Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Zulhelmi Amir.
Microbial enhanced oil recovery (MEOR) involves the utilization of microbes and their by-products, such as biosurfactants, biopolymers, biogenic acids, solvents, biogases, biomass, and emulsifiers, to stimulate the production of oil by mobilizing residual reserves.
- MEOR
- microbial consortium
- mechanism
- effect
1. Introduction
Crude oil is the primary energy source within the intricate capillary network in porous reservoir media. Enhanced oil recovery aims to economically extract the maximum amount of original oil in place (OOIP). It can be categorized into three stages based on the development process: primary recovery (natural energy extraction), secondary recovery (injecting water or gas to maintain reservoir pressure), and tertiary recovery (known as enhanced oil recovery or EOR) [1]. Tertiary recovery methods include chemical flooding (including polymer flooding, surfactant flooding, and alkaline flooding [2]), thermal flooding, microbial enhanced oil recovery (MEOR), and miscible flooding, which are designed to increase the amount of oil recovered by altering the reservoir conditions or fluid properties [3,4][3][4]. Figure 1 illustrates the different stages of oil recovery and their respective operating mechanisms.
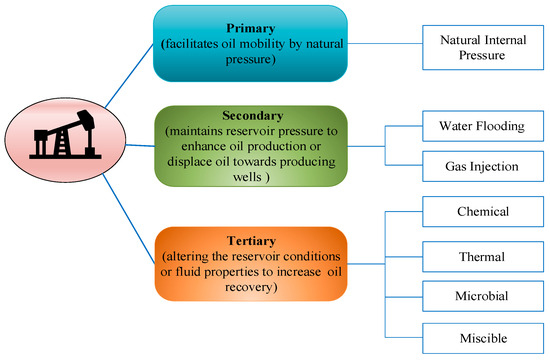
Figure 1.
The distinct stages of oil recovery and their corresponding operational mechanisms.
In generally, primary production allows for recovery of approximately 10% of the OOIP, and secondary recovery techniques can improve the overall oil recovery by 12% [5]. Tertiary recovery techniques come into play when primary and secondary recovery methods have become ineffective and significant amounts of oil remain trapped in the reservoir. As one of the methods of tertiary oil recovery, polymer flooding technology, particularly using partially hydrolyzed polyacrylamide (HPAM), has emerged as a crucial tertiary oil recovery method and has gained widespread application [6]. This technique involves the application of HPAM to block large channels, increase the viscosity of the displacing phase, and effectively enhance the oil recovery rate by 8–15% [7]. However, polymer flooding encounters challenges related to severe plugging issues and a reduction in oil recovery. This is a consequence of the polymer solution’s potential reactivity with metal ions in the original formation, resulting in the formation of compounds that are difficult to degrade [8].
2. Microbial Enhanced Oil Recovery
Microbial enhanced oil recovery (MEOR) involves the utilization of microbes and their by-products, such as biosurfactants, biopolymers, biogenic acids, solvents, biogases, biomass, and emulsifiers, to stimulate the production of oil by mobilizing residual reserves [14][9]. According to Quraishi et al. [22][10], microbial flooding can be categorized into exogenous and indigenous. The category is based on the source of the strains. Exogenous microbial flooding involves the injection of microbes that have been screened under conditions similar to, but not within, the reservoir. These microbes are introduced underground to enhance oil production through their propagation and metabolites. Indigenous microbial flooding involves the utilization of remaining oil as a carbon source by microbes, making use of the active substances present in the formations. During water injection, air, inorganic salts, a phosphorus source, and a nitrogen source are introduced to facilitate the proliferation of these native microorganisms [14][9]. The key aspect of exogenous microbial flooding lies in developing effective production strains, and challenges include ensuring the compatibility of microorganisms, performance degradation, and high costs [3]. Indigenous microbial flooding shows excellent adaptability but lacks a subsequent procedure for developing production strains. In the process of MEOR implementation, microorganisms are commonly cultured ex situ and subsequently introduced into the reservoir by injection. As the injected water is transported, these microorganisms build up in the caprock pores, where oil is present at the interface between oil and water. After the injection of MEOR bacteria, in situ production persists, resulting in continuous alterations in the oil and reservoir properties, which facilitates the mobilization of tightly trapped oil to the surface [23][11]. The MEOR diagram in Figure 2 illustrates introducing a water mixture, including bacteria and/or biosurfactants, accompanied by a nutrient medium, into the reservoir as part of the MEOR process. Inside the reservoir, the bacteria facilitate the biodegradation of heavy crude oil into light components through biosurfactants. This degradation process aids in improving the movement of oil toward the production well. Furthermore, the injected bacteria experience metabolic activities that result in the production of metabolites. These metabolites contribute to multiple mechanisms, including reducing oil viscosity, lowering interfacial tension (IFT), promoting emulsification, and re-pressurizing the reservoir. These mechanisms collectively enhance the recovery of residual oil.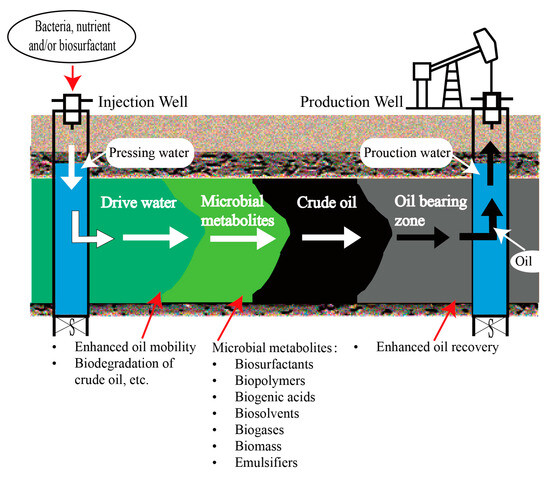
Figure 2.
Diagram of MEOR.
2.1. Microorganism
Oil reservoirs can be viewed as extensive geo-bioreactors primarily populated by sulfate-reducing bacteria (SRB), methanogens, syntrophic bacteria, and fermentative bacteria [24][12]. Accordingly, the dominant microbial processes observed in oil field ecosystems affected by petroleum hydrocarbons include sulfate reduction, fermentation, acetogenesis, methanogenesis, nitrate reduction, as well as iron and manganese reduction [25,26][13][14]. In addition, oil reservoirs contain a significant number of organic substances, including various inorganic ions such as sulfate and nitrate, as well as organic compounds like alkanes, alkenes, cycloalkanes, and aromatic hydrocarbons [27][15]. The microbial activities in oil reservoirs significantly impact oil’s chemical composition and physical–chemical properties [28][16]. This influence can be either positive, such as reducing viscosity in heavy oil to enhance its exploitation, or negative, leading to corrosion of drilling equipment or reservoir souring [29,30][17][18]. A large number of studies have highlighted the significance of microbial community dynamics within petroleum oilfield ecosystems [27][15]. The spatial distribution of microbial communities within long cores plays a role in the mechanism of oil displacement by microorganisms [31][19]. Generally, in the vicinity of the injection water, aerobic oil-displacement functional bacteria can decrease oil–water IFT by producing biosurfactants and emulsifying crude oil [12][20]. Additionally, aerobic hydrocarbon-loving microorganisms are abundant in the aerobic zone, which enhances crude oil’s physical properties and residual oil fluidity through aerobic hydrocarbon metabolism. Within the middle section of the reservoir, facultative and anaerobic microorganisms coexist and produce H2, CO2, small molecule acids, and alcohols through anaerobic fermentation [32][21]. Within the anoxic conditions of the reservoir’s deep environment, methanogenic microbes increase crude oil fluidity by generating CH4 [33][22]. Figure 3 depicts the distribution of microorganisms in MEOR.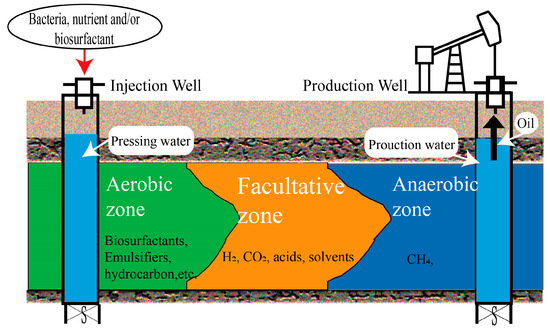
Figure 3.
The spatial distribution of microorganisms in MEOR.
2.2. Microbial Products
The effectiveness of the MEOR process relies on metabolite production by either exogenous or indigenous microorganisms [42][31]. These metabolites play a crucial role in altering the rock properties of the reservoir, including permeability, porosity, wettability, and oil viscosity. This alteration facilitates oil recovery by reducing viscosity, emulsifying the oil, pressuring the reservoir, and dissolving residual oil [3]. The bioproducts can generally be categorized into seven main groups: biosurfactants, biopolymers, biogenic acids, biosolvents, biogas, biomass, and emulsifiers.2.2.1. Biosurfactants
Biosurfactants, a group of surface-active molecules that are synthesized by microorganisms via fermentation processes, can be categorized into five primary groups, including glycolipids, phospholipids and fatty acids, particulate surfactants, lipopeptides and lipoproteins, and polymeric surfactants [43][32]. These molecules exhibit great potential for improving oil recovery on account of their ability to lower IFT and surface tension, alter wettability, and create water/oil or oil/water emulsions, thereby facilitating the mobilization of oil to the surface [44,45][33][34]. The abilities mentioned above result from biosurfactants forming biofilms that interact with reservoir rocks and water/oil formations, bringing about modifications in their characteristics. Pseudomonas aeruginosa is an illustrative example of a biosurfactant-producing microorganism that exhibited the capability to degrade alkanes, including hexadecane and octadecane, within a 28-day incubation period [46][35]. As stated by Niu et al. [47][36], biosurfactants with lower molecular weights can reduce surface tension and IFT, whereas those with higher molecular weights display emulsifying activities, forming stable emulsions at the interface due to the close arrangement of surfactant molecules. The effectiveness of reducing IFT relies on the number of biosurfactants required to attain the desired reduction and the adsorption of biosurfactants onto caprocks. To achieve significant oil production, it is necessary to reduce the IFT to a range of approximately 10−2 to 10−3 mN/m, which corresponds to a capillary number of 10−3 to 10−4 [48][37]. Numerous studies have reported significant oil recovery achieved through the use of biosurfactants. To produce biosurfactants of high quality with minimal losses, careful consideration of various conditions, including temperature, salinity, pH, oxygen, nutrient composition, and physical and chemical parameters, is necessary. Additionally, it is essential to investigate the characteristics of compounds such as carbon sources and nitrogen sources, as well as the C:N ratio and the selection of bacterial strains, to ensure the production of biosurfactants that are effective [49][38]. Prior studies have shown that in situ production of surfactants is typically restricted by the requirement of most surfactant-producing microorganisms for oxygen to support their growth. In the past few years, numerous experimental studies have shown that specific microorganisms have the ability to generate surfactants under anaerobic conditions. This ability is exceedingly advantageous for extracting trapped oil residues situated within oxygen-depleted reservoirs’ deep pores. It facilitates surfactant production and subsequently improves oil recovery without needing oxygen during metabolic processes. Biosurfactants offer several advantages for EOR applications, including their biodegradability, outstanding surface and interfacial activities, low toxicity and capital cost, enough raw materials for production, and substantial productivity. These attributes make biosurfactants a cost-effective and efficient approach for achieving enhanced oil recovery.2.2.2. Biopolymers
Injected microorganisms and their metabolites encounter significant limitations in migrating within low- and high permeability zones; they face hindrances in contacting residual oil caused by preferential fluid flow [50][39]. To resolve this problem, biopolymers as plugging agents are employed to selectively plug high permeability zones and redirect injected water towards low permeability zones to enhance the recovery of residual oil [45,51][34][40]. Figure 4 visually illustrates the mechanism of selective plugging. The left-hand side diagram initially portrays water flowing effortlessly through wide pore channels, neglecting the narrow and low permeable zones resulting in poor sweep efficiency. In contrast, the right-hand side diagram illustrates the effective plugging of the wide pore channel via biopolymers. When the biopolymers adhere and grow, they deviate the water flow towards the less permeable zones, facilitating oil displacement and sweep efficiency [52][41]. The biopolymer plugging process can be accomplished through either the injection of bacteria or the in-situ production of bacteria in the reservoir. As mentioned by Sen [53][42], microorganisms enter the reservoir via high permeability zones and establish themselves in specific laminae. The presence of the growth of biopolymers in the pore throat leads to the plugging of the pore space, causing a reduction in the permeability rate. As a result, plugging pore throats with biopolymer growth helps in restoring a balanced permeability across the reservoir, thereby allowing water-flooding operations to resume with conventional sweep efficiency.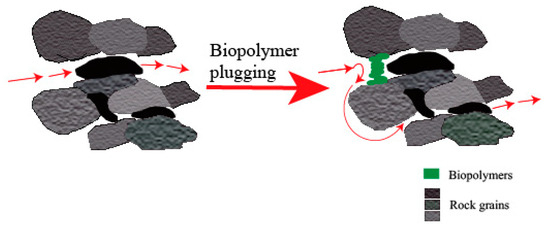
Figure 4.
Illustration of the selective plugging mechanism.
2.2.3. Biogenic Acids
The production of bioacids contributes to the dissolution of carbonate rocks in the reservoir, leading to increased porosity and permeability and assisting oil displacement into the remaining reservoir [18][45]. Generally, low molecular weight organic acids like formic acid, acetic acid, and propionic acid are commonly employed in MEOR [19][46]. It has been reported that organic acids serve as the active metabolites responsible for reducing oil viscosity, thereby elevating their mobility. Laboratory experiments conducted in Daqing Oilfield demonstrated a 36% reduction in viscosity through the utilization of microbial acids [14][9].2.2.4. Others
- (1)
-
Biosolvents
- (2)
-
Biogases
- (3)
-
Biomass
- (4)
-
EmulsifiersTable 1.Microbial products.
Microbial Products Representative Microorganisms Effect Limitation Type of Formation/Reservoir Reference -
Biosurfactant (Alasan, Surfactin, Rhamnolipid, Emulsan)
-
Acinetobacter Arthrobacter paraffineus sp.
-
Calcoaceticus sp.
-
Emulsify crude oil into an oil–water mixture.
-
Decrease IFT.
-
Modify rock wettability.
-
Low displacement efficiency at the microscopic level.
-
Reservoirs composed of sandstone or carbonate formations with temperatures below 50 °C and containing lighter oils with an API gravity greater than 25.
[57][49] -
Biopolymers (Xanthan gum, Pullulan, Levan)
-
Bacillus polymyxa sp.
-
Pseudomonas sp.
-
Brevibacterium viscogenes sp.
-
Raise the viscosity of the displacing fluid while diminishing the mobility ratio between water and oil.
-
Optimize the sweep regions and effectiveness with selective plugging.
-
Low efficiency in sweeping fluids across the volume.
-
Stratified reservoirs exhibiting distinct permeable zones.
[58][50] -
Bioacids (Acetic acid, formic acid, propionic acid)
-
Clostridium sp.
-
Enterobacter aerogenes sp.
-
Methanobacterium sp.
-
Improve the rock’s dissolution in the pore throats to increase porosity and permeability.
65 -
Low porosity.
-
Inefficient fluid drainage.
-
Reservoir impairment.
-
Carbonate or carbonaceous reservoirs
][59][51] -
Biogases (CO2, H2, CH4, N2)
-
Clostridium sp.
-
Enterobacter aerogenes sp.
-
Enhance the mobility of crude oil by reducing its viscosity.
Gordonia amici C18–C36 - Re-establish reservoir pressure
-
Gas injection for partial or miscible displacement.
[74][ -
Equipment corrosion.
-
Reservoir souring.
66] -
Formations containing heavy crude oil with an API gravity of less than 25.
[22][10] -
Biosolvents (Alcohols, ketones, acetone, butanol)
-
Zymomonas mobilis sp.
-
Clostridium acetobutylicum sp.
-
Klebsiella sp.
-
Decrease the viscosity of oil through its dissolution in crude oil.
-
Alcohols and ketones can act as co-surfactants in the formation of micelles, leading to a decrease in critical micelle concentration (CMC) and IFT, thereby facilitating emulsification.
-
Optimize the porosity and permeability by dissolving heavy oil in the pore throats.
-
Low displacement efficiency at the microscopic level.
-
Formations containing heavy crude oil with an API gravity of less than 25.
-
Reservoirs with high oil wettability have undergone water flooding.
[60][52] -
Biomass (Microbial cells, biofilms, EPS)
-
Licheniformis
-
Bacillus
-
Xanthomonas
-
Leuconostocmesenteroides
-
Campestris
-
Change rock wettability.
-
Employ selective plugging to optimize sweep effectiveness.
-
Gas-based biopolymers.
-
Specific decomposition of heavy oil.
-
Low displacement efficiency at the microscopic level.
-
Stratified reservoirs exhibiting distinct permeable zones.
[61][53] -
Emulsifiers
-
Candida
-
Acinetobacter sp.
-
Bacillus sp.
-
Pseudomonas sp.
-
Emulsification of oil with water.
-
Sludge buildup.
-
Low displacement efficiency at the microscopic level.
-
Formation containing high-molecular-weight waxy oil (>C22 alkanes), paraffinic oil, and asphaltene.
[62,63][54][55] 2.3. Mechanisms of Microbial Enhanced Oil Recovery
MEOR is founded on two fundamental principles. The first principle involves modifying the interfacial properties of oil–water minerals to enhance oil movement through porous media, thereby improving displacement efficiency (IFT reduction or permeability increase), driving force (reservoir pressure), fluidity (miscible flooding, viscosity reduction), and sweep efficiency (selective plugging, mobility control). The second principle focuses on microbial activity for the degradation and removal of sulfur and heavy metals from heavy oils [64][56]. Due to the diverse range of strains and metabolites involved, the mechanism of MEOR is intricate and comprehensive, encompassing processes such as biodegradation, emulsification, IFT reduction, and alteration of reservoir rock properties [19][46]. A thorough comprehension of the mechanisms of MEOR lays the groundwork for evaluating its practicability and prospective benefits.2.3.1. Biodegradation
Biodegradation is of paramount importance in MEOR. The main aspect of this mechanism involves the breakdown of crude oil’s long-chain hydrocarbons into shorter hydrocarbons, which results in an increase of up to 30% in the light components of crude oil [14][9]. From one perspective, through this process, bacteria can obtain carbon for energy, growth, and reproduction by degrading hydrocarbons in crude oil [65][57]. From another perspective, converting heavy components into lighter ones brings about fundamental alterations in crude oil properties, leading to a reduction in viscosity and an enhancement in fluidity. Consequently, this contributes to improved oil recovery rates. This biodegradation mechanism entails direct cell contact and the involvement of biosurfactants, which decrease IFT and facilitate the emulsification of hydrocarbons [66][58]. Biosurfactants are commonly utilized to degrade the heavy fractions of crude oil. Biodegradation pathways can be classified into aerobic and anaerobic biodegradation [19][46]. Typically, aerobic biodegradation occurs near the injection wells, while anaerobic biodegradation takes place in deep reservoirs. Aerobic biodegradation involves dissolving oxygen and injecting it into the reservoir alongside water rich in nutrients in MEOR, which has been an established and extensively employed technique for many years. Field trials have been conducted in China’s Daqing Oilfield, where dissolved oxygen was injected to stimulate aerobic bacteria for degrading heavy components, thereby enhancing oil recovery [14][9]. During aerobic biodegradation, Pseudomonas putida with alkB genes on the OCT plasmid is capable of degrading aliphatic alkanes. This degradation process involves the enzyme alkane hydroxylase, which consists of rubredoxin reductase, oxygenase, and membrane-bound rubredoxin. These cofactors facilitate the transport of electrons from nicotinamide adenine dinucleotide phosphate to hydrocarbon substrates. The degradation of alkanols occurs through alcohol dehydrogenase, which converts them into alkanals. Subsequently, alkanals are transformed into fatty acids by aldehyde dehydrogenase and further converted to acetyl CoA by its synthetase [41][30]. Cell biomass biosynthesis occurs in the central precursor metabolites such as acetyl-CoA, succinate, and pyruvate [67][59], as depicted in Figure 5.Anaerobic degradation has demonstrated the capability of biodegrading various hydrocarbons, including benzene, toluene, naphthalene, asphaltene, phenanthrene, alkane, branched alkane, and hydrocarbon mixtures [69][61]. Nonetheless, the application of anaerobic degradation in MEOR is restricted, possibly because of the choice of unsuitable oil-degrading microorganisms or suboptimal conditions [70][62]. Of all the options, the anaerobic degradation of long-chain alkanes holds the greatest promise for achieving enhanced oil recovery. Table 2 displays the commonly encountered long-chain degrading microorganisms and the scope of hydrocarbons they can degrade. Furthermore, oil-degrading microorganisms that have the ability to degrade asphaltenes are frequently regarded as highly effective, while hydrocarbon-degrading microorganisms generating biosurfactants are considered optimal for MEOR [69][61].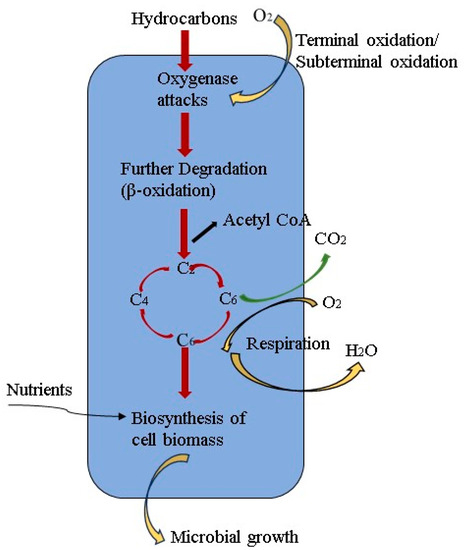 Figure 5. The fundamental concept underlying aerobic hydrocarbon degradation by microorganisms [68].Since reservoirs are generally anaerobic environments, anaerobic degradation predominantly governs the biodegradation of hydrocarbons in deep reservoirs. The thorough examination of this process has led to a clear understanding of the biochemical mechanisms involved in the anaerobic biodegradation of organisms. An illustration of this is the work by Purwasena et al. [77][69], who effectively isolated a thermophilic anaerobic bacterium strain belonging to the Petrotoga sp. from a Japanese oil reservoir. Through core flooding methods, their findings demonstrated that the bacterium could degrade long-chain n-alkanes, reduce oil viscosity, and enhance oil recovery under 80 °C. Additionally, researchers have effectively identified the key metabolites and associated genes involved in this specific biodegradation mechanism [19][46]. Nevertheless, a complete understanding of the biochemical processes involved in anaerobic hydrocarbon mechanisms is still being explored and investigated. In summary, biodegradation plays a crucial role in MEOR, as research indicates that microorganisms and their products can degrade carbon atoms by utilizing them as a carbon source for metabolic processes. As a consequence, the viscosity of the oil is reduced, and the flow properties are improved; hence, promoting oil displacement towards production wells for recovery. While aerobic and anaerobic biodegradation pathways exist, anaerobic degradation predominantly controls the process. The duration of the biodegradation process varies based on factors such as hydrocarbon composition, bacterial population, reservoir environment, and the amount of oxygen available or introduced into the wells.Table 2.Microorganisms that exclusively use long-chain n-alkanes as their carbon source.
Figure 5. The fundamental concept underlying aerobic hydrocarbon degradation by microorganisms [68].Since reservoirs are generally anaerobic environments, anaerobic degradation predominantly governs the biodegradation of hydrocarbons in deep reservoirs. The thorough examination of this process has led to a clear understanding of the biochemical mechanisms involved in the anaerobic biodegradation of organisms. An illustration of this is the work by Purwasena et al. [77][69], who effectively isolated a thermophilic anaerobic bacterium strain belonging to the Petrotoga sp. from a Japanese oil reservoir. Through core flooding methods, their findings demonstrated that the bacterium could degrade long-chain n-alkanes, reduce oil viscosity, and enhance oil recovery under 80 °C. Additionally, researchers have effectively identified the key metabolites and associated genes involved in this specific biodegradation mechanism [19][46]. Nevertheless, a complete understanding of the biochemical processes involved in anaerobic hydrocarbon mechanisms is still being explored and investigated. In summary, biodegradation plays a crucial role in MEOR, as research indicates that microorganisms and their products can degrade carbon atoms by utilizing them as a carbon source for metabolic processes. As a consequence, the viscosity of the oil is reduced, and the flow properties are improved; hence, promoting oil displacement towards production wells for recovery. While aerobic and anaerobic biodegradation pathways exist, anaerobic degradation predominantly controls the process. The duration of the biodegradation process varies based on factors such as hydrocarbon composition, bacterial population, reservoir environment, and the amount of oxygen available or introduced into the wells.Table 2.Microorganisms that exclusively use long-chain n-alkanes as their carbon source.2.3.2. Emulsification
Emulsions have an impact on increasing the oil–water interface, leading to enhance the accessibility of oil [78][70]. Different types of emulsions are classified based on particle size, including transparent microemulsion (nm), translucent colloid emulsion (below 1 μm), milky white emulsion (1–2 μm), fine dispersion (1 mm), and coarse emulsion (100 mm) [79][71]. Emulsification can change the reservoir’s wettability, shifting it from oil-wet to water-wet, thus making trapped oil easier to recover [14][9]. Figure 6 provides a general visual representation of the emulsification process. On the left-hand side of the figure, water containing biosurfactant comes into contact with oil. Biosurfactants composed of both hydrophobic and hydrophilic structures, through their hydrophobic interaction properties, facilitate the formation of steady oil emulsions in water, as depicted on the right-hand side of Figure 6. These emulsions allow for the coexistence of both liquids by creating amphiphilic films at the oil–water interface [80][72]. To ensure a consistent dispersion of oil in water, biosurfactants are incorporated to provide stabilization. The hydrophobic interaction properties of biosurfactants enable them to attach to the interface between oil and water, improving the dispersion and facilitating movement until the biosurfactant is either diluted or completely adsorbed on the caprocks. Emulsification of crude oil leads to the generation of various droplet sizes, exhibiting tensile, deformed, and seepage flow characteristics, which alter the permeability of the reservoir, and increase the contact area between the droplets and the reservoir medium, thereby facilitating the flow and recovery of oil [14][9]. Additionally, the structural alteration of bioacids contributes to changes in wettability, converting water-in-oil emulsion into oil-in-water emulsion, followed by a subsequent reduction in oil viscosity.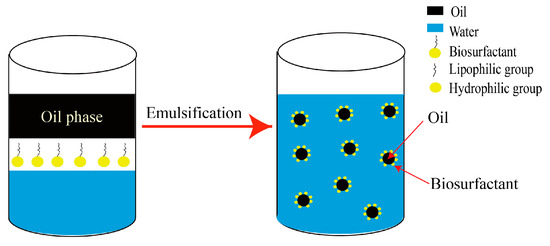 Figure 6.Schematic diagram illustrating the emulsification process.
Figure 6.Schematic diagram illustrating the emulsification process.2.3.3. Interfacial Tension Reduction
Reducing the IFT is an alternative mechanism that significantly enhances oil recovery. One of the three components contributing to the reduction in IFT is the work of adhesion, which represents the energy required to separate an oil film from an oil-wet rock surface [81][73]. Secondly, the elasticity of the oil/water interface is changed, enabling the two phases to mix more easily [82][74]. Thirdly, biosurfactants, characterized by their amphiphilic nature, have the ability to accumulate at the interface between immiscible fluids like oil and water or water and oil. This accumulation leads to a reduction in IFT, ultimately strengthening capillary forces and making oil easier to flow [83][75]. The formation of foams using organic materials also can lead to a reduction in IFT at the oil/water interface [23][11]. A visual representation of reducing IFT can be seen in Figure 7.IFT reduction and wettability alteration are closely related mechanisms that occur concurrently and contribute significantly to EOR [84][76]. The reduction in IFT increases the capillary number by 3–4 orders of magnitude, resulting in improved oil flow. Simultaneously, wettability alteration promotes oil production by supporting the mobilization and displacement of oils attached to the rock surface or trapped in the pores [85,86][77][78]. The wettability condition of the rocks significantly influences the oil recovery [84][76]. In the case of oil-wet rocks, IFT reduction helps decrease the negative capillary force that hinders the entry of injection fluids. For intermediate-wet rocks, IFT reduction facilitates oil desorption and promotes mixing between oil and water. In the case of water-wet rocks, IFT reduction reduces positive capillary force and weakens interface elasticity, allowing trapped oil droplets to flow more easily [84][76]. Figure 8 depicts the process of alteration in wettability.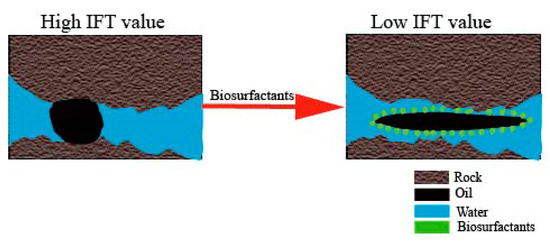 Figure 7.Graph of IFT reduction.
Figure 7.Graph of IFT reduction.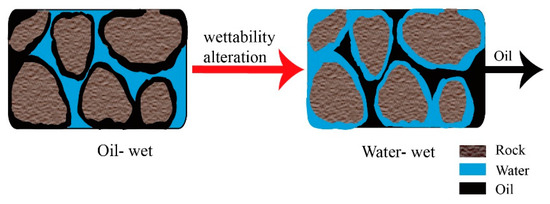 Figure 8.Illustration of alteration in wettability.
Figure 8.Illustration of alteration in wettability.2.3.4. Altering Reservoir Rock Properties
By altering the properties of reservoir rocks, it is possible to increase their permeability and porosity. Bioacids have the potential to significantly decrease the permeability of reservoir rocks, reducing it from 284 mD to 24 mD while also diminishing the viscosity by approximately tenfold [14][9]. The mechanism is believed to predominantly occur in carbonate reservoirs [87][79]. The porosity and permeability modification can be seen in Figure 9.Biosurfactants can undergo adsorption onto the pores of the reservoir rocks, leading to modifications in the wetting characteristics of the porous media. Interfacial forces dictate fluid flow at the microscopic level, making pore-scale wettability a crucial factor in enhancing oil recovery [88][80]. This phenomenon occurs due to the influence of the amphiprotic groups in biosurfactants. Biosurfactants primarily alter the reservoir rock’s wettability or aid in the displacement of cells and biofilms within pore structures by adhering to them [87][79]. The modification of wettability accelerates oil production by improving the permeability of injected water within the reservoir matrix and boosting the capillary pressure [89][81]. In porous media, fluids’ location, distribution, and flow are governed by the wettability of rock/fluid systems [90][82]. In non-fractured reservoirs, the transition from highly wet conditions to neutral wettability enhances oil extraction efficiency. In fractured reservoirs, changing the wettability to water-wet leads to favorable capillary forces, enabling the displacement of trapped oil through water infiltration into the rock formations in the opposite direction [91][83]. Nevertheless, the precise mechanisms underlying the alteration of reservoir wettability during MEOR processes remain insufficiently understood and necessitate additional research and exploration. In practical terms, a mechanism must ultimately accomplish two objectives: (1) a substantial enhancement in oil recovery and (2) a financially viable yield ratio that ensures the incremental yield of oil surpasses the input material for MEOR. When these criteria are fulfilled, a mechanism can be regarded as promising and deserving of further investigation. The feasibility and robustness of each mechanism in the field are crucial considerations. Considering the reservoir conditions, it is essential to access fundamental reservoir data such as porosity, permeability, pressure, temperature, pH, viscosity, wettability, etc. These data are critical to determining which mechanisms are most suitable and effective for the specific reservoir conditions.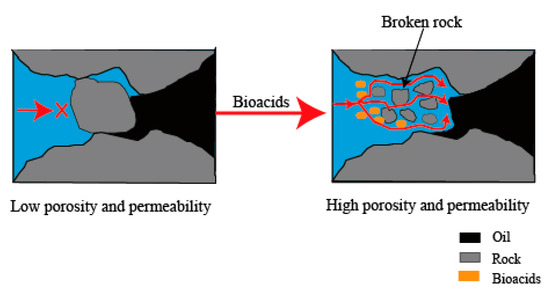 Figure 9.Diagram of the porosity and permeability modification.
Figure 9.Diagram of the porosity and permeability modification.2.4. Advantages and Disadvantages of MEOR
Given the prevailing low oil prices, MEOR holds significant potential, particularly for marginal or uneconomical reservoirs. Microbial flooding has emerged as a viable substitute for other EOR techniques, showing a remarkably high success rate [92][84]. Compared to thermal flooding and gas flooding, microbial flooding exhibits notable advantages, primarily its eco-friendly attributes and cost-effectiveness for augmenting oil production [50][39]. Compared to other EOR technologies, microbial flooding possesses distinct features. Firstly, microbial products employed in the process are typically biodegradable and harmless. Secondly, implementing microbial processes is relatively straightforward in the field, as it necessitates minimal adjustments to existing facilities [93][85]. Thirdly, unlike thermal processes that demand significant energy consumption, MEOR is not energy-intensive, making it a cost-effective EOR method [3]. Figure 10 illustrates various cost estimates for different EOR techniques.There are also some disadvantages of MEOR. For example, the presence of SRB in MEOR is recognized for its detrimental effects [94][86], leading to corrosion, plugging, and reservoir souring issues in oil production, transportation, and storage [26][14]. Moreover, the hydrogen sulfide (H2S) generated by SRB acts as an inhibitor, impeding the growth and metabolism of numerous bacteria, including those responsible for producing rhamnolipids [95][87]. Table 3 displays the current advantages and disadvantages of MEOR technology.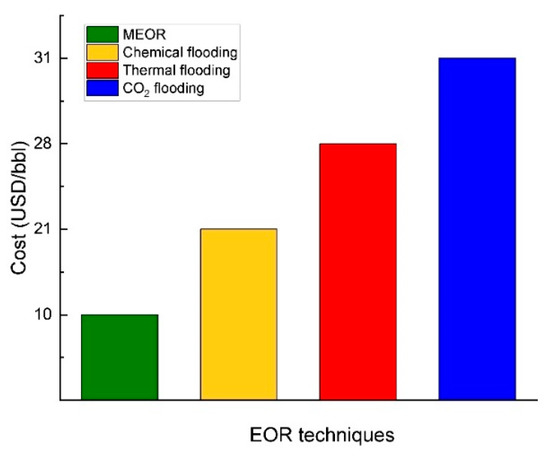 Table 3.Benefits and drawbacks of MEOR technology.
Table 3.Benefits and drawbacks of MEOR technology.Benefits Drawbacks -
Cost-effectiveness and simplicity of facility setup
-
Equipment corrosion
-
Affordable injection cost of materials
-
Minimal energy consumption associated with microbial metabolic activities
-
Minimal environmental pollution
-
Progressive enhancement of microbial metabolic activities over time
-
Achieving improved effects through the concurrent activation of multiple mechanisms
-
Significantly effective in sandstone and carbonate reservoirs
-
Microorganisms’ constrained ability to withstand reservoir conditions
-
The toxicity of microorganisms induced by the heavy metal ions
-
Challenges in constructing a comprehensive model that covers every aspect of MEOR
-
Restricted applications on offshore platforms due to the high demand for sugar in anaerobic bacteria activities
-
Ability to apply to both light and heavy crude oils
References
- Lourdes, R.S.; Cheng, S.Y.; Chew, K.W.; Ma, Z.; Show, P.L. Prospects of microbial enhanced oil recovery: Mechanisms and environmental sustainability. Sustain. Energy Technol. Assess. 2022, 53, 102527.
- Abbas, A.H.; Ajunwa, O.M.; Mazhit, B.; Martyushev, D.A.; Bou-Hamdan, K.F.; Alsaheb, R.A.A. Evaluation of OKRA (Abelmoschus esculentus) Macromolecular Solution for Enhanced Oil Recovery in Kazakhstan Carbonate Reservoir. Energies 2022, 15, 6827.
- Guo, H.; Li, Y.; Yiran, Z.; Wang, F.; Wang, Y.; Yu, Z.; Haicheng, S.; Yuanyuan, G.; Chuyi, J.; Xian, G. Progress of Microbial Enhanced Oil Recovery in China. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015.
- El-Masry, J.F.; Bou-Hamdan, K.F.; Abbas, A.H.; Martyushev, D.A. A Comprehensive Review on Utilizing Nanomaterials in Enhanced Oil Recovery Applications. Energies 2023, 16, 691.
- Seright, R.S.; Wang, D. Polymer flooding: Current status and future directions. Pet. Sci. 2023, 20, 910–921.
- Li, X.; Zhang, F.; Liu, G. Review on Polymer Flooding Technology. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Xiamen, China, 17–19 November 2021; IOP Publishing: Xiamen, China, 2021; p. 012199.
- Gao, P.; Li, G.; Le, J.; Liu, X.; Liu, F.; Ma, T. Succession of microbial communities and changes of incremental oil in a post-polymer flooded reservoir with nutrient stimulation. Appl. Microbiol. Biotechnol. 2018, 102, 2007–2017.
- Mogollón, J.L.; Lokhandwala, T. Rejuvenating Viscous Oil Reservoirs by Polymer Injection: Lessons Learned in the Field. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013.
- She, H.; Kong, D.; Li, Y.; Hu, Z.; Guo, H. Recent Advance of Microbial Enhanced Oil Recovery (MEOR) in China. Geofluids 2019, 2019, 1871392.
- Quraishi, M.; Bhatia, S.K.; Pandit, S.; Gupta, P.K.; Rangarajan, V.; Lahiri, D.; Varjani, S.; Mehariya, S.; Yang, Y.-H. Exploiting microbes in the petroleum field: Analyzing the credibility of microbial enhanced oil recovery (MEOR). Energies 2021, 14, 4684.
- Yernazarova, A.; Kayirmanova, G.; Baubekova, A.; Zhubanova, A. Microbial enhanced oil recovery. In Chemical Enhanced Oil Recovery (cEOR)—A Practical Overview; InTech: Rijeka, Croatia, 2016; pp. 147–167.
- Liang, B.; Wang, L.; Zhou, Z.; Mbadinga, S.M.; Zhou, L.; Liu, J.; Yang, S.-Z.; Gu, J.; Mu, B. High frequency of Thermodesulfovibrio spp. and Anaerolineaceae in association with Methanoculleus spp. in a long-term incubation of n-alkanes-degrading methanogenic enrichment culture. Front. Microbiol. 2016, 7, 1431.
- Lv, L.; Zhou, L.; Wang, L.-Y.; Liu, J.-F.; Gu, J.-D.; Mu, B.; Yang, S.Z. Selective inhibition of methanogenesis by sulfate in enrichment culture with production water from low-temperature oil reservoir. Int. Biodeterior. Biodegrad. 2016, 108, 133–141.
- Zhao, F.; Zhou, J.D.; Ma, F.; Shi, R.J.; Han, S.Q.; Zhang, J.; Zhang, Y. Simultaneous inhibition of sulfate-reducing bacteria, removal of H2S and production of rhamnolipid by recombinant Pseudomonas stutzeri Rhl: Applications for microbial enhanced oil recovery. Bioresour. Technol. 2016, 207, 24–30.
- Yang, G.-C.; Zhou, L.; Mbadinga, S.M.; You, J.; Yang, H.-Z.; Liu, J.-F.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Activation of CO2-reducing methanogens in oil reservoir after addition of nutrient. J. Biosci. Bioeng. 2016, 122, 740–747.
- Varjani, S.J.; Upasani, V.N. A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int. Biodeterior. Biodegrad. 2017, 120, 71–83.
- Varjani, S.J.; Gnansounou, E. Microbial dynamics in petroleum oilfields and their relationship with physiological properties of petroleum oil reservoirs. Bioresour. Technol. 2017, 245 Pt. A, 1258–1265.
- Hussain, A.; Hasan, A.; Javid, A.; Qazi, J.I. Exploited application of sulfate-reducing bacteria for concomitant treatment of metallic and non-metallic wastes: A mini review. 3 Biotech. 2016, 6, 119.
- Song, Z.; Zhu, W.; Sun, G.; Blanckaert, K. Dynamic investigation of nutrient consumption and injection strategy in microbial enhanced oil recovery (MEOR) by means of large-scale experiments. Appl. Microbiol. Biotechnol. 2015, 99, 6551–6561.
- Mu, B.Z.; Nazina, T.N. Recent Advances in Petroleum Microbiology. Microorganisms 2022, 10, 1706.
- Varjani, S.J.; Upasani, V.N. Core Flood study for enhanced oil recovery through ex-situ bioaugmentation with thermo- and halo-tolerant rhamnolipid produced by Pseudomonas aeruginosa NCIM 5514. Bioresour. Technol. 2016, 220, 175–182.
- Li, J.; Liu, J.; Trefry, M.G.; Park, J.; Liu, K.; Haq, B.; Johnston, C.D.; Volk, H. Interactions of microbial-enhanced oil recovery processes. Transp. Porous Media 2011, 87, 77–104.
- Li, H.; Wang, X.-L.; Mu, B.-Z.; Gu, J.-D.; Liu, Y.-D.; Lin, K.-F.; Lu, S.-G.; Lu, Q.; Li, B.-Z.; Li, Y.-Y.; et al. Molecular detection, quantification and distribution of alkane-degrading bacteria in production water from low temperature oilfields. Int. Biodeterior. Biodegrad. 2013, 76, 49–57.
- Robles-Fernández, A.; Areias, C.; Daffonchio, D.; Vahrenkamp, V.C.; Sánchez-Román, M. The Role of Microorganisms in the Nucleation of Carbonates, Environmental Implications and Applications. Minerals 2022, 12, 1562.
- Billings, N.; Birjiniuk, A.; Samad, T.S.; Doyle, P.S.; Ribbeck, K. Material properties of biofilms-a review of methods for understanding permeability and mechanics. Rep. Prog. Phys. 2015, 78, 036601.
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575.
- Persat, A.; Nadell, C.D.; Kim, M.K.; Ingremeau, F.; Siryaporn, A.; Drescher, K.; Wingreen, N.S.; Bassler, B.L.; Gitai, Z.; Stone, H.A. The mechanical world of bacteria. Cell 2015, 161, 988–997.
- Salama, Y.; Chennaoui, M.; Sylla, A.; Mountadar, M.; Rihani, M.; Assobhei, O. Characterization, structure, and function of extracellular polymeric substances (EPS) of microbial biofilm in biological wastewater treatment systems: A review. Desalin. Water Treat. 2016, 57, 16220–16237.
- Antonietta, Q.; Uta, P.; Wei-Chun, C.; Chen, X.; Shawn, D.; Laura, B.; Manoj, K.; Alicia, K.W.; Jason, B.S.; Zoe, V.F.; et al. The role of microbial exopolymers in determining the fate of oil and chemical dispersants in the ocean. Limnol. Oceanogr. Lett. 2016, 1, 3–26.
- Bacosa, H.P.; Kamalanathan, M.; Labonté, J.M.; Hala, D.; Quigg, A.; Chiu, M.H.; Tsai, S.M.; Chin, W.C.; Sun, L.; Schwehr, K.A.; et al. Extracellular polymeric substances (EPS) producing and oil degrading bacteria isolated from the northern Gulf of Mexico. PLoS ONE 2018, 13, e0208406.
- Kapse, N.G.; Paliwal, V.; Dagar, S.S.; Rana, D.P.; Dhakephalkar, P.K. Genomics and simulated laboratory studies reveal Thermococcus sp. 101C5 as a novel hyperthermophilic archaeon possessing a specialized metabolic arsenal for enhanced oil recovery. Antonie Van Leeuwenhoek 2022, 115, 19–31.
- Aboelkhair, H.; Diaz, P.; Attia, A. Biosurfactant production using Egyptian oil fields indigenous bacteria for microbial enhanced oil recovery. J. Pet. Sci. Eng. 2022, 208, 109601.
- Geetha, S.; Banat, I.M.; Joshi, S.J. Biosurfactants: Production and potential applications in microbial enhanced oil recovery (MEOR). Biocatal. Agric. Biotechnol. 2018, 14, 23–32.
- Wood, D.A. Microbial improved and enhanced oil recovery (MIEOR): Review of a set of technologies diversifying their applications. Adv. Geo-Energy Res. 2019, 3, 122–140.
- Karlapudi, A.P.; Venkateswarulu, T.; Tammineedi, J.; Kanumuri, L.; Ravuru, B.K.; Ramu Dirisala, V.; Kodali, V.P. Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 2018, 4, 241–249.
- Moeininia, N. Improvement of a Full Field Reservoir Model for a MEOR Application. Master’s Thesis, University of Leoben, Leoben, Austria, 2018.
- Massarweh, O.; Abushaikha, A.S. The use of surfactants in enhanced oil recovery: A review of recent advances. Energy Rep. 2020, 6, 3150–3178.
- Reis, R.; Pacheco, G.; Pereira, A.; Freire, D. Biosurfactants: Production and applications. In Biodegradation-Life of Science; InTech: Rijeka, Croatia, 2013; pp. 31–61.
- Ke, C.-Y.; Lu, G.-M.; Li, Y.-B.; Sun, W.-J.; Zhang, Q.-Z.; Zhang, X.-L. A pilot study on large-scale microbial enhanced oil recovery (MEOR) in Baolige Oilfield. Int. Biodeterior. Biodegrad. 2018, 127, 247–253.
- Jeong, M.S.; Lee, Y.W.; Lee, H.S.; Lee, K.S. Simulation-based optimization of microbial enhanced oil recovery with a model integrating temperature, pressure, and salinity effects. Energies 2021, 14, 1131.
- Halim, A.Y.; Nielsen, S.M.; Lantz, A.E.; Suicmez, V.S.; Lindeloff, N.; Shapiro, A. Investigation of spore forming bacterial flooding for enhanced oil recovery in a North Sea chalk Reservoir. J. Pet. Sci. Eng. 2015, 133, 444–454.
- Sen, R. Biotechnology in petroleum recovery: The microbial EOR. Prog. Energy Combust. Sci. 2008, 34, 714–724.
- Dhanarajan, G.; Rangarajan, V.; Bandi, C.; Dixit, A.; Das, S.; Ale, K.; Sen, R. Biosurfactant-biopolymer driven microbial enhanced oil recovery (MEOR) and its optimization by an ANN-GA hybrid technique. J. Biotechnol. 2017, 256, 46–56.
- Eldeen, A.E.G.; Alhadi, A.M.A.A.; Mergani, M.A. Microbial Enhanced Oil Recovery (MEOR); University of Khartoum: Khartom, Sudan, 2013.
- Patel, J.; Borgohain, S.; Kumar, M.; Rangarajan, V.; Somasundaran, P.; Sen, R. Recent developments in microbial enhanced oil recovery. Renew. Sustain. Energy Rev. 2015, 52, 1539–1558.
- Niu, J.; Liu, Q.; Lv, J.; Peng, B. Review on microbial enhanced oil recovery: Mechanisms, modeling and field trials. J. Pet. Sci. Eng. 2020, 192, 107350.
- Bahadori, A. Fundamentals of Enhanced Oil and Gas Recovery from Conventional and Unconventional Reservoirs; Gulf Professional Publishing: Cambridge, MA, USA, 2018.
- Cui, K.; Zhang, Z.; Zhang, Z.; Sun, S.; Li, H.; Fu, P. Stimulation of indigenous microbes by optimizing the water cut in low permeability reservoirs for green and enhanced oil recovery. Sci. Rep. 2019, 9, 15772.
- Mujumdar, S.; Joshi, P.; Karve, N. Production, characterization, and applications of bioemulsifiers (BE) and biosurfactants (BS) produced by Acinetobacter spp.: A review. J. Basic. Microbiol. 2019, 59, 277–287.
- Elakkiya, V.T.; SureshKumar, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Govindarajan, M. Swift production of rhamnolipid biosurfactant, biopolymer and synthesis of biosurfactant-wrapped silver nanoparticles and its enhanced oil recovery. Saudi J. Biol. Sci. 2020, 27, 1892–1899.
- Morais, I.; Cordeiro, A.; Teixeira, G.; Domingues, V.; Nardi, R.; Monteiro, A.; Alves, R.; Siqueira, E.; Santos, V. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P6A and Lactobacillus gasseri P65. Microb. Cell Fact. 2017, 16, 155.
- Dowluru, S. Prospects for Industrial and Microbial Biotechnology in Oil Industry. Biotechnology 2014, 3, 37–39.
- Jahanbani Veshareh, M.; Ganji Azad, E.; Deihimi, T.; Niazi, A.; Ayatollahi, S. Isolation and screening of Bacillus subtilis MJ01 for MEOR application: Biosurfactant characterization, production optimization and wetting effect on carbonate surfaces. J. Pet. Explor. Prod. Technol. 2019, 9, 233–245.
- Borah, D.; Chaubey, A.; Sonowal, A.; Gogoi, B.; Kumar, R. Microbial biosurfactants and their potential applications: An overview. In Microbial Biosurfactants: Preparation, Properties and Applications; Inamuddin, A.M.I., Prasad, R., Eds.; Springer: Singapore, 2021; pp. 91–116.
- Jadeja, N.B.; Moharir, P.; Kapley, A. Genome sequencing and analysis of strains Bacillus sp. AKBS9 and Acinetobacter sp. AKBS16 for biosurfactant production and bioremediation. Appl. Biochem. Biotechnol. 2019, 187, 518–530.
- Shibulal, B.; Al-Bahry, S.N.; Al-Wahaibi, Y.M.; Elshafie, A.E.; Al-Bemani, A.S.; Joshi, S.J. Microbial Enhanced Heavy Oil Recovery by the Aid of Inhabitant Spore-Forming Bacteria: An Insight Review. Sci. World J. 2014, 2014, 309159.
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 2885.
- Al-Sulaimani, H.; Joshi, S.; Al-Wahaibi, Y.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A. Microbial biotechnology for enhancing oil recovery: Current developments and future prospects. Biotechnol. Bioinf. Bioeng. 2011, 1, 147–158.
- Olajire, A.; Essien, J. Aerobic degradation of petroleum components by microbial consortia. J. Pet. Environ. Biotechnol. 2014, 5, 1.
- Bacosa, H.P.; Evans, M.M.; Wang, Q.; Liu, Z. Chapter 28—Assessing the Role of Environmental Conditions on the Degradation of Oil Following the Deepwater Horizon Oil Spill. In Oil Spill Environmental Forensics Case Studies; Stout, S.A., Wang, Z., Eds.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 617–637.
- Gudina, E.J.; Pereira, J.F.; Costa, R.; Coutinho, J.A.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant-producing and oil-degrading Bacillus subtilis strains enhance oil recovery in laboratory sand-pack columns. J. Hazard. Mater. 2013, 261, 106–113.
- Gao, H.; Zhang, J.; Lai, H.; Xue, Q. Degradation of asphaltenes by two Pseudomonas aeruginosa strains and their effects on physicochemical properties of crude oil. Int. Biodeterior. Biodegrad. 2017, 122, 12–22.
- Margesin, R.; Moertelmaier, C.; Mair, J. Low-temperature biodegradation of petroleum hydrocarbons (n-alkanes, phenol, anthracene, pyrene) by four actinobacterial strains. Int. Biodeterior. Biodegrad. 2013, 84, 185–191.
- Li, Y.; Pan, J.; Ma, Y. Elucidation of multiple alkane hydroxylase systems in biodegradation of crude oil n-alkane pollution by Pseudomonas aeruginosa DN1. J. Appl. Microbiol. 2020, 128, 151–160.
- Sakthipriya, N.; Doble, M.; Sangwai, J.S. Influence of thermophilic Bacillus subtilis YB7 on the biodegradation of long chain paraffinic hydrocarbons (C16 H34 to C36 H74). RSC Adv. 2016, 6, 82541–82552.
- Zhang, J.H.; Xue, Q.H.; Gao, H.; Ma, X.; Wang, P. Degradation of crude oil by fungal enzyme preparations from Aspergillus spp. for potential use in enhanced oil recovery. J. Chem. Technol. Biotechnol. 2016, 91, 865–875.
- Ke, C.-Y.; Lu, G.-M.; Wei, Y.-L.; Sun, W.-J.; Hui, J.-F.; Zheng, X.-Y.; Zhang, Q.-Z.; Zhang, X.-L. Biodegradation of crude oil by Chelatococcus daeguensis HB-4 and its potential for microbial enhanced oil recovery (MEOR) in heavy oil reservoirs. Bioresour. Technol. 2019, 287, 121442.
- Wang, Y.; Nie, M.; Wan, Y.; Tian, X.; Nie, H.; Zi, J.; Ma, X. Functional characterization of two alkane hydroxylases in a versatile Pseudomonas aeruginosa strain NY3. Ann. Microbiol. 2017, 67, 459–468.
- Purwasena, I.A.; Sugai, Y.; Sasaki, K. Estimation of the potential of an anaerobic thermophilic oil-degrading bacterium as a candidate for MEOR. J. Pet. Explor. Prod. Technol. 2014, 4, 189–200.
- Vijayakumar, S.; Saravanan, V. Biosurfactants-types, sources and applications. Res. J. Microbiol. 2015, 10, 181.
- Suthar, H.; Hingurao, K.; Desai, A.; Nerurkar, A. Evaluation of bioemulsifier mediated microbial enhanced oil recovery using sand pack column. J. Microbiol. Methods 2008, 75, 225–230.
- Reuvers, B. Emulsifying ionic apolar polymer in water: Understanding the process. J. Coat. Technol. Res. 2020, 17, 1131–1143.
- Hou, B.; Wang, Y.; Cao, X.; Zhang, J.; Song, X.; Ding, M.; Chen, W. Mechanisms of enhanced oil recovery by surfactant-induced wettability alteration. J. Dispers. Sci. Technol. 2016, 37, 1259–1267.
- Tetteh, J.; Janjang, N.M.; Barati, R. Wettability alteration and enhanced oil recovery using low salinity waterflooding in limestone rocks: A mechanistic study. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 23–26 April 2018; OnePetro: Dammam, Saudi Arabia, 2018.
- Najafi-Marghmaleki, A.; Kord, S.; Hashemi, A.; Motamedi, H. Experimental investigation of efficiency of MEOR process in a carbonate oil reservoir using Alcaligenes faecalis: Impact of interfacial tension reduction and wettability alteration mechanisms. Fuel 2018, 232, 27–35.
- Deng, X.; Tariq, Z.; Murtaza, M.; Patil, S.; Mahmoud, M.; Kamal, M.S. Relative contribution of wettability Alteration and interfacial tension reduction in EOR: A critical review. J. Mol. Liq. 2021, 325, 115175.
- Joonaki, E.; Ghanaatian, S. The application of nanofluids for enhanced oil recovery: Effects on interfacial tension and coreflooding process. Pet. Sci. Technol. 2014, 32, 2599–2607.
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S. Review on surfactant flooding: Phase behavior, retention, IFT, and field applications. Energy Fuels 2017, 31, 7701–7720.
- Kögler, F.; Mahler, E.; Dopffel, N.; Schulze-Makuch, D.; Borovina, A.; Visser, F.; Herold, A.; Alkan, H. The Microbial Enhanced Oil Recovery (MEOR) potential of Halanaerobiales under dynamic conditions in different porous media. Pet. Sci. Eng. 2021, 196, 107578.
- Khajepour, H.; Mahmoodi, M.; Biria, D.; Ayatollahi, S. Investigation of wettability alteration through relative permeability measurement during MEOR process: A micromodel study. J. Pet. Sci. Eng. 2014, 120, 10–17.
- Abolhasanzadeh, A.; Khaz’ali, A.R.; Hashemi, R.; Jazini, M. Experimental study of microbial enhanced oil recovery in oil-wet fractured porous media. Oil Gas Sci. Technol—Rev. d’IFP Energ. Nouv. 2020, 75, 10.
- Karimi, M.; Mahmoodi, M.; Niazi, A.; Al-Wahaibi, Y.; Ayatollahi, S. Investigating wettability alteration during MEOR process, a micro/macro scale analysis. Colloid. Surf. B-Biointerfaces 2012, 95, 129–136.
- Donaldson, E.C.; Alam, W. Wettability; Gulf Publishing Company: Houston, TX, USA, 2013.
- Gao, C. Experiences of microbial enhanced oil recovery in Chinese oil fields. J. Pet. Sci. Eng. 2018, 166, 55–62.
- Zhang, J.; Gao, H.; Xue, Q. Potential applications of microbial enhanced oil recovery to heavy oil. Crit. Rev. Biotechnol. 2020, 40, 459–474.
- Guan, J.; Xia, L.-P.; Wang, L.-Y.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Diversity and distribution of sulfate-reducing bacteria in four petroleum reservoirs detected by using 16S rRNA and dsrAB genes. Int. Biodeterior. Biodegrad. 2013, 76, 58–66.
- Zhao, F.; Ma, F.; Shi, R.; Zhang, J.; Han, S.; Zhang, Y. Production of rhamnolipids by Pseudomonas aeruginosa is inhibited by H2S but resumes in a co-culture with P. stutzeri: Applications for microbial enhanced oil recovery. Biotechnol. Lett. 2015, 37, 1803–1808.
More
