Pancreatic Ductal Adenocarcinoma (PDAC) is a ravaging disease with a poor prognosis, requiring a more detailed understanding of its biology to foster the development of effective therapies. The unsatisfactory results of treatments targeting cell proliferation and its related mechanisms suggest a shift in focus towards the tumor microenvironment (TME). Here, we discuss tThe role of cancer-secreted proteins in the complex TME tumor-stroma crosstalk, shedding lights on druggable molecular targets for the development of innovative, safer and more efficient therapeutic strategies is discussed here.
- pancreatic ductal adenocarcinoma
- secretome
- cell signaling
- tumor microenvironment
- small molecules
1. Pancreatic Cancer Fact Sheet
2. Proteins Secreted by Pancreatic Cancer Cells: Messages Sent to the Neighborhood
Cell communication in multicellular organisms allows cells to adapt their phenotypes and function. A number of secreted factors, whether soluble or associated with membranes, mediate critical molecular mechanisms involved in tissue and organism homeostasis. Typically, proteins follow the conventional protein secretion pathway, which involves the endoplasmic reticulum (ER) and the Golgi complex. However, some proteins use alternative routes, such as Unconventional Protein Secretion (UPS) pathways, induced by cellular stress such as nutrient deficiency, mechanical stress, inflammation, and ER stress. When the pathways leading to protein secretion, mediating both short- and long-range signals, are dysregulated, it accelerates disease pathogenesis. In addition, for some cancers, there is growing interest in intracellular proteins that, if secreted, play distinct functions, demonstrating that UPS pathways are still not fully understood [32][9]. In this context, tumor secretomes are able to influence the behavior of both neoplastic and non- neoplastic cells, providing a promising source of potential biomarkers useful in patient management. In fact, the alteration of the secretome mirrors disrupted cell-cell signaling in the pancreatic cancer milieu and participated in the reshaping of a fibrotic and inflammatory micro-environment that promotes cancer development and progression [33][10]. The obtained dataset represented and summarized the coexistence of cytokines, growth factors, extracellular matrix proteins, proteases and protease inhibitors, membrane and extracellular vesicle-associated proteins, and metabolic enzymes in the neoplastic milieu (Figure 1). Enzymes accounted for 32% of the proteins reported in the study, whereas enzyme inhibitors accounted for 5%. Cell signaling molecules accounted for 19%, while specific cell-matrix adhesion molecules accounted for 20%. Proteins with multiple roles accounted for 19% of the studied dataset, with the remaining 5% representing gene expression regulators and 6% representing transporters.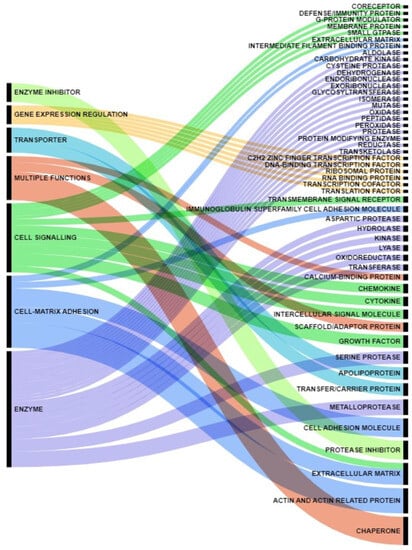
| Term | p-Value | q-Value | Overlaps Genes |
|---|---|---|---|
| Extracellular Matrix Disassembly (GO:0022617) | 7.05 × 10−9 | 2.09 × 10−6 | [MMP12, PRSS1, GSN, MMP7, MMP2, MMP9, MMP10] |
| Regulation of Apoptotic Process (GO:0042981) | 2.42 × 10−11 | 3.59 × 10−8 | [HSP90AA1, GSTP1, ANXA5, PARK7, IGF1, CLU, MMP9, THBS1, ACTB, NME1, LGALS1, AXL, BAG3, CEACAM5, ARHGDIA, CFL1, ALB, PPT1, FLNA, CALR, PPIA, CTSD, TGM2] |
| Neutrophil Chemotaxis (GO:0030593) | 1.94 × 10−7 | 2.88 × 10−5 | [LGALS3, CCL24, CXCL8, SAA1, PPBP, PPIA, PF4] |
| Carbohydrate Catabolic Process (GO:0016052) | 4.19 × 10−9 | 1.56 × 10−6 | [LDHA, TPI1, PKM, PGAM1, PGK1, ENO1, ENO2] |
As a result of the analysis, it was possible to highlight a set of proteases responsible for extracellular matrix and cellular component disassembly. As previously reported, the acellular components of the pancreatic tumor mass, as well as their changes over time, drive the tumor’s progression. In this regard, MMP-2 and MMP-9 (Matrix MetalloProteinases) gelatinases are abnormally and contemporarily upregulated in pancreatic cancer [48][11], but the clinical relevance measured by the correlation between their expression and survival, metastasis, or tumor stage is debatable [49][12]. Instead, the expression of the matrilysin MMP-7 in tumor samples was linked to a poorer prognosis in patients [50][13] and an unfavorable pathologic response to neoadjuvant therapy [51][14].
Some apoptosis-regulating proteins with very different biochemical functions have also been identified, and some of them have been linked to a role in pancreatic cancer. For example, Hsp90AA1 (Heat Shock Protein 90 Alpha Family Class A Member 1) is one of the most abundant proteins expressed in cells, interacting with several secreted client proteins. Hsp90AA1 promotes tumor aggressiveness and chemoresistance by activating AKT through LRP-1 (Low-density lipoprotein Receptor-related Protein 1) [54][15]. Among other chaperones, PARK7 (Parkinson protein 7) [55][16] and PPIA (Peptidylprolyl Isomerase A) [42][17] are upregulated and secreted by cancer cells. While PARK7 has been described for its ability to counteract environmental oxidative stress [56][18], PPIA is known to act through the CD47 and NF-kB pathway, thus promoting cell proliferation [57][19]. In addition, the extracellular chaperone Clusterin (CLU) has been shown to be a mediator of chemoresistance in pancreatic cancer [58][20]. The overexpression of the co-chaperone BAG3 (BCL2 Associated Athanogene 3) has also been described as associated with pancreatic cancer aggressiveness [59][21], and its sera levels are measurable in pancreatic cancer patients [45,60][22][23].
An additional functional cluster of secreted proteins was linked to neutrophil chemotaxis. Tumor-infiltrating neutrophils indicate a poor prognosis for patients, and activated neutrophils can generate neutrophil extracellular traps (NETs), which are emerging in several cancers as markers of cancer progression and immunosuppression [71,72][24][25]. As a first example, extracellular Galectin-3 (LGALS3), detected in the blood of PDAC patients [73][26], has been associated with neutrophils recruitment and inflammation exacerbation in several infectious diseases [74][27].
Moreover, altered carbohydrate catabolism has been recognized as the major metabolic alteration in pancreatic cancer [81][28], but the role of those enzymes in the pancreatic cancer milieu has not been fully elucidated yet. The evidence that secretory PKM (Pyruvate Kinase M1/2) promotes tumor angiogenesis by facilitating endothelial cell proliferation and new vessel formation via the PI3K/AKT and Wnt/-catenin signaling pathways provides some hints [82][29].
3. Targeting Pancreatic Cancer-Secreted Proteins
3.1. Communications Breakdown Operated by Small Molecule Drugs
The DGIdb (The Drug Gene Interaction Database, accessed on 28 June 2023) was queried, screened and integrated with a literature search for available molecules possibly having inhibitory activity on pancreatic cancer-secreted proteins illustrated above; the obtained search results are described below. The response to synthetic inhibitors of MMPs (MMPIs) was studied in the past decades in several solid tumors. However, despite promising preclinical data, all trials were unsuccessful in reducing tumor mass or improving overall survival [89][30]. Clusterin expression was challenged using the drug OGX-011, an antisense oligonucleotide that showed a potentiating effect on various FDA-approved anticancer chemotherapeutics during clinical trials [90][31]; however, no trial in pancreatic cancer has been programmed yet [91][32]. Ganetespib (STA-9090) is a small molecule that interferes with HSP90 client protein binding, thus promoting the inactivation and degradation of the signaling proteins that regulate cancer progression. Unfortunately, a Phase II study carried out in refractory metastatic pancreatic cancer failed to prove its clinical efficacy [92][33]. More clinical trials as a neoadjuvant treatment and/or in combination with chemotherapy are expected [93][34].3.2. Communications Breakdown Operated by Monoclonal Antibodies
The target specificity of monoclonal antibodies (mAbs) makes them powerful tools for a wide spectrum of biomedical and clinical application. As previously stated, the use of DGIdb was supported and integrated by a literature search to identify available mAbs able to bind and neutralize the secreted proteins here selected for discussion. Xentuzumab, an IgG1 monoclonal antibody with high affinity for IGF-1 and IGF-2 currently tested in preclinical models for the treatment of advanced solid tumors, allowed the collection of several interesting data [96,97][35][36]. A phase I trial enrolling patients affected by different advanced solid tumors, including PDAC, allowed verifying its safety, tolerability, and clinical manageability. On the other hand, in a phase II study in metastatic breast cancer, treatments with Xentuzumab combined with everolimus and exemestane did not show a significant impact on PFS (Progression Free Survival) [98][37]. Another strategy, aimed at neutralizing VEGF with the monoclonal antibody Bevacizumab, showed promising results in preclinical studies [99][38], but it did not show appreciable benefits when combined with gemcitabine in clinical trials [100][39]. Thanks to its high safety profile, trials were further extended to a third compound, erlotinib, but still without satisfying results [101][40]. In this context, the neutralization of extracellular BAG3 is another promising strategy supported by studies carried out in several murine preclinical models treated with an anti-BAG3 mAb, which showed its ability in reducing PDAC tumor growth as monotherapy [61,104][41][42]. But even more striking results were observed in combined treatments with the ICIs (Immune Check-point Inhibitors) anti-PD-1 [105][43] and anti-SIRP-alpha [106][44]. An anti-IL-8 antibody was also tested in a humanized mouse model of PDAC in combination with anti-PD-1. The treatment resulted in significantly reduced tumor growth, as well as an increased innate immune response and type I cytokine expression in myeloid cells, revealing a novel function of the IL-8 antibody in myeloid cell “re-education” [109][45]. HuMax-IL8 was tested in a Phase I trial on solid tumors, showing its safety and tolerability, while further studies are ongoing to evaluate the efficacy of anti-IL-8 treatments combined with other immunotherapies [110][46]. Another strategic perspective aims at targeting the asTF protein by a first-in-class humanized antibody, which exerted a significant effect on tumor growth in an animal model, downregulating several gene function categories, including focal adhesion, cell motility, cell proliferation, cytoskeleton, regulatory proteases and cell death, many of which are known to be TF- associated [111][47]. In this case, XB002, a novel, investigational ADC (Antibody Drug Conjugate), is currently being tested as a single-agent and combination therapy in subjects with inoperable locally advanced, or metastatic solid tumors in a Phase I trial; results are expected in late 2024 (NCT04925284) (Figure 2).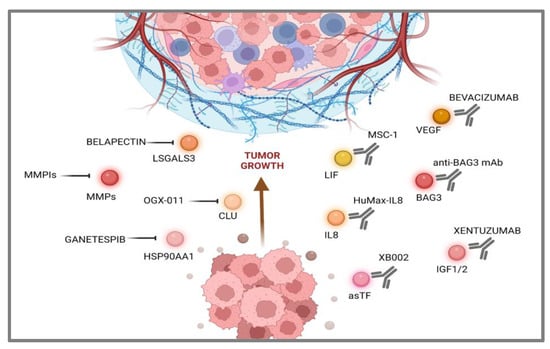
References
- Khalaf, N.; El-Serag, H.B.; Abrams, H.R.; Thrift, A.P. Burden of Pancreatic Cancer: From Epidemiology to Practice. Clin. Gastroenterol. Hepatol. 2021, 19, 876–884.
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer. JAMA 2021, 326, 851.
- Lippi, G.; Mattiuzzi, C. The global burden of pancreatic cancer. Arch. Med. Sci. 2020, 16, 820–824.
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA: A Cancer J. Clin. 2023, 73, 17–48.
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708.
- Kindler, H.L. A Glimmer of Hope for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2463–2464.
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218.
- Schepis, T.; De Lucia, S.S.; Pellegrino, A.; Del Gaudio, A.; Maresca, R.; Coppola, G.; Chiappetta, M.F.; Gasbarrini, A.; Franceschi, F. State-of-the-Art and Upcoming Innovations in Pancreatic Cancer Care: A Step Forward to Precision Medicine. Cancers 2023, 15, 3423.
- Padgaonkar, M.; Shendre, S.; Chatterjee, P.; Banerjee, S. Cancer secretome: Finding out hidden messages in extracellular secretions. Clin. Transl. Oncol. 2022, 25, 1145–1155.
- Espinet, E.; Klein, L.; Puré, E.; Singh, S.K. Mechanisms of PDAC subtype heterogeneity and therapy response. Trends in Cancer. 2022, 8, 1060–1071.
- Xu, T.; Xu, X.; Liu, P.C.; Mao, H.; Ju, S. Transcriptomic Analyses and Potential Therapeutic Targets of Pancreatic Cancer with Concomitant Diabetes. Front. Oncol. 2020, 10, 563527.
- Slapak, E.J.; Duitman, J.; Tekin, C.; Bijlsma, M.F.; Spek, C.A. Matrix Metalloproteases in Pancreatic Ductal Adenocarcinoma: Key Drivers of Disease Progression? Biology 2020, 9, 80.
- Jones, L.E.; Humphreys, M.J.; Campbell, F.; Neoptolemos, J.P.; Boyd, M.T. Comprehensive Analysis of Matrix Metalloproteinase and Tissue Inhibitor Expression in Pancreatic Cancer. Clin. Cancer Res. 2004, 10, 2832–2845.
- Shoucair, S.; Chen, J.; Martinson, J.R.; Habib, J.R.; Kinny-Köster, B.; Pu, N.; van Oosten, A.F.; Javed, A.A.; Shin, E.J.; Ali, S.Z.; et al. Association of Matrix Metalloproteinase 7 Expression with Pathologic Response After Neoadjuvant Treatment in Patients with Resected Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2022, 157, e221362.
- Xue, N.; Du, T.; Lai, F.; Jin, J.; Ji, M.; Chen, X. Secreted HSP90α-LRP1 Signaling Promotes Tumor Metastasis and Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2022, 23, 5532.
- Chen, Y.; Kang, M.; Lu, W.; Guo, Q.; Zhang, B.; Xie, Q.; Wu, Y. DJ-1, a novel biomarker and a selected target gene for overcoming chemoresistance in pancreatic cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 1463–1474.
- Kong, K.; Guo, M.; Liu, Y.; Zheng, J. Progress in Animal Models of Pancreatic Ductal Adenocarcinoma. J. Cancer 2020, 11, 1555–1567.
- Li, M.; Zhai, Q.; Bharadwaj, U.; Li, F.; Fisher, W.E.; Chen, C.; Yao, Q. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer 2006, 106, 2284–2294.
- Han, J.M.; Jung, H.J. Cyclophilin A/CD147 Interaction: A Promising Target for Anticancer Therapy. Int. J. Mol. Sci. 2022, 23, 69341.
- Chen, Q.; Wang, Z.; Zhang, K.; Liu, X.; Cao, W.; Zhang, L.; Zhang, S.; Yan, B.; Wang, Y.; Xia, C. Erratum to: Clusterin confers gemcitabine resistance in pancreatic cancer. World J. Surg. Oncol. 2013, 11, 149.
- Rosati, A.; Bersani, S.; Tavano, F.; Dalla Pozza, E.; De Marco, M.; Palmieri, M.; De Laurenzi, V.; Franco, R.; Scognamiglio, G.; Palaia, R.; et al. Expression of the Antiapoptotic Protein BAG3 Is a Feature of Pancreatic Adenocarcinoma and Its Overexpression Is Associated with Poorer Survival. Am. J. Pathol. 2012, 181, 1524–1529.
- Firpo, M.A.; Boucher, K.M.; Bleicher, J.; Khanderao, G.D.; Rosati, A.; Poruk, K.E.; Kamal, S.; Marzullo, L.; De Marco, M.; Falco, A.; et al. Multianalyte Serum Biomarker Panel for Early Detection of Pancreatic Adenocarcinoma. JCO Clin. Cancer Inform. 2023, 7, e2200160.
- Falco, A.; Rosati, A.; Festa, M.; Basile, A.; De Marco, M.; d’Avenia, M.; Pascale, M.; Dal Piaz, F.; Tavano, F.; Di Mola, F.F.; et al. BAG3 Is a Novel Serum Biomarker for Pancreatic Adenocarcinomas. Am. J. Gastroenterol. 2013, 108, 1178–1180.
- Jiang, W.; Li, X.; Xiang, C.; Zhou, W. Neutrophils in pancreatic cancer: Potential therapeutic targets. Front. Oncol. 2022, 12, 1025805.
- Jin, W.; Xu, H.X.; Zhang, S.R.; Li, H.; Wang, W.-Q.; Gao, H.-L.; Wu, C.-T.; Xu, J.-Z.; Qi, Z.-H.; Li, S.; et al. Tumor-Infiltrating NETs Predict Postsurgical Survival in Patients with Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2018, 26, 635–643.
- Yi, N.; Zhao, X.; Ji, J.; Xu, M.; Jiao, Y.; Qian, T.; Zhu, S.; Jiang, F.; Chen, J.; Xiao, M. Serum galectin-3 as a biomarker for screening, early diagnosis, prognosis and therapeutic effect evaluation of pancreatic cancer. J. Cell. Mol. Med. 2020, 24, 11583–11591.
- Guo, Y.; Shen, R.; Yu, L.; Zheng, X.; Cui, R.; Song, Y.; Wang, D. Roles of galectin-3 in the tumor microenvironment and tumor metabolism (Review). Oncol. Rep. 2020, 44, 1799–1809.
- Liu, Y.H.; Hu, C.M.; Hsu, Y.S.; Lee, W.H. Interplays of glucose metabolism and KRAS mutation in pancreatic ductal adenocarcinoma. Cell Death Dis. 2022, 13, 817.
- Yang, P.; Li, Z.; Wang, Y.; Zhang, L.; Wu, H.; Li, Z. Secreted pyruvate kinase M2 facilitates cell migration via PI3K/Akt and Wnt/β-catenin pathway in colon cancer cells. Biochem. Biophys. Res. Commun. 2015, 459, 327–332.
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155.
- Praharaj, P.P.; Patra, S.; Panigrahi, D.P.; Patra, S.K.; Bhutia, S.K. Clusterin as modulator of carcinogenesis: A potential avenue for targeted cancer therapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188500.
- Zhang, Y.; Lu, L.; Song, F.; Zou, X.; Liu, Y.; Zheng, X.; Qian, J.; Gu, C.; Huang, P.; Yang, Y. Research progress on non-protein-targeted drugs for cancer therapy. J. Exp. Clin. Cancer Res. 2023, 42, 62.
- Cardin, D.B.; Thota, R.; Goff, L.W.; Berlin, J.D.; Jones, C.M.; Ayers, G.D.; Whisenant, J.G.; Chan, E. A Phase II Study of Ganetespib as Second-line or Third-line Therapy for Metastatic Pancreatic Cancer. Am. J. Clin. Oncol. 2018, 41, 772–776.
- Lang, J.E.; Forero-Torres, A.; Yee, D.; Yau, C.; Wolf, D.; Park, J.; Parker, B.A.; Chien, A.J.; Wallace, A.M.; Murthy, R.; et al. Safety and efficacy of HSP90 inhibitor ganetespib for neoadjuvant treatment of stage II/III breast cancer. NPJ Breast Cancer 2022, 8, 128.
- Friedbichler, K.; Hofmann, M.H.; Kroez, M.; Ostermann, E.; Lamche, H.R.; Koessl, C.; Borges, E.; Pollak, M.N.; Adolf, G.; Adam, P.J. Data from Pharmacodynamic and Antineoplastic Activity of BI 836845, a Fully Human IGF Ligand-Neutralizing Antibody, and Mechanistic Rationale for Combination with Rapamycin; American Association for Cancer Research (AACR): Philadelphia, PA, USA, 2023.
- Weyer-Czernilofsky, U.; Hofmann, M.H.; Friedbichler, K.; Baumgartinger, R.; Adam, P.J.; Solca, F.; Kraut, N.; Nguyen, H.M.; Corey, E.; Liu, G.; et al. Data from Antitumor Activity of the IGF-1/IGF-2–Neutralizing Antibody Xentuzumab (BI 836845) in Combination with Enzalutamide in Prostate Cancer Models; American Association for Cancer Research (AACR): Philadelphia, PA, USA, 2023.
- Schmid, P.; Cortes, J.; Joaquim, A.; Jañez, N.M.; Morales, S.; Díaz-Redondo, T.; Blau, S.; Neven, P.; Lemieux, J.; García-Sáenz, J.Á.; et al. XENERA-1: A randomised double-blind Phase II trial of xentuzumab in combination with everolimus and exemestane versus everolimus and exemestane in patients with hormone receptor-positive/HER2-negative metastatic breast cancer and non-visceral disease. Breast Cancer Res. 2023, 25, 67.
- Sullivan, L.A.; Carbon, J.G.; Roland, C.L.; Toombs, J.E.; Nyquist-Andersen, M.; Kavlie, A.; Schlunegger, K.; Richardson, J.A.; Brekken, R.A. r84, a Novel Therapeutic Antibody against Mouse and Human VEGF with Potent Anti-Tumor Activity and Limited Toxicity Induction. PLoS ONE 2010, 5, e12031.
- Kindler, H.L.; Niedzwiecki, D.; Hollis, D.; Sutherland, S.; Schrag, D.; Hurwitz, H.; Innocenti, F.; Mulcahy, M.F.; O’Reilly, E.; Wozniak, T.F.; et al. Gemcitabine Plus Bevacizumab Compared with Gemcitabine Plus Placebo in Patients with Advanced Pancreatic Cancer: Phase III Trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol. 2010, 28, 3617–3622.
- Van Cutsem, E.; Vervenne, W.L.; Bennouna, J.; Humblet, Y.; Gill, S.; Van Laethem, J.L.; Verslype, C.; Scheithauer, W.; Shang, A.; Cosaert, J.; et al. Phase III Trial of Bevacizumab in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. J. Clin. Oncol. 2009, 27, 2231–2237.
- Rosati, A.; Basile, A.; D’Auria, R.; d’Avenia, M.; De Marco, M.; Falco, A.; Festa, M.; Guerriero, L.; Iorio, V.; Parente, R.; et al. BAG3 promotes pancreatic ductal adenocarcinoma growth by activating stromal macrophages. Nat. Commun. 2015, 6, 8695.
- Basile, A.; De Marco, M.; Festa, M.; Falco, A.; Iorio, V.; Guerriero, L.; Eletto, D.; Rea, D.; Arra, C.; Lamolinara, A.; et al. Development of an anti-BAG3 humanized antibody for treatment of pancreatic cancer. Mol. Oncol. 2019, 13, 1388–1399.
- Iorio, V.; Rosati, A.; D’Auria, R.; De Marco, M.; Marzullo, L.; Basile, A.; Festa, M.; Pascale, M.; Remondelli, P.; Capunzo, M.; et al. Combined effect of anti-BAG3 and anti-PD-1 treatment on macrophage infiltrate, CD8+ Tcell number and tumour growth in pancreatic cancer. Gut 2017, 67, 314225.
- De Marco, M.; Gauttier, V.; Pengam, S.; Mary, C.; Ranieri, B.; Basile, A.; Festa, M.; Falco, A.; Reppucci, F.; Cammarota, A.L.; et al. Concerted BAG3 and SIRPα blockade impairs pancreatic tumor growth. Cell Death Discov. 2022, 8, 94.
- Li, P.; Rozich, N.; Wang, J.; Wang, J.; Xu, Y.; Herbst, B.; Yu, R.; Muth, S.; Niu, N.; Li, K.; et al. Anti-IL-8 antibody activates myeloid cells and potentiates the anti-tumor activity of anti-PD-1 antibody in the humanized pancreatic cancer murine model. Cancer Lett. 2022, 539, 215722.
- Bilusic, M.; Heery, C.R.; Collins, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Marté, J.L.; Strauss, J.; Gatti-Mays, M.E.; et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunotherapy Cancer 2019, 7, 240.
- Lewis, C.S.; Karve, A.; Matiash, K.; Stone, T.; Li, J.; Wang, J.K.; Versteeg, H.H.; Aronow, B.J.; Ahmad, S.A.; Desai, P.B.; et al. A First-In-Class, Humanized Antibody Targeting Alternatively Spliced Tissue Factor: Preclinical Evaluation in an Orthotopic Model of Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2021, 11, 691685.
