Biomass-based solutions have been discussed as having the potential to replace fossil-based solutions in the iron and steel industry. To produce the biocarbon required in these processes, thermochemical treatment, pyrolysis, typically takes place. There are various ways to produce biocarbon, alongside other products, which are called pyrolysis oil and pyrolysis gas. These conversion methods can be divided into conventional and non-conventional methods.
- biocarbon
- biocarbon yield
- pyrolysis
1. Introduction
There are two ways to convert biomass into biofuels: biochemical or thermochemical routes. The difference between these two routes is that the biochemical process takes days to complete, whereas, in the case of a thermochemical process, it is finished within seconds to hours [12][1]. Typically, in the thermochemical process, pyrolysis is used when biocarbon is produced [13][2]. Depending on the process parameters such as the final pyrolysis temperature, heating rate, and residence time, pyrolysis can be divided into three main categories: slow (often referred to as conventional pyrolysis), fast, and flash pyrolysis [14,15][3][4]. The other way to categorize pyrolysis is based on the technology used—for example, solar- or microwave-based technologies [16][5].
2. Pyrolysis Methods and Technologies
2.1. Pyrolysis Stages and Biocarbon Formation
Biocarbon formation consists of three temperature-dependent stages. These are called pre-pyrolysis, main pyrolysis, and the production of carbonaceous products [13][2]. When wood-based material is turned into biocarbon, the first stage takes place at temperatures under 200 °C. At these temperatures, moisture and light volatiles are evaporated [36][18]. In the second stage, the organic compounds such as hemicellulose and cellulose are devolatilized. This stage takes place in the temperature range of 200–500 °C [36,37][18][19]. The last stage occurs at temperatures over 500 °C. In this stage, the chemical compounds with strong chemical bonds, such as lignin, decompose into other compounds [36][18]. It is also noteworthy that lignin decomposes at lower temperatures, starting at 160 °C [19][8]. Biomass typically contains three main compounds: cellulose, hemicellulose, and lignin. In addition, there are three minor compounds: proteins, sugars/aliphatic acid, and fats. The combustion and degradation behavior of these compounds differs depending on the biomass type and composition [18][7]. For example, studies have shown that a high lignin content in the feedstock increases biocarbon yield [23,38][12][20]. According to Yang et al., cellulose, hemicellulose, and lignin decomposition occur following temperature-dependent stages [19][8].-
Cellulose decomposes into pyrolysis oil, gaseous products, and biocarbon at 315–400 °C [19,41][8][23]. The decomposition products depend on the feedstock heating rate. At slow heating rates, the process favors biocarbon formation. Rapid volatilization occurs at high heating rates, leading to the formation of levoglucosan, which breaks down further into liquid and gas products [40][22].
2.2. Pyrolysis Methods
2.2.1. Slow Pyrolysis
Slow pyrolysis has been used for thousands of years for biocarbon production. A typical process feature is a slow heating rate (0.1–1 °C/s) combined with a low pyrolysis temperature (300–700 °C) and a long vapor residence time, between 10 and 100 min. Compared to fast pyrolysis, the vapors do not escape as fast. Thus, different components in the vapor phase continue to react with each other as the biocarbon and pyrolysis oil is formed. It is also possible to remove vapors continuously through pyrolysis and thus decrease vapor residence time [14,15][3][4].2.2.2. Fast Pyrolysis
In fast pyrolysis, the feedstock is heated rapidly in the absence of oxygen with a very short vapor residence time. Because of these process features, the biomass decomposes and forms pyrolysis oil, biocarbon, and pyrolysis gas [15][4]. Depending on the source, the temperature range of fast pyrolysis varies from 400–800 °C [14][3] to 850–1250 °C [45][25]. Typically, the process involves high heating rates of 10–200 °C/s and is combined with a very short vapor residence time, under 2 s. These parameters favor pyrolysis oil formation, and a typical product yield distribution is 30% pyrolysis gas, 20% biocarbon, and 50% pyrolysis oil [14][3].2.2.3. Flash Pyrolysis
Flash pyrolysis uses a very high heating rate, up to 2500 °C/s. The vapor residence time is very short, below 0.5 s. The temperatures used are typically around 1000 °C. The primary product is pyrolysis oil. The main difference between flash and fast pyrolysis is that the heating rate is considerably higher than in fast pyrolysis [46][26]. Given the product yields, flash pyrolysis leads to more pyrolysis oil generated during pyrolysis than fast pyrolysis [14][3].2.2.4. Intermediate Pyrolysis
In some publications, intermediate pyrolysis is separated by its own pyrolysis type because the heating rate, 1–10 °C/s, settles between slow and fast pyrolysis [47,48][27][28]. Other operation conditions are also between slow and fast pyrolysis: temperature range is typically between 400 °C and 650 °C with several suggested vapor residence times starting from 0.5–20 s [47][27] to 300–1000 s [45][25]. Operating pressure is typically 0.1 MPa [45,47][25][27]. When intermediate pyrolysis is used, it is possible to obtain a higher biocarbon and pyrolysis oil yield and a lower pyrolysis gas yield compared to conventional pyrolysis, according to Kazawadi et al. [47][27]. These pyrolysis gases contain little or no dust or tar, and the pyrolysis gas can be used directly for generating electricity and heat [45,48][25][28].2.2.5. Segmented Heating
Segmented heating is not its own pyrolysis type. Rather, it can be described as an alternative way to heat feedstock to the final pyrolysis temperature [49][29]. Overall, pyrolysis is an endothermic process, but in the early stages, around 280 °C, pyrolysis is exothermic [43,50][30][31]. Because of the exothermicity, it has been shown that it is possible to utilize heat released from these reactions to meet the energy requirements in the stage where the reactions are endothermic. Based on the exothermicity of pyrolysis in the early stages, Lam et al. [49][29] introduced the two-stage pyrolysis concept in 2010. This approach was later developed further by Cheung et al. [50][31]. In both concepts, the pyrolysis process is divided into several stages, for example, a heating stage, followed by an adiabatic stage, a second heating stage, and a second adiabatic stage. This type of segmented pyrolysis could reduce pyrolysis energy consumption by 22.5% compared to conventional pyrolysis. [50][31].2.3. Pyrolysis Technologies
2.3.1. Microwave Pyrolysis
Microwave-assisted pyrolysis is an alternative pyrolysis method compared to the traditionally used pyrolysis methods. The main difference is that it does not require any external temperature field. Instead of an external field, it is based on the use of electromagnetic radiation. The electromagnetic radiation wavelength varies from 1 mm to 1 m. In the case of frequency, it varies between 300 MHz and 30 GHz. Industrial and domestic microwave applications typically operate at 2.45 GHz, corresponding to a wavelength of 12.2 cm [16,57][5][32]. The penetration depth of these waves varies due to several things: material type, microstructure, and temperature. Another example is water: at a frequency of 2.54 GHz, the penetration depth is 1 cm–4 cm, but it increases to 5 cm–7 cm when the temperature rises from 25 °C to 95 °C. Because of this, the biomass’s density and water content should also be considered when pyrolyzing biomass using microwaves [58][33]. In order to improve the absorption of microwaves, an absorbent material is used. Usually, this is mixed into the feedstock before pyrolysis [59][34]. Graphite and silicon carbide are typically used as absorbent materials [60,61][35][36]. Compared to conventional pyrolysis, the heating mechanism of microwave-assisted pyrolysis is different. This is because, in conventional pyrolysis, the heat is transferred from the surface to the core, and in microwave pyrolysis, the electromagnetic energy is converted into heat from the core to the surface [57,63,64][32][37][38]. Therefore, the heating mechanism of microwave-assisted pyrolysis can be described as an energy conversion method rather than heat transfer. The difference between these two mechanisms is illustrated in Figure 1.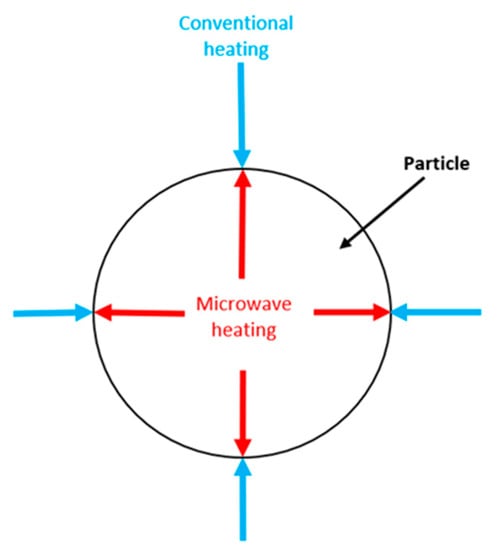
2.3.2. Solar Pyrolysis
In solar pyrolysis, feedstock with a low energy density is converted into energy-denser products [77,78][44][45]. This pyrolysis type represents fast pyrolysis, and it is an endothermic process based on the utilization of solar energy. The basic principle of solar pyrolysis is quite simple. Solar radiation is used to heat a reactor, and pyrolysis occurs in an inert environment [79][46]. This can be performed by using different types of concentrators and reactor configurations; for example, using parabolic dishes or parabolic troughs [79,80][46][47]. Because of the unique features of solar pyrolysis, it offers some advantages compared to traditionally used technologies. The concept used for performing pyrolysis allows fast shutdowns and startups of reactors [81][48]. The process maximizes the amount of products formed during pyrolysis. This means that in conventional pyrolysis, a small amount of feedstock is burned for heat to maintain the pyrolysis process. This does not occur in solar pyrolysis because the heat for the pyrolysis is maintained by solar radiation [81,82][48][49]. The highest possible operating temperature and the heating rate depend on the technology used. When parabolic mirrors are used, the operating temperature is from 400 °C to 700 °C, whereas in the case of parabolic dishes, the operating temperature rises over 1200 °C [84][50]. However, the limited size of parabolic dishes sets challenges for large-scale applications. Therefore, it seems that on a larger scale, another option, such as solar tower systems, would be better [85][51]. Generally, the technology used to carry out solar pyrolysis is divided into three main groups: directly heated reactors, indirectly heated reactors, and separated reactor systems. The difference between the first two is that solar radiation is directly concentrated onto the feedstock in the directly heated reactor. In contrast, in indirectly heated reactors, solar energy is first concentrated onto the reactor surface and then transferred to feedstock. The third main type is a separated reactor system, where solar radiation is first used to heat a transfer fluid, which is then used to heat the reactor directly [79][46].2.3.3. Plasma Pyrolysis
Plasma pyrolysis represents a form of flash pyrolysis and is used to pyrolyze a carbon-based material [92,93,94,95][52][53][54][55]. Typically, the operating temperature varies from 4273 °C to 5273 °C when using microwave-powered plasma and up to 10,273–12,273 °C if plasma is inductively coupled or arc discharge plasma [96][56]. Compared to conventional pyrolysis methods, plasma pyrolysis offers a high heating rate and short residence time [94][54]. Because of the high heating rates, organic compounds are decomposed, and inorganic materials are melted thus forming slag [94][54]. Plasma pyrolysis produces a relatively high pyrolysis gas yield, and the produced gas has a good heating value. Because of this, the produced gas is suitable for energy generation [97,98][57][58]. However, there are some downsides related to the use of plasma pyrolysis. The reactor has high energy consumption, and because of heat loss during pyrolysis, the overall efficiency of the process is reduced [99][59].2.3.4. Vacuum Pyrolysis
Vacuum pyrolysis is based on using vacuum conditions during pyrolysis. Based on the heating rate used, it represents either slow or fast pyrolysis [100,101,102][60][61][62]. Because of the vacuum conditions, there are no molecules or just a small number of molecules that disturb the reactions. This makes the decomposition products uniform and accelerates the reaction rate [100,101][60][61]. Because the reaction rate is fast, the process consumes less energy than traditional pyrolysis methods [101][61]. A second characteristic feature of this process is that the vapor residence time is short. This leads to fewer side reactions and, therefore, pyrolysis oil with higher quality and yields than other pyrolysis methods [99,103][59][63]. The downside of using vacuum pyrolysis is that it is complicated, so the investment cost and maintenance are high [104][64].2.4. Reactor Types
The reactor type determines the final product yield and its distribution during pyrolysis alongside the operating conditions. The desired product yield depends on the reactor configuration used and the residence time of the volatiles [105][65]. Pyrolysis reactors can be divided into two main groups: fixed bed reactors, where the feedstock does not move, and moving bed reactors, where movement could be caused by a mechanical force or fluid flow [106][66]. Several other reactor types are under these two main groups, such as a fluidized bed, vacuum pyrolizer, and ablative pyrolizer [107][67].2.4.1. Fluidized Bed Reactor
Based on operating parameters such as vapor residence time and pyrolysis temperature, a fluidized bed reactor conducts fast pyrolysis [110][68]. In this reactor type, gasifying agents maintain the biomass in a fluidized state. The biomass is mixed with an inert bed material, improving heat transfer. This bed enables a uniform temperature in the conversion zone. In the conversion zone, the devolatilization, drying, oxidation, and gasification co-occur [111][69]. The typical operating temperature varies from 700 °C to 900 °C [112][70]. According to some publications, the operating temperature could also be higher, between 1000 °C and 1050 °C [113,114][71][72].2.4.2. Ablative Plate Reactor
An ablative plate reactor type is also used for fast pyrolysis [115][73]. In an ablative reactor, the feedstock is pressed onto a hot surface, and the heating is conducted by using hot flue gas. The gas is produced by pyrolysis combustion gases [116][74]. The main product is pyrolysis oil [117][75]. The reaction rate is affected by the reactor surface temperature and pressure. The disadvantage of this type of reactor is related to heat transfer efficiency from the surface of the reactor to the feedstock. Additionally, feedstock properties such as particle size impose restrictions because the feedstock must be pressed onto the reactor’s surface [12,108][1][76].2.4.3. Auger Reactor
Based on the pyrolysis temperature and residence time, an auger reactor represents a slow pyrolysis [118][77]. In an auger-type reactor, the feedstock is fed into the reactor with a screw. The screw mixes the particles, moves them to the reactor, and at the same time controls the residence time in the reactor by its speed. The reaction heat is carried out by heating the wall around the screw. The advantage of auger-type reactors is that they control the mass flow well. The disadvantages are the risk of plugging, mechanical wear to the moving parts at high temperatures, and the possibility of heat transfer problems [119][78].2.4.4. Rotating Cone Reactor
Rotating cone reactors represent a fast pyrolysis [120][79]. In a rotating cone reactor, the feedstock and sand are fed into the bottom of the reactor. Because of the reactor’s constant motion, the feedstock is forced against the wall [108][76]. The wall is heated, and in this area, pyrolysis occurs. After pyrolysis, the biocarbon and sand are collected, and the biocarbon is burned to heat the sand. Typically, when this reactor type is used, a liquid yield of 60%–70% is achieved [120][79].2.4.5. Cyclone/Vortex Reactor
Cyclone/vortex-type reactors represent fast pyrolysis [121][80]. The feedstock is fed into the reactor alongside hot steam or nitrogen gas. After entering the reactor, the feedstock is forced into contact with the reactor wall at high speed caused by centrifugal forces. This reactor type leads to pyrolysis oil yields of up to 65% [109][81].3. The Effect of Reaction Conditions and Process Parameters
3.1. Effect of Final Temperature and Heating Rate
The pyrolysis temperature and heating rate are two main features that affect biocarbon yield during pyrolysis [23][12]. For example, biocarbon produced at higher temperatures has a higher carbon content and greater pore volume. The heating rate, conversely, defines the type of pyrolysis, i.e., if it is slow, fast, or flash. It also affects the product distribution during pyrolysis. Higher heating rates lead to increased pyrolysis gas production [23,128][12][82]. These parameters also affect the biocarbon yield, porosity, and surface area [23,128][12][82]. Higher heating rates tend to favor high pyrolysis oil yields [128][82] whereas slow heating rates tend to favor biocarbon formation [129][83].3.2. Vapor and Biomass Residence Time
Generally, longer vapor residence times favor biocarbon formation, whereas shorter times tend to favor pyrolysis oil and pyrolysis gas formation [15][4]. Organic vapors are quickly removed when shorter vapor residence times are used, preventing secondary reactions [137][84]. On the contrary, when the vapor residence time is long, it causes secondary cracking reactions to take place and thus leads to an increased biocarbon yield [138][85].3.3. Feedstock Particle Size
Feedstock particle size and particle size distribution are important factors that affect biocarbon yield during pyrolysis [43,140][30][86]. Kirubakaran et al. [141][87] suggested that in view of heat transfer, a smaller particle size below 0.2 cm would be ideal. This is because, at this particle size, achieving a uniform temperature is possible throughout the particles, thus allowing chemical reactions to occur through the particle. Şensöz et al. [142][88] found that the biocarbon yield was independent of the particle size when fast pyrolysis was carried out at a temperature of 500 °C. Liu et al. [139][89] reported that smaller particle sizes generated less biocarbon when pyrolysis took place at 600 °C. According to Liu et al. [139][89], this is possible because a greater temperature gradient is achieved when the particle size is larger. It leads to more biocarbon and less pyrolysis oil and pyrolysis gas produced during pyrolysis.3.4. Reaction Atmosphere
Usually, the pyrolysis of biomass is carried out in an inert, non-oxidizing gas atmosphere [137][84]. Other gases, such as water, nitrogen, hydrogen, carbon dioxide, and carbon monoxide, can also be used to modify the pyrolysis process [145,146][90][91]. The effect of mixing carbon dioxide with other gases has also been evaluated [147][92]. Minkova et al. showed that when a water vapor flow is present during slow pyrolysis, it increases pyrolysis oil yield but decreases the biocarbon and pyrolysis gas yield. The produced biocarbon had a higher surface area together with a good adsorption capacity [146][91]. Özbal et al. [148][93] confirmed Minkova’s observation of an increased oil yield when steam was used. However, the use of steam has a negative impact on biocarbon yield. The reason behind the increased pyrolysis oil content and decreased biocarbon yield is that the presence of steam prevents secondary cracking reactions [148][93].3.5. Pressure
Typically, pyrolysis is performed at an atmospheric pressure [23][12]. Only a few studies focused on using different pressures during pyrolysis and their effect on the biocarbon yield during pyrolysis. Basile et al. [154][94] studied the effect of pressure on solid biocarbon yield in the pyrolysis of three different raw materials; namely, corn, poplar, and switchgrass. The pressure range in the experiments was between 0.1 MPa and 4 MPa, while the pyrolysis temperature remained at 500 °C in all the experiments. They found that increasing the pressure from 0.1 MPa to 4 MPa increased the biocarbon yield in all cases. They also found that the reactions may shift from endothermic to exothermic when the pressure is increased.3.6. Catalyst
Generally, a catalyst is used in pyrolytic processes to modify the composition, chemical, and physical properties of pyrolysis products [157][95]. According to Tripathi et al. [45][25], it is hard to state any general rule on how different catalysts affect product yield. This is because each biomass is different in composition, ash, and water content; thus, each catalyst’s reactions may differ. Still, it is possible to say that using acidic catalysts leads to increased biocarbon yields, and using basic catalysts leads to lower biocarbon yields [45][25]. In recent years, catalyst-related studies have focused on increasing pyrolysis oil yield and its quality rather than biocarbon yield and quality [158,159,160,161][96][97][98][99]. Despite this, several studies still show that the biocarbon yield was increased by using different catalysts.3.7. Binders
Binders are used when the material undergoes briquetting [165][100]. This technique is used for compacting loose material into a more energy-dense material [166][101]. The binder can be added before [167][102] or after pyrolysis [168][103]. When binders are added before pyrolysis, they are mixed with feedstock and pelletized. This aims to improve the pellet properties, such as their tensile strength, and achieve a higher heating value [169][104]. When binders are added after pyrolysis, the feedstock is first pyrolyzed, and then the binders are mixed with biocarbon and pelletized. This affects pellet properties, such as energy and bulk density [168][103].4. Summary
Process parameters such as heating rate and final pyrolysis temperature must be optimized for selected biomass to achieve better biocarbon yield during pyrolysis. Biomass typically contains three main compounds: cellulose, hemicellulose, and lignin. The amount of these varies from feedstock to feedstock, and each has its own effect on biocarbon yield during pyrolysis. For example, it is suggested that the high lignin content of biomass-based feedstock leads to increased biocarbon yield [14,19,22,26,38][3][8][11][15][20]. Pyrolysis temperature and heating rate play key roles in biocarbon formation during pyrolysis. Using low temperatures below 400 °C favors biocarbon formation and thus improves biocarbon yield during pyrolysis. This increased biocarbon yield during low-temperature pyrolysis is because biocarbon yield at higher temperatures is affected by the occurrence of secondary reactions [14,130][3][106]. Feedstock particle size also has its own impact on biocarbon yield during pyrolysis. It is suggested that using a small particle size below 0.2 cm increases biocarbon yield during pyrolysis. This is because, in this particle size, it is easier to achieve uniform temperature throughout the particle, thus allowing a reaction to take place through the particle [141][87]. Microwave-assisted pyrolysis has shown great potential to increase biocarbon yield during pyrolysis, and other properties such as carbon content and calorific value are increased compared to conventional pyrolysis. However, microwave pyrolysis has faced problems scaling from laboratory to industrial scale [30,70,71][40][107][108]. Solar-based pyrolysis is a promising technology for producing biocarbon with good yield at relatively low temperatures. However, this technology is not applicable globally because the efficiency of the process is highly dependent on the day and current season [86,91][109][110].Other technologies such as plasma and vacuum pyrolysis do not produce biocarbon with a high yield. These technologies focus on producing pyrolysis oil and pyrolysis gases rather than biocarbon. Moreover, they also have some disadvantages. For example, plasma pyrolysis consumes high energy, and vacuum pyrolysis has high maintenance costs due to reactor configuration [97,98,99,103][57][58][59][63].References
- Dhyani, V.; Bhaskar, T. A Comprehensive Review on the Pyrolysis of Lignocellulosic Biomass. Renew. Energy 2018, 129, 695–716.
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215.
- Nanda, S.; Mohammad, J.; Reddy, S.N.; Kozinski, J.A.; Dalai, A.K. Pathways of Lignocellulosic Biomass Conversion to Renewable Fuels. Biomass. Convers. Biorefin. 2014, 5, 157–191.
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-Oil: A Critical Review. Energy Fuels 2006, 20, 848–889.
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578.
- Zhang, Y.; Chen, P.; Liu, S.; Peng, P.; Min, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Fan, L.; Liu, C.; et al. Effects of Feedstock Characteristics on Microwave-Assisted Pyrolysis—A Review. Bioresour. Technol. 2017, 230, 143–151.
- Akhtar, A.; Krepl, V.; Ivanova, T. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy Fuels 2018, 32, 7294–7318.
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788.
- Choo, M.Y.; Oi, L.E.; Ling, T.C.; Ng, E.P.; Lee, H.V.; Juan, J.C. Conversion of Microalgae Biomass to Biofuels. In Microalgae Cultivation for Biofuels Production; Yousuf, A., Ed.; Elsevier: London, UK, 2019; pp. 149–161. ISBN 9780128175361.
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of Wood. Part 2. Analysis of Products. J. Anal. Appl. Pyrolysis 2006, 77, 35–40.
- Piloni, R.V.; Brunetti, V.; Urcelay, R.C.; Daga, I.C.; Moyano, E.L. Chemical Properties of Biosilica and Bio-Oil Derived from Fast Pyrolysis of Melosira Varians. J. Anal. Appl. Pyrolysis 2017, 127, 402–410.
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A Critical Review of the Production and Advanced Utilization of Biochar via Selective Pyrolysis of Lignocellulosic Biomass. Bioresour. Technol. 2020, 312, 123614.
- Altamer, D.H.; Al-Irhayim, A.N.; Saeed, L.I. Bio-Based Liquids and Solids from Sustainable Feedstock: Production and Analysis. J. Anal. Appl. Pyrolysis 2021, 157, 105224.
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33.
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnol. Rep. 2020, 28, e00570.
- Wang, Y.; He, T.; Liu, K.; Wu, J.; Fang, Y. From Biomass to Advanced Bio-Fuel by Catalytic Pyrolysis/Hydro-Processing: Hydrodeoxygenation of Bio-Oil Derived from Biomass Catalytic Pyrolysis. Bioresour. Technol. 2012, 108, 280–284.
- Carrier, M.; Hardie, A.G.; Uras, Ü.; Görgens, J.; Knoetze, J. Production of Char from Vacuum Pyrolysis of South-African Sugar Cane Bagasse and Its Characterization as Activated Carbon and Biochar. J. Anal. Appl. Pyrolysis 2012, 96, 24–32.
- Lee, X.J.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Ng, H.K. Biochar Potential Evaluation of Palm Oil Wastes through Slow Pyrolysis: Thermochemical Characterization and Pyrolytic Kinetic Studies. Bioresour. Technol. 2017, 236, 155–163.
- Cárdenas- Aguiar, E.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. The Effect of Biochar and Compost from Urban Organic Waste on Plant Biomass and Properties of an Artificially Copper Polluted Soil. Int. Biodeterior. Biodegrad. 2017, 124, 223–232.
- Demirbas, A. Effects of Temperature and Particle Size on Bio-Char Yield from Pyrolysis of Agricultural Residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248.
- Peng, Y.; Wu, S. The Structural and Thermal Characteristics of Wheat Straw Hemicellulose. J. Anal. Appl. Pyrolysis 2010, 88, 134–139.
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. A Comprehensive Assessment of the Method for Producing Biochar, Its Characterization, Stability, and Potential Applications in Regenerative Economic Sustainability—A Review. Clean. Mater. 2022, 3, 100045.
- Xia, C.; Cai, L.; Zhang, H.; Zuo, L.; Shi, S.Q.; Shiung Lam, S. A Review on the Modeling and Validation of Biomass Pyrolysis with a Focus on Product Yield and Composition. Biofuel Res. J. 2021, 29, 1296–1315.
- Brebu, M.; Vasile, C. Thermal Degradation of Lignin-A Review. Cellul. Chem. Technol. 2010, 44, 353–363.
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2016, 55, 467–481.
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass Pyrolysis: Past, Present, and Future. Environ. Dev. Sustain. 2020, 22, 17–32.
- Kazawadi, D.; Ntalikwa, J.; Kombe, G. A Review of Intermediate Pyrolysis as a Technology of Biomass Conversion for Coproduction of Biooil and Adsorption Biochar. J. Renew. Energy 2021, 143, 1939–1948.
- Hornung, A. Intermediate Pyrolysis of Biomass. In Biomass Combustion Science, Technology and Engineering; Rosendahl, L., Ed.; Woodhead Publishing: Cambridge, UK, 2013; Volume 143, pp. 172–186. ISBN 9780857091314.
- Lam, K.-L.; Lee, C.-W.; Hui, C.-W. Multi-Stage Waste Tyre Pyrolysis: An Optimisation Approach. Chem. Eng. Trans. 2010, 21, 853.
- Nachenius, R.W.; Ronsse, F.; Venderbosch, R.H.; Prins, W. Biomass Pyrolysis. In Advances in Chemical Engineering; Marin, G.B., West, D.H., Li, J., Narasimhan, S., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 42, pp. 75–139.
- Cheung, K.Y.; Lee, K.L.; Lam, K.L.; Chan, T.Y.; Lee, C.W.; Hui, C.W. Operation Strategy for Multi-Stage Pyrolysis. J. Anal. Appl. Pyrolysis 2011, 91, 165–182.
- Ethaib, S.; Omar, R.; Mazlina, S.; Kamal, M.; Radiah, D.; Biak, A.; Zubaidi, S.L. Microwave-Assisted Pyrolysis of Biomass Waste: A Mini Review. Processes 2020, 8, 1190.
- Mushtaq, F.; Mat, R.; Ani, F.N. A Review on Microwave Assisted Pyrolysis of Coal and Biomass for Fuel Production. Renew. Sustain. Energy Rev. 2014, 39, 555–574.
- Zhang, Y.; Cui, Y.; Liu, S.; Fan, L.; Zhou, N.; Peng, P.; Wang, Y.; Guo, F.; Min, M.; Cheng, Y.; et al. Fast Microwave-Assisted Pyrolysis of Wastes for Biofuels Production—A Review. Bioresour. Technol. 2020, 297, 122480.
- Macquarrie, D.J.; Clark, J.H.; Fitzpatrick, E. The Microwave Pyrolysis of Biomass. Biofuels Bioprod. Biorefin. 2012, 6, 549–560.
- Omar, R.; Mokhtar, N.M.; Ethaib, S. Effect of Microwave Absorbers on the Products of Microwave Pyrolysis of Oily Sludge. J. Eng. Sci. Technol. 2018, 13, 3313–3330.
- Budarin, V.L.; Shuttleworth, P.S.; De Bruyn, M.; Farmer, T.J.; Gronnow, M.J.; Pfaltzgraff, L.; Macquarrie, D.J.; Clark, J.H. The Potential of Microwave Technology for the Recovery, Synthesis and Manufacturing of Chemicals from Bio-Wastes. Catal. Today 2015, 239, 80–89.
- Motasemi, F.; Afzal, M.T. A Review on the Microwave-Assisted Pyrolysis Technique. Renew. Sustain. Energy Rev. 2013, 28, 317–330.
- Ingole, P.M.; Ranveer, A.C.; Deshmukh, S.M.; Deshmukh, S.K. Microwave Assisted Pyrolysis of Biomass: A Review. Int. J. Adv. Technol. Eng. Sci. 2016, 4, 78–84.
- Luque, R.; Men, J.A.; Arenillas, A.; Cot, J. Microwave-Assisted Pyrolysis of Biomass Feedstocks: The Way Forward? Energy Environ. Sci. 2012, 5, 5481–5488.
- Wu, C.; Budarin, V.L.; Gronnow, M.J.; De Bruyn, M.; Onwudili, J.A.; Clark, J.H.; Williams, P.T. Conventional and Microwave-Assisted Pyrolysis of Biomass under Different Heating Rates. J. Anal. Appl. Pyrolysis 2014, 107, 276–283.
- Zhao, X.; Wang, M.; Liu, H.; Zhao, C.; Ma, C.; Song, Z. Effect of Temperature and Additives on the Yields of Products and Microwave Pyrolysis Behaviors of Wheat Straw. J. Anal. Appl. Pyrolysis 2013, 100, 49–55.
- Wallace, C.A.; Afzal, M.T.; Saha, G.C. Effect of Feedstock and Microwave Pyrolysis Temperature on Physio-Chemical and Nano-Scale Mechanical Properties of Biochar. Bioresour. Bioprocess. 2019, 6, 33.
- Morales, S.; Miranda, R.; Bustos, D.; Cazares, T.; Tran, H. Solar Biomass Pyrolysis for the Production of Bio-Fuels and Chemical Commodities. J. Anal. Appl. Pyrolysis 2014, 109, 65–78.
- Bashir, M.; Yu, X.; Hassan, M.; Makkawi, Y. Modeling and Performance Analysis of Biomass Fast Pyrolysis in a Solar-Thermal Reactor. ACS Sustain. Chem. Eng. 2017, 5, 3795–3807.
- Rahman, M.A.; Parvej, A.M.; Aziz, M.A. Concentrating Technologies with Reactor Integration and Effect of Process Variables on Solar Assisted Pyrolysis: A Critical Review. Therm. Sci. Eng. Prog. 2021, 25, 100957.
- Li, R.; Zeng, K.; Soria, J.; Mazza, G.; Gauthier, D.; Rodriguez, R.; Flamant, G. Product Distribution from Solar Pyrolysis of Agricultural and Forestry Biomass Residues. Renew. Energy 2016, 89, 27–35.
- Zeng, K.; Gauthier, D.; Soria, J.; Mazza, G.; Flamant, G. Solar Pyrolysis of Carbonaceous Feedstocks: A Review. Sol. Energy 2017, 156, 73–92.
- Ayala-Cortés, A.; Lobato-Peralta, D.R.; Arreola-Ramos, C.E.; Martínez-Casillas, D.C.; Pacheco-Catalán, D.E.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Villafán-Vidales, H.I. Exploring the Influence of Solar Pyrolysis Operation Parameters on Characteristics of Carbon Materials. J. Anal. Appl. Pyrolysis 2019, 140, 290–298.
- Parthasarathy, P.; Al-Ansari, T.; Mackey, H.R.; Sheeba Narayanan, K.; McKay, G. A Review on Prominent Animal and Municipal Wastes as Potential Feedstocks for Solar Pyrolysis for Biochar Production. Fuel 2022, 316, 123378.
- Nzihou, A.; Flamant, G.; Stanmore, B. Synthetic Fuels from Biomass Using Concentrated Solar Energy—A Review. Energy 2012, 42, 121–131.
- Ciuta, S.; Tsiamis, D.; Castaldi, M.J. Fundamentals of Gasification and Pyrolysis. In Gasification of Waste Materials: Technologies for Generating Energy, Gas, and Chemicals from Municipal Solid Waste, Biomass, Nonrecycled Plastics, Sludges, and Wet Solid Wastes; Ciuta, S., Tsiamis, D., Castaldi, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 13–36. ISBN 9780128127162.
- Hrabovsky, M. Thermal Plasma Gasification of Biomass. In Progress in Biomass and Bioenergy Production; Shaukat, S.S., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 39–62. ISBN 978-953-307-491-7.
- Shie, J.L.; Tsou, F.J.; Lin, K.L.; Chang, C.Y. Bioenergy and Products from Thermal Pyrolysis of Rice Straw Using Plasma Torch. Bioresour. Technol. 2010, 101, 761–768.
- Cheng, Y.; Yan, B.H.; Cao, C.X.; Cheng, Y.; Jin, Y. Experimental Investigation on Coal Devolatilization at High Temperatures with Different Heating Rates. Fuel 2014, 117, 1215–1222.
- Sturmn, G.S.J.; Muños, A.N.; Aravind, P.V.; Stefanidis, G.D. Microwave-Driven Plasma Gasification for Biomass Waste Treatment at Miniature Scale. IEEE Trans. Plasma Sci. 2016, 44, 670–678.
- Tang, L.; Huang, H. Biomass Gasification Using Capacitively Coupled RF Plasma Technology. Fuel 2005, 84, 2055–2063.
- Huang, H.; Tang, L. Treatment of Organic Waste Using Thermal Plasma Pyrolysis Technology. Energy Convers. Manag. 2007, 48, 1331–1337.
- Soria-Verdugo, A. Pyrolysis of Sludge and Biomass Residues. In Wastewater Treatment Residues as Resources for Biorefinery Products and Biofuels; Olivares, J.A., Puyol, D., Melero, J.A., Dufour, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 155–181. ISBN 9780128162040.
- Liu, L.; Xiong, B.; Zhang, X.; Ye, L. Vacuum Pyrolysis of Ammonium Paratungstate: Study on Reaction Mechanism and Morphology Changes of Product. J. Anal. Appl. Pyrolysis 2021, 157, 105168.
- Ruan, J.; Huang, J.; Qin, B.; Dong, L. Heat Transfer in Vacuum Pyrolysis of Decomposing Hazardous Plastic Wastes. ACS Sustain. Chem. Eng. 2018, 6, 5424–5430.
- Carrier, M.; Hugo, T.; Gorgens, J.; Knoetze, H. Comparison of Slow and Vacuum Pyrolysis of Sugar Cane Bagasse. J. Anal. Appl. Pyrolysis 2011, 90, 18–26.
- Dusso, D.; Téllez, J.F.; Fuertes, V.C.; De Paoli, J.M.; Moyano, E.L. Vacuum Pyrolysis of Chia Flour Residues: An Alternative Way to Obtain Omega-3/Omega-6 Fatty Acids and Calcium-Enriched Biochars. J. Anal. Appl. Pyrolysis 2022, 161, 105379.
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Johan, R.B. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; Samer, M., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 3–36. ISBN 978-953-51-3312-4.
- Collard, F.X.; Carrier, M.; Görgens, J.F. Fractionation of Lignocellulosic Material With Pyrolysis Processing. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 81–101. ISBN 9780128025611.
- Yadav, K.; Jagadevan, S.; Yadav, K.; Jagadevan, S. Influence of Process Parameters on Synthesis of Biochar by Pyrolysis of Biomass: An Alternative Source of Energy. In Recent Advances in Pyrolysis; Hassan, A.-H.I., Ed.; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-78984-064-3.
- Shah, A.T.; Attique, S.; Batool, M.; Godini, H.R.; Goerke, O. Role of Polyoxometalates in Converting Plastic Waste into Fuel Oil. In Advanced Technology for the Conversion of Waste into Fuels and Chemicals: Volume 2: Chemical Processes; Khan, A., Pizzi, A., Jawaid, M., Azum, N., Asiri, A., Isa, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 333–355. ISBN 9780323901505.
- Wang, X.; Kersten, S.R.A.; Prins, W.; Van Swaaij, W.P.M. Biomass Pyrolysis in a Fluidized Bed Reactor. Part 2: Experimental Validation of Model Results. Ind. Eng. Chem. Res. 2005, 44, 8786–8795.
- Bermudez, J.M.; Fidalgo, B. Production of Bio-Syngas and Bio-Hydrogen via Gasification. In Handbook of Biofuels Production: Processes and Technologies; Lugue, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Woodhead Publishing: Duxford, UK, 2016; pp. 431–494. ISBN 9780081004562.
- Ram, M.; Mondal, M.K. Biomass Gasification: A Step toward Cleaner Fuel and Chemicals. In Biofuels and Bioenergy: Opportunities and Challenges; Gurunathan, B., Sahadevan, R., Zakaria, Z.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 253–276. ISBN 978-0-323-85269-2.
- Mallick, D.; Sharma, S.D.; Kushwaha, A.; Brahma, H.S.; Nath, R.; Bhowmik, R. Emerging Commercial Opportunities for Conversion of Waste to Energy: Aspect of Gasification Technology. In Waste-to-Energy Approaches Towards Zero Waste; Hussain, C.M., Singht, S., Goswami, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 105–127. ISBN 978-0-323-85387-3.
- Zhu, Y.; Frey, H.C. Integrated Gasification Combined Cycle (IGCC) Power Plant Design and Technology. In Advanced Power Plant Materials, Design and Technology; Roddy, D., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 54–88. ISBN 978-1-84569-515-6.
- Khuenkaeo, N.; Tippayawong, N. Bio-Oil Production from Ablative Pyrolysis of Corncob Pellets in a Rotating Blade Reactor. IOP Conf. Ser. Earth Environ. Sci. 2018, 159, 012037.
- Mei Wu, L.; Hui Zhou, C.; Shen Tong, D.; Hua Yu, W. Catalytic Thermochemical Processes for Biomass Conversion to Biofuels and Chemicals. In Bioenergy Research: Advances and Applications; Gupta, V.K., Tuohy, M.G., Kubicek, C.P., Saddler, J., Xu, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 243–254. ISBN 9780444595614.
- Hu, X.; Gholizadeh, M. Biomass Pyrolysis: A Review of the Process Development and Challenges from Initial Researches up to the Commercialisation Stage. J. Energy Chem. 2019, 39, 109–143.
- Dhyani, V.; Bhaskar, T. Pyrolysis of Biomass. In Biomass, Biofuels, Biochemicals: Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Elsevier: London, UK, 2019; pp. 217–244. ISBN 9780128168561.
- Brassard, P.; Godbout, S.; Raghavan, V.; Palacios, J.H.; Grenier, M.; Zegan, D. The Production of Engineered Biochars in a Vertical Auger Pyrolysis Reactor for Carbon Sequestration. Energies 2017, 10, 288.
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger Reactors for Pyrolysis of Biomass and Wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409.
- Bridgwater, A.V. Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68–94.
- Nunez Manzano, M.; Gonzalez Quiroga, A.; Perreault, P.; Madanikashani, S.; Vandewalle, L.A.; Marin, G.B.; Heynderickx, G.J.; Van Geem, K.M. Biomass Fast Pyrolysis in an Innovative Gas-Solid Vortex Reactor: Experimental Proof of Concept. J. Anal. Appl. Pyrolysis 2021, 156, 105165.
- Rizzo, A.M.; Nistri, R.; Buffi, M.; Marsili Libelli, I.; Bettucci, L.; Prussi, M.; Chiaramonti, D. Effect of Feedstock Composition on Quality and Yield of Bio-Oil from the Pyrolysis of Three Microalgae Species from Open Pond and Closed Photobioreactor. In Proceedings of the 21st European Biomass Conference and Exhibitions, Copenghagen, Denmark, 3 June 2013; pp. 494–499.
- Akhtar, J.; Amin, N.S. A Review on Operating Parameters for Optimum Liquid Oil Yield in Biomass Pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109.
- Angin, D. Effect of Pyrolysis Temperature and Heating Rate on Biochar Obtained from Pyrolysis of Safflower Seed Press Cake. Bioresour. Technol. 2013, 128, 593–597.
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic Biomass Pyrolysis: A Review of Product Properties and Effects of Pyrolysis Parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140.
- Bridgwater, A.V. Principles and Practice of Biomass Fast Pyrolysis Processes for Liquids. J. Anal. Appl. Pyrolysis 1999, 51, 3–22.
- Mlonka-Mędrala, A.; Magdziarz, A.; Dziok, T.; Sieradzka, M.; Nowak, W. Laboratory Studies on the Influence of Biomass Particle Size on Pyrolysis and Combustion Using TG GC/MS. Fuel 2019, 252, 635–645.
- Kirubakaran, V.; Sivaramakrishnan, V.; Nalini, R.; Sekar, T.; Premalatha, M.; Subramanian, P. A Review on Gasification of Biomass. Renew. Sustain. Energy Rev. 2009, 13, 179–186.
- Şensöz, S.; Angin, D.; Yorgun, S. Influence of Particle Size on the Pyrolysis of Rapeseed (Brassica Napus L.): Fuel Properties of Bio-Oil. Biomass Bioenergy 2000, 19, 271–279.
- Liu, R.; Liu, G.; Yousaf, B.; Abbas, Q. Operating Conditions-Induced Changes in Product Yield and Characteristics during Thermal-Conversion of Peanut Shell to Biochar in Relation to Economic Analysis. J. Clean. Prod. 2018, 193, 479–490.
- Mellin, P.; Yu, X.; Yang, W.; Blasiak, W. Influence of Reaction Atmosphere (H2O, N2, H2, CO2, CO) on Fluidized-Bed Fast Pyrolysis of Biomass Using Detailed Tar Vapor Chemistry in Computational Fluid Dynamics. Ind. Eng. Chem. Res. 2015, 54, 8344–8355.
- Minkova, V.; Razvigorova, M.; Bjornbom, E.; Zanzi, R.; Budinova, T.; Petrov, N. Effect of Water Vapour and Biomass Nature on the Yield and Quality of the Pyrolysis Products from Biomass. Fuel Process. Technol. 2001, 70, 53–61.
- Bach, Q.V.; Trinh, T.N.; Tran, K.Q.; Thi, N.B.D. Pyrolysis Characteristics and Kinetics of Biomass Torrefied in Various Atmospheres. Energy Convers. Manag. 2017, 141, 72–78.
- Özbay, N.; Uzun, B.B.; Varol, E.A.; Pütün, A.E. Comparative Analysis of Pyrolysis Oils and Its Subfractions under Different Atmospheric Conditions. Fuel Process. Technol. 2006, 87, 1013–1019.
- Basile, L.; Tugnoli, A.; Stramigioli, C.; Cozzani, V. Influence of Pressure on the Heat of Biomass Pyrolysis. Fuel 2014, 137, 277–284.
- Ren, X.; Shanb Ghazani, M.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and Opportunities in Microwave-Assisted Catalytic Pyrolysis of Biomass: A Review. Appl. Energy 2022, 315, 118970.
- Nishu; Liu, R.; Rahman, M.M.; Sarker, M.; Chai, M.; Li, C.; Cai, J. A Review on the Catalytic Pyrolysis of Biomass for the Bio-Oil Production with ZSM-5: Focus on Structure. Fuel Process. Technol. 2020, 199, 106301.
- Wan, Y.; Chen, P.; Zhang, B.; Yang, C.; Liu, Y.; Lin, X.; Ruan, R. Microwave-Assisted Pyrolysis of Biomass: Catalysts to Improve Product Selectivity. J. Anal. Appl. Pyrolysis 2009, 86, 161–167.
- Chen, W.; Fang, Y.; Li, K.; Chen, Z.; Xia, M.; Gong, M.; Chen, Y.; Yang, H.; Tu, X.; Chen, H. Bamboo Wastes Catalytic Pyrolysis with N-Doped Biochar Catalyst for Phenols Products. Appl. Energy 2020, 260, 114242.
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic Pyrolysis of Lignocellulosic Biomass for Bio-Oil Production: A Review. Chemosphere 2022, 297, 134181.
- Zhang, G.; Sun, Y.; Xu, Y. Review of Briquette Binders and Briquetting Mechanism. Renew. Sustain. Energy Rev. 2018, 82, 477–487.
- Kataki, R.; Kataki, M.D. Weeds as a Renewable Bioresource: Prospects for Bioconversion to Biofuels and Biomaterials through a Cascade of Approaches. In Biofuels and Bioenergy; Gurunathan, B., Sahadevan, R., Zakaria, Z.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 437–461. ISBN 978-0-323-85269-2.
- Ahn, B.J.; Chang, H.S.; Lee, S.M.; Choi, D.H.; Cho, S.T.; Han, G.S.; Yang, I. Effect of Binders on the Durability of Wood Pellets Fabricated from Larix kaemferi C. and Liriodendron tulipifera L. Sawdust. Renew. Energy 2014, 62, 18–23.
- Hu, Q.; Shao, J.; Yang, H.; Yao, D.; Wang, X.; Chen, H. Effects of Binders on the Properties of Bio-Char Pellets. Appl. Energy 2015, 157, 508–516.
- Lu, D.; Tabil, L.G.; Wang, D.; Wang, G.; Emami, S. Experimental Trials to Make Wheat Straw Pellets with Wood Residue and Binders. Biomass Bioenergy 2014, 69, 287–296.
- Obi, O.F.; Pecenka, R.; Clifford, M.J. A Review of Biomass Briquette Binders and Quality Parameters. Energies 2022, 15, 2426.
- Solar, J.; de Marco, I.; Caballero, B.M.; Lopez-Urionabarrenechea, A.; Rodriguez, N.; Agirre, I.; Adrados, A. Influence of Temperature and Residence Time in the Pyrolysis of Woody Biomass Waste in a Continuous Screw Reactor. Biomass Bioenergy 2016, 95, 416–423.
- Mašek, O.; Budarin, V.; Gronnow, M.; Crombie, K.; Brownsort, P.; Fitzpatrick, E.; Hurst, P. Microwave and Slow Pyrolysis Biochar—Comparison of Physical and Functional Properties. J. Anal. Appl. Pyrolysis 2013, 100, 41–48.
- Shirvanimoghaddam, K.; Czech, Z.; Abdikheibari, S.; Brodie, G.; Ko, M.; Krzyszczak, A.; Al-Othman, A.; Naebe, M. Microwave Synthesis of Biochar for Environmental Applications. J. Anal. Appl. Pyrolysis 2022, 161, 105415.
- Chintala, V. Production, Upgradation and Utilization of Solar Assisted Pyrolysis Fuels from Biomass—A Technical Review. Renew. Sustain. Energy Rev. 2018, 90, 120–130.
- Singh, Y.; Singla, A.; Singh, K.; Sharma, A. Production and Feasibility Characterization of Bio-Oil from Jojoba Seed-Based Biomass through Solar Thermal Energy Pyrolysis Process. Biomass Convers. Biorefin. 2022, 1, 1–13.
