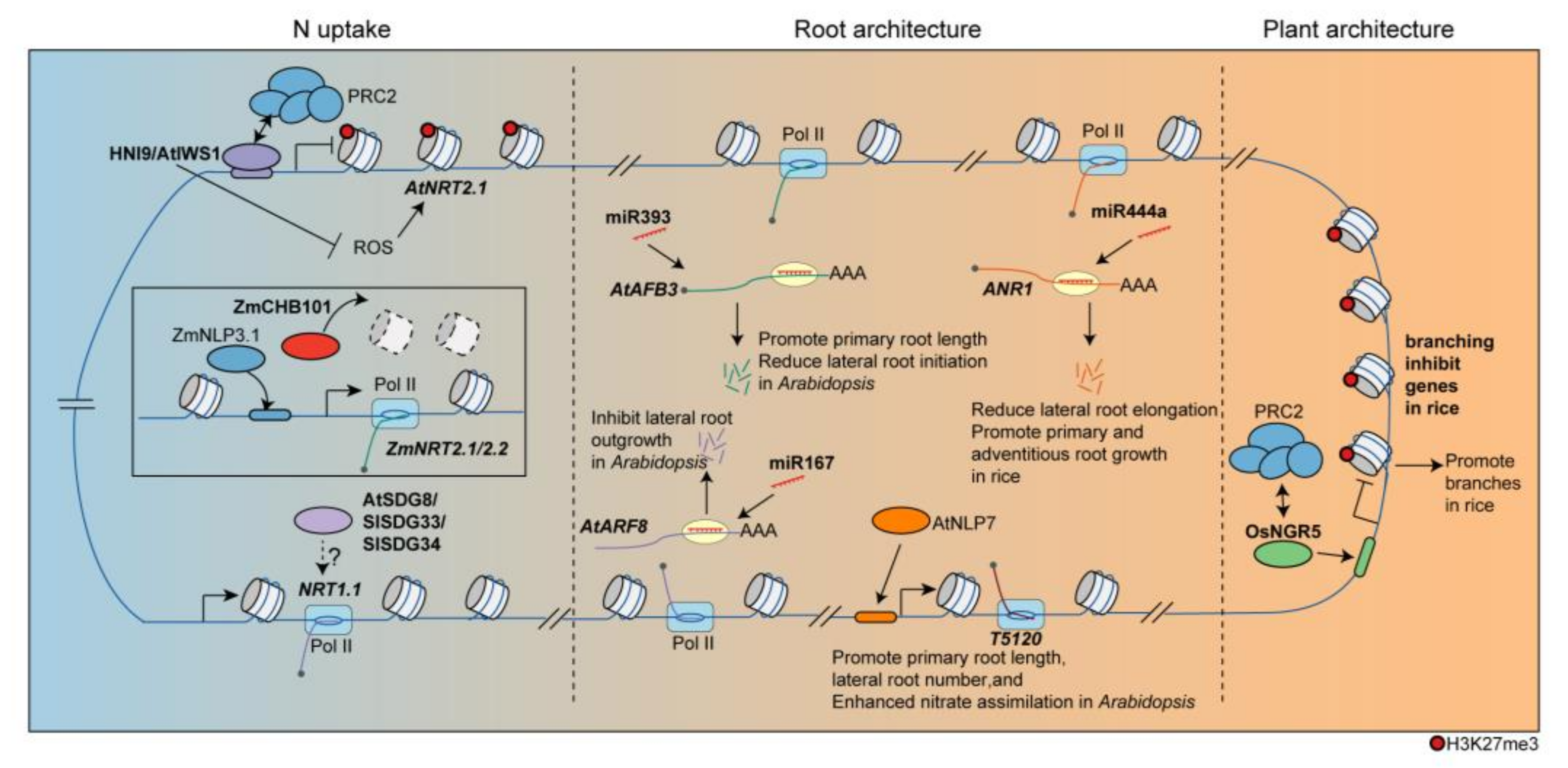
Figure 1. Epigenetic regulation of divergent processes in response to N. HNI9/AtIWS1 binds to the AtNRT2.1 locus and recruits PRC2 to suppress its expression. Conversely, ZmCHB101 regulates ZmNRT2.1 by reducing nucleosome densities. The homologs of SDG8 in tomatoes, SlSDG33 and SlSDG34, also regulate NRT1.1 under N supply. Epigenetic factors are involved in the regulation of N-dependent development processes as well. And miR444a reduces lateral root elongation by targeting ANR1 in rice. In Arabidopsis, miRNAs, such as miR167 and miR393, target transcripts involved in root development, particularly in response to N, thereby influencing root growth. Additionally, NLP7 modulates the expression of T5120, a long non-coding RNA. In rice, OsNGR5, which recruits PRC2 to inhibit branching-inhibition genes, influence plant architecture.
In monocots, N uptake was regulated by changes in chromatin rearrangement. CHROMATIN REMODELING COMPLEX SUBUNIT B 101 (CHB101) encodes a subunit of the ATP-dependent chromatin remodeling complex known as the SWITCH/SUCROSE NONFERMENTING (SWI/SNF) complex [40,41][17][18]. In maize, ZmCHB101 has been found to govern the expression of nitrate transport genes, including ZmNRT2.1 and ZmNRT2.2, in response to nitrate supply [17][9] (Figure 1). The ZmCHB101-RNAi lines showed significantly reduced nucleosome densities at the −1 and +1 nucleosome regions of ZmNRT2.1 and ZmNRT2.2 as compared to WT under nitrate treatment. These low nucleosome densities resulted in enhanced ZmNLP3.1 binding to the promoter regions of ZmNRT2.1 and ZmNRT2.2, further regulating their expression [17][9] (Figure 1).
2. Epigenetic Regulation of Root Architecture in Response to N
The N signal greatly influences the root system, which serves as the sensory organ for detecting external nitrogen. Nitrate, in particular, has been shown to modulate various aspects of root development, including primary root growth [42[19][20],43], lateral root initiation and elongation [44,45,46,47][21][22][23][24].
Recent discoveries highlight the regulatory role of microRNAs (miRNAs) on key TFs involved in root development in monocots. One such TF is ARABIDOPSIS NITRATE REGULATED 1 (ANR1), a member of the MIKC-type MADS (MCM1/AGAMOUS/DEFICIENS/SRFl) box family of TFs, which mediates the root response to external N [48][25]. It stimulates lateral root growth, leading to an increase in lateral root number and length, as well as total shoot fresh weight [49][26]. The conservation of ANR1 function has been observed in various species, including rice [50][27] and chrysanthemum [51][28]. Overexpression of miR444a in rice reduced lateral root elongation in a nitrate-dependent manner by targeting ANR1 [52][29] (Figure 1). However, miR444a overexpression promoted primary and adventitious root growth and improved nitrate accumulation under high nitrate concentration conditions [52][29].
In dicots, in particular Arabidopsis, the presence of several ncRNAs has been observed in response to N. The miR393 also regulates primary root length and lateral root density in response to N availability in Arabidopsis by targeting AUXIN SIGNALING F-BOX 3 (AFB3) [53][30]. Similarly, miR167 inhibits lateral root outgrowth in response to N by targeting AUXIN RESPONSE FACTOR 8 (ARF8) in pericycle cells in Arabidopsis [37][13] (Figure 1). Additionally, T5120, a long non-coding RNA modulated by NLP7 and NRT1.1, promotes nitrate assimilation and root growth (primary root length and lateral root number) in Arabidopsis [22][31] (Figure 1). Interestingly, the miRNA/target modules are responsive to downstream N metabolites, with miR393 and ARF8 being induced by an N metabolite produced during N reduction, coordinating plant growth and development in response to both external and internal N availability [53][30].
3. Epigenetic Regulation of Plant Architecture in Response to N
N supply exerts an epigenetic influence not only on root development but also on plant architecture, particularly for tillering in monocots. In rice, the application of N fertilizer induces changes in the genome-wide H3K27me3 pattern through NITROGEN-MEDIATED TILLER GROWTH RESPONSE 5 (NGR5). This mechanism facilitates the recruitment of PRC2, thus promoting the repression of branching-inhibitory genes (Dwarf14 (D14) and SQUAMOSA-promoter binding protein-like 14 (SPL14)) through H3K27me3 modification [54][32] (Figure 1). Interestingly, PRC2 has been reported to associate with the regulation of rice tillering with normal N condition. In particular, VIN3-LIKE 2 (OsVIL2) suppresses the expression of TEOSINTE BRANCHED1 (OsTB1), which is a branching-inhibitory gene, by recruiting PRC2 and promoting bud outgrowth [55][33]. In this context, NRG5 acts as a missing link, connecting N signaling with the regulation of tillering through PRC2. Consequently, exploring the connection between N signaling and chromatin response to N can uncover crucial factors, like NGR5, involved in the regulation of NUE.
In conclusion, the integration of epigenetic regulations involving histone modification, chromatin remodeling, and miRNA regulation in a plant’s response to N underscores the profound influence of N on the epigenome, transcriptional processes, and subsequent divergence in growth and metabolism. Moreover, establishing the link between N signaling and chromatin response holds great significance. Considering the findings discussed above, exploring the role of epigenomic changes in plants adapted to different N conditions, such as LN, becomes particularly intriguing.
References
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutierrez, R.A. Nitrate Transport, Sensing, and Responses in Plants. Mol. Plant 2016, 9, 837–856.
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate Transport, Signaling, and Use Efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122.
- Maeda, Y.; Konishi, M.; Kiba, T.; Sakuraba, Y.; Sawaki, N.; Kurai, T.; Ueda, Y.; Sakakibara, H.; Yanagisawa, S. A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat. Commun. 2018, 9, 1376.
- Chen, J.; Liu, X.; Liu, S.; Fan, X.; Zhao, L.; Song, M.; Fan, X.; Xu, G. Co-Overexpression of OsNAR2.1 and OsNRT2.3a Increased Agronomic Nitrogen Use Efficiency in Transgenic Rice Plants. Front. Plant Sci. 2020, 11, 1245.
- Chen, K.E.; Chen, H.Y.; Tseng, C.S.; Tsay, Y.F. Improving nitrogen use efficiency by manipulating nitrate remobilization in plants. Nat. Plants 2020, 6, 1126–1135.
- Fan, X.; Tang, Z.; Tan, Y.; Zhang, Y.; Luo, B.; Yang, M.; Lian, X.; Shen, Q.; Miller, A.J.; Xu, G. Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc. Natl. Acad. Sci. USA 2016, 113, 7118–7123.
- Gao, Z.; Wang, Y.; Chen, G.; Zhang, A.; Yang, S.; Shang, L.; Wang, D.; Ruan, B.; Liu, C.; Jiang, H.; et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 2019, 10, 5207.
- Hu, M.; Zhao, X.; Liu, Q.; Hong, X.; Zhang, W.; Zhang, Y.; Sun, L.; Li, H.; Tong, Y. Transgenic expression of plastidic glutamine synthetase increases nitrogen uptake and yield in wheat. Plant Biotechnol. J. 2018, 16, 1858–1867.
- Meng, X.; Yu, X.; Wu, Y.; Kim, D.H.; Nan, N.; Cong, W.; Wang, S.; Liu, B.; Xu, Z.Y. Chromatin Remodeling Protein ZmCHB101 Regulates Nitrate-Responsive Gene Expression in Maize. Front. Plant Sci. 2020, 11, 52.
- Widiez, T.; El Kafafi, S.; Girin, T.; Berr, A.; Ruffel, S.; Krouk, G.; Vayssieres, A.; Shen, W.H.; Coruzzi, G.M.; Gojon, A.; et al. High nitrogen insensitive 9 (HNI9)-mediated systemic repression of root NO3- uptake is associated with changes in histone methylation. Proc. Natl. Acad. Sci. USA 2011, 108, 13329–13334.
- Bvindi, C.; Tang, L.; Lee, S.; Patrick, R.M.; Yee, Z.R.; Mengiste, T.; Li, Y. Histone methyltransferases SDG33 and SDG34 regulate organ-specific nitrogen responses in tomato. Front. Plant Sci. 2022, 13, 1005077.
- Li, Y.; Mukherjee, I.; Thum, K.E.; Tanurdzic, M.; Katari, M.S.; Obertello, M.; Edwards, M.B.; McCombie, W.R.; Martienssen, R.A.; Coruzzi, G.M. The histone methyltransferase SDG8 mediates the epigenetic modification of light and carbon responsive genes in plants. Genome Biol. 2015, 16, 79.
- Gifford, M.L.; Dean, A.; Gutierrez, R.A.; Coruzzi, G.M.; Birnbaum, K.D. Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. USA 2008, 105, 803–808.
- Li, Y.; Brooks, M.; Yeoh-Wang, J.; McCoy, R.M.; Rock, T.M.; Pasquino, A.; Moon, C.I.; Patrick, R.M.; Tanurdzic, M.; Ruffel, S.; et al. SDG8-Mediated Histone Methylation and RNA Processing Function in the Response to Nitrate Signaling. Plant Physiol. 2020, 182, 215–227.
- Bellegarde, F.; Herbert, L.; Sere, D.; Caillieux, E.; Boucherez, J.; Fizames, C.; Roudier, F.; Gojon, A.; Martin, A. Polycomb Repressive Complex 2 attenuates the very high expression of the Arabidopsis gene NRT2.1. Sci. Rep. 2018, 8, 7905.
- Bellegarde, F.; Maghiaoui, A.; Boucherez, J.; Krouk, G.; Lejay, L.; Bach, L.; Gojon, A.; Martin, A. The Chromatin Factor HNI9 and ELONGATED HYPOCOTYL5 Maintain ROS Homeostasis under High Nitrogen Provision. Plant Physiol. 2019, 180, 582–592.
- Yu, X.; Jiang, L.; Wu, R.; Meng, X.; Zhang, A.; Li, N.; Xia, Q.; Qi, X.; Pang, J.; Xu, Z.-Y.; et al. The Core Subunit of A Chromatin-Remodeling Complex, ZmCHB101, Plays Essential Roles in Maize Growth and Development. Sci. Rep. 2016, 6, 38504.
- Yu, X.; Meng, X.; Liu, Y.; Li, N.; Zhang, A.; Wang, T.-J.; Jiang, L.; Pang, J.; Zhao, X.; Qi, X.; et al. The chromatin remodeler ZmCHB101 impacts expression of osmotic stress-responsive genes in maize. Plant Mol. Biol. 2018, 97, 451–465.
- Patterson, K.; Walters, L.A.; Cooper, A.M.; Olvera, J.G.; Rosas, M.A.; Rasmusson, A.G.; Escobar, M.A. Nitrate-Regulated Glutaredoxins Control Arabidopsis Primary Root Growth. Plant Physiol. 2016, 170, 989–999.
- Walch-Liu, P.; Forde, B.G. Nitrate signalling mediated by the NRT1.1 nitrate transporter antagonises l-glutamate-induced changes in root architecture. Plant J. 2008, 54, 820–828.
- Asim, M.; Ullah, Z.; Oluwaseun, A.; Wang, Q.; Liu, H. Signalling Overlaps between Nitrate and Auxin in Regulation of The Root System Architecture: Insights from the Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 2880.
- Asim, M.; Ullah, Z.; Xu, F.; An, L.; Aluko, O.O.; Wang, Q.; Liu, H. Nitrate Signaling, Functions, and Regulation of Root System Architecture: Insights from Arabidopsis thaliana. Genes 2020, 11, 633.
- Liu, B.; Wu, J.; Yang, S.; Schiefelbein, J.; Gan, Y. Nitrate regulation of lateral root and root hair development in plants. J. Exp. Bot. 2020, 71, 4405–4414.
- Sun, C.H.; Yu, J.Q.; Hu, D.G. Nitrate: A Crucial Signal during Lateral Roots Development. Front. Plant Sci. 2017, 8, 485.
- Zhang, H.; Forde, B.G. An Arabidopsis MADS Box Gene That Controls Nutrient-Induced Changes in Root Architecture. Science 1998, 279, 407–409.
- Gan, Y.; Bernreiter, A.; Filleur, S.; Abram, B.; Forde, B.G. Overexpressing the ANR1 MADS-Box Gene in Transgenic Plants Provides New Insights into its Role in the Nitrate Regulation of Root Development. Plant Cell Physiol. 2012, 53, 1003–1016.
- Yu, C.; Liu, Y.; Zhang, A.; Su, S.; Yan, A.; Huang, L.; Ali, I.; Liu, Y.; Forde, B.G.; Gan, Y. MADS-box transcription factor OsMADS25 regulates root development through affection of nitrate accumulation in rice. PLoS ONE 2015, 10, e0135196.
- Sun, C.H.; Yu, J.Q.; Duan, X.; Wang, J.H.; Zhang, Q.Y.; Gu, K.D.; Hu, D.G.; Zheng, C.S. The MADS transcription factor CmANR1 positively modulates root system development by directly regulating CmPIN2 in chrysanthemum. Hortic. Res. 2018, 5, 52.
- Yan, Y.; Wang, H.; Hamera, S.; Chen, X.; Fang, R. miR444a has multiple functions in the rice nitrate-signaling pathway. Plant J. 2014, 78, 44–55.
- Vidal, E.A.; Araus, V.; Lu, C.; Parry, G.; Green, P.J.; Coruzzi, G.M.; Gutierrez, R.A. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 4477–4482.
- Liu, F.; Xu, Y.; Chang, K.; Li, S.; Liu, Z.; Qi, S.; Jia, J.; Zhang, M.; Crawford, N.M.; Wang, Y. The long noncoding RNA T5120 regulates nitrate response and assimilation in Arabidopsis. New Phytol. 2019, 224, 117–131.
- Wu, K.; Wang, S.; Song, W.; Zhang, J.; Wang, Y.; Liu, Q.; Yu, J.; Ye, Y.; Li, S.; Chen, J.; et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science 2020, 367, eaaz2046.
- Yoon, J.; Cho, L.H.; Lee, S.; Pasriga, R.; Tun, W.; Yang, J.; Yoon, H.; Jeong, H.J.; Jeon, J.S.; An, G. Chromatin Interacting Factor OsVIL2 Is Required for Outgrowth of Axillary Buds in Rice. Mol. Cells 2019, 42, 858–868.
More
