Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Paraskev Todorov Nedialkov.
Naturally occurring benzophenones represent a relatively small group of plant metabolites with narrow distribution, mainly in members of Clusiaceae, Gentianaceae, Hypericaceae, Polygalaceae, Myrtaceae, etc.
- simple oxygenated benzophenones
- C-glycosides
- O-glycosides
- structural diversity
1. Introduction
Naturally occurring benzophenones are considered compounds derived from diphenylmethanone (Figure 1), in which one or several protons are replaced by hydroxyl, alkyl, alkyloxy groups, or halogen atoms. So far, unsubstituted benzophenone has been found in nature only in Iris adriatica [1] and Hemidesmus indicus [2].
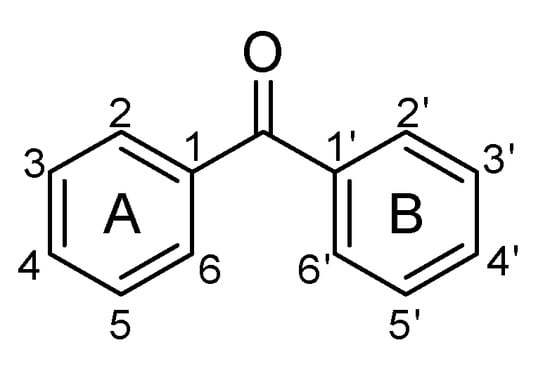
Figure 1.
The structure of unsubstituted benzophenone.
Despite the large number of isolated compounds over the past 50 years, the few published reviews focused almost entirely on prenylated benzophenones [3,4,5][3][4][5]. Singh and Bharate reviewed naturally occurring benzophenones with a phloroglucinol ring up to December 2005 [6]. Up to now, a systematic review of the existing naturally occurring simple oxygenated benzophenones and their glycosides has not been performed. This revisewarch compiles references from 1850 to 2023 using databases like Chemical Abstracts, Scopus, Google Scholar, PubMed, and ResearchGate. The main goal of this reviewsearch is to summarize the available information on structural diversity of simple oxygenated benzophenones. A consensus classification of this group of compounds is also lacking. If the biogenesis of xanthones is considered, it can be seen that ring A and the attached CO group are produced by the shikimate pathway, while ring B is formed in the acetate–malonate pathway [7], yielding an oxygenated benzophenone intermediate, which subsequently undergoes molecular dehydration to give xanthone. Furthermore, it has been reported that the transformation can be accomplished from a benzophenone glycoside by elimination of the glycosidic moiety and subsequent dehydration [8]. Therefore, simple oxygenated benzophenones and their glycosides are precursors in the biogenesis of xanthones. Thus, the classification for the biosynthetically related xanthones provided by Mandal et al. [9] is used in this revisewarch. Special attention is given to benzophenone C- and O-glycosides. The second goal of this reviewsearch is to summarize the published information on the distribution of simple oxygenated benzophenones and their glycosides. Some synthetic hydroxy and methoxy benzophenones are heavily used in sunscreens and in personal care cosmetic products and both associated with allergy and photoallergy [10].
2. Monooxygenated Benzophenones
Only two naturally occurring benzophenones (Figure 2), which have a hydroxyl or methoxy group in para position to carbonyl function, were reported in the literature. p-Hydroxybenzophenone 1 was isolated from 1,2-dichloroethylene extract of Talauma mexicana (DC.) G.Don leaves [11] and p-methoxybenzophenone 2 was found as a component of the essential oil of Costus speciosus (J.Koenig) Sm. [12].
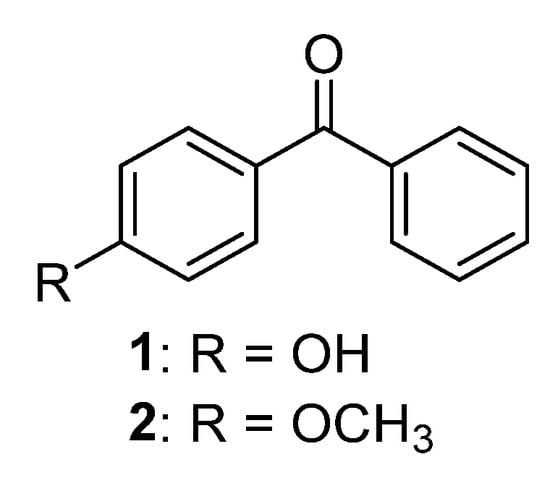
Figure 2.
The structures of monooxygenated benzophenones.
3. Dioxygenated Bezophenones
The literature survey showed three compounds, 3–5 (Figure 3), isolated from the endophytic moss Polytrichastrum formosum (Hedw.) G.L. Smith [13], while 6 was found in Pogonatum inflexum (Lindb.) Sande Lac. [14]. The nitrogen-containing derivatives of 2,4-dihydrobenzophenone 7 and 8 were isolated from the ethanol extract of the moss Polytrichum commune Hedw. [15]. Compounds 9–11 were found in Polytrichastrum formosum [13,16][13][16] and compound 12 was detected in Pogonatum inflexum [14]. All of these compounds possess 2,4-dioxygenation pattern only in one of the phenyl rings. O-glycosidic forms of the deoxygenated benzophenones have not been discovered so far, but there was a report of a C-glycoside, namely hyperinone 13, isolated from Hypericum styphelioides A.Rich. [17].
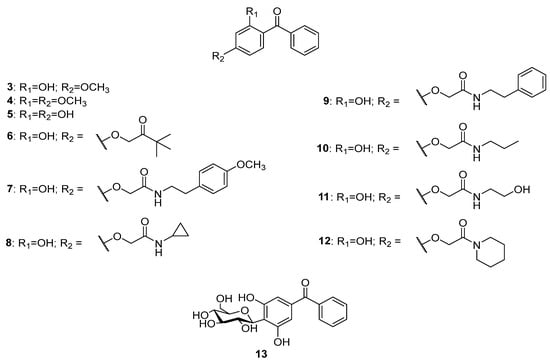
Figure 3.
The structures of dioxygenated benzophenones.
4. Trioxygenated Bezophenones
The first reports of isolated trioxygenated simple benzophenones date from nearly a century and a half ago, when Jobst and Hesse began to study the composition of Coto and Paracoto barks. Although the origin of the two barks remains still unknown, it can be safely assumed that they belong to the Lauraceae family [18]. The first isolated compound was cotoin 14 (Figure 4), which was obtained from a diethyl ether extract of Coto bark [19,20][19][20]. Hydrocotoine 15 and methylhydrocotoine 16 were subsequently isolated from an alcoholic extract of Paracoto bark [20]. More than a decade later, thanks to the outstanding work of Ciamiciani and Silber, the structures of these compounds were finally established [21,22,23,24][21][22][23][24]. 2,4,6-Trihydroxybenzophenone 17 and 2,4-dihydroxy-6-methoxybenzophenone 18 were found to be major components in the methanol extract of leaves and stems of Helichrysum triplinerve DC. [25].
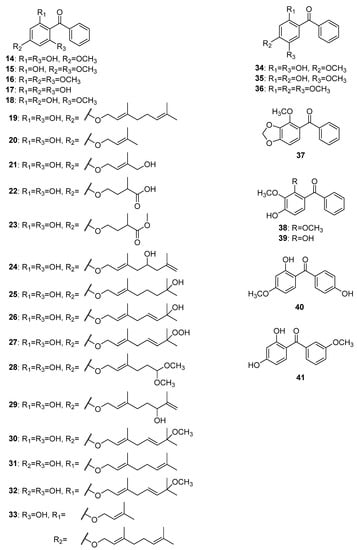
Figure 4.
The structures of hydroxy, methoxy, and alkyloxy derivatives of trioxygenated benzophenones.
Most of the simply oxygenated naturally occurring benzophenones with 2,4,6-oxygenation patterns were alkyloxy derivatives. Sixteen compounds from this group have been reported to date, fourteen of which have been found in species of the genus Hypericum. The first alkyloxy benzophenone 19 was discovered in 1978 by Bohlmann and Suwita in the root of Leontonyx squarrosus DC and aerial parts of L. spathulatus Less. [26]. Eleven years later, four more compounds, 20–23, belonging to this group were isolated from Helichrysum asperum (Thunb.) Hilliard and B.L.Burtt [27]. Phytochemical studies of the aerial parts of Hypericum sampsonii Hance led to the isolation of seven new compounds, 24, 25 [28]; sampbenzophenones D-G (26–29) [29]; and 33 [30]. The compounds hyperinokone 30, elegaphenone 31, and hyperinagasone 32 were isolated from H. nokoense Ohwi [31], H. elegans Steph. ex Willd. [32] and H. nagasawae Hayata [33], respectively. In 1968, the isolation of a trioxygenated benzophenone cearoin 34 from extracts of Dalbergia cearensis Ducke and D. miscolobium Benth. was reported [34]. It was the first natural benzophenone with 2,4,5-trioxygenation pattern. In addition, two compounds with the same oxygenation pattern, 35 and 36, were isolated from D. odorifera T. C. Chen [35] and Securidaca longipedunculata Fresen [36], respectively. Two benzophenones, 37 and 38, possessing 2,3,4-trioxygenation patterns were isolated from S. inappendiculata Hassk [37]. In addition, compound 39 with the same oxygenation was isolated from the dichloromethane extract of S. diversifolia (L.) Blake [38]. The only two trioxygenated benzophenones with oxygenation on both rings were 40 and 41 isolated from Tolpis webbii Sch. Bip. [39] and Securidaca longepedunculata Fresen. [36], respectively.
Fourteen glycosides of trioxygenated benzophenones (Figure 5) mostly sharing a common 2,4,6-trioxygenation pattern have been reported to date. The first tryoxygenated C-glycoside malaferin A 42 was isolated from an ethanolic extract of twigs and leaves of Malania oleifera Chun and S.K.Lee [40], while a di-C-glycoside arrilanin G 43 was obtained from an ethanolic extract of the stem bark of Polygala rillate Buch.-Ham. ex D.Don [41]. 2,4,6-Trihydroxybenzophenone 17 was the most common aglycon of the O-glycosides of the of trioxygenated benzophenones. Garcimangosone D 44 was its first O-glycoside isolated from the ethanol extract of Garcinia mangostana L. fruits [42]. Furthermore, four 6-O-biosides of 17 including tricornoside A 46 (from Polygala tricornis Gagnep.) [43], pyrafortunoside B 47, and C 48 (from Pyracantha fortuneana (Maxim.) H. Li) [44] as well as piperwallioside A 49 (from Piper wallichii (Miq.) Hand.-Mazz.) [45] were isolated from the respective species. Two 4-O-glycosides of 17 (50 and 51) were isolated from the ethanol extract of the Psidium guajava L. leaves [46,47][46][47]. The phytochemical investigations of Polygala tenuifolia Willd. led to the isolation of three O-glycosides of 2,4-dihydroxy-6-methoxybenzophenone 18, namely tenuiside B 52 and C 45 [48] as well as the O-bioside tenuiphenone A 53 [49]. In addition, a O-bioside 54 of 4-hydroxy-2,6-dimethoxybenzophenone was found in P. sibirica var. megalopha Franch. [50]. An O-glycoside pruniflonone B 55 having an unusual aglycone (3,4,5-trihydroxybenzophenone) was isolated from the methanol extract of Cratoxylum formosum (Jack) Dyer roots [51].
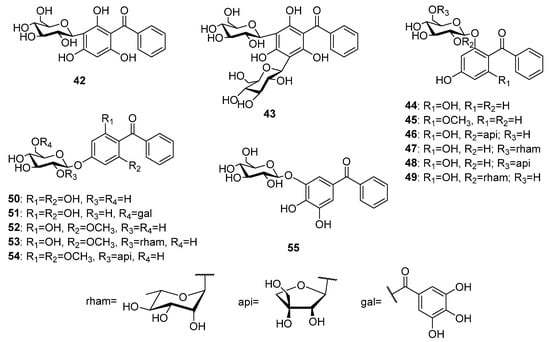
Figure 5.
The structures of C- and O-glycosides of trioxygenated benzophenones.
5. Tetraoxygenated Bezophenones
This group includes eighteen hydroxy and methoxy tetraoxygenated benzophenones (Figure 6). Only three compounds (56–58) have substituents on ring A but not in B and share a common 2,3,4,5-tetraoxygenation pattern. Compounds 56 and 57 were isolated from the wood of Machaerium scleroxylon Tul. [52[52][53],53], while 58 was found in the roots of Securidaca longipedunculata [54]. Four compounds (59–62) share a common phloroglucinol oxygenation pattern in ring A and a monooxygenation in ring B. The first occurrence in nature of compounds 59–62 was reported for Morus alba L. [55], Aniba duckei Kosterm. [56], Gentiana lutea L. [57], and for Allanblackia floribunda Oliv. [58], respectively. Compounds 63–65 possess a rare 2,4,5-trioxynation pattern of ring A and structurally differ by the position of the hydroxyl group in ring B. The former two compounds, 64 and 65, were isolated from the heartwood of Dalbergia melanoxylon Guill. and Perr. [59,60][59][60] while the latter 66 was found in D. cochinchinensis Pierre [61]. Compounds 66–69 shared a common pyrogallol-like ring A and monooxygenated ring B. These compounds were reported as the first to occur in biogenetically different plant species: 66 in Securidaca diversifolia [38]; 67 in Anemarrhena asphodeloides Bunge [62]; 68 in Oroxylum indicum (L.) (L.) Benth. ex Kurz [63]; and 69 in Garcinia mangostana [64]. Compounds 70–73 possess di-oxygenation on both rings and were found in Garcinia cantleyana Whit. var. cantleyana [65], Hypericum lanceolatum Lam. [66], Garcinia eugenifolia Wall. ex T. Anderson [67], and Syzygium polyanthum (Wight) Walp. [68], respectively.
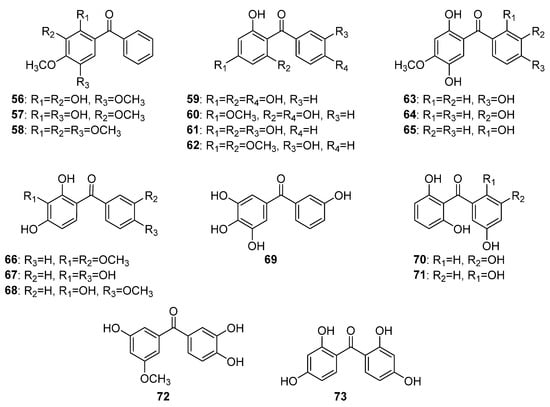
Figure 6.
The structures of hydroxy and methoxy tetraoxygenated benzophenones.
To date, 24 C-glycosides of tetraoxygenated benzophenones (Figure 7) have been isolated from the plant sources. The aglycone of most of these compounds is iriflophenone 59. The first occurrence of iriflofenone-3-C-β-D-glucoside 74 was reported for the leaf extracts of the ferns Hypodematium fauriei (Kodama) Tagawa and H. crenatum (Forssk.) Kuhn [69]. Two phytochemical studies on mango tree bark and leaves led to the isolation of the galloyl esters 75–77 [70,71][70][71] and one p-hydroxybenzoyl ester 78 [71] of 74. Further galloyl and p-hydroxybenzoyl esters 79–84, as well as an ester of 3,4-dihydroxy-5-methoxybenzoic acid 85, were isolated from the ethanol extract of the leaves of Mangifera indica [72,73][72][73]. A mixed C,O-diglycoside 86 was isolated from water extract of aerial parts of Cyclopia genistoides (L.) R.Br. [74]. A di-C-glycoside 87 of iriflophenone 59 was isolated from the leaves extract of Aquilaria sinensis (Lour.) Spreng. [75]. Five C-glycosides 88–92 of 2,4′,6-trihydoxy-4-methoxybenzophenone 60 were found in leaves of Mangifera indica [72,73][72][73]. The only C-galactosyl benzophenone 93 known up to now was isolated from the leaves of the mango tree [76]. The only C-glycoside of 2,6,3′-trihydroxy-4-methoxybenzophenone rhodanthenone C 94 was isolated from a methanolic extract of the aerial parts of Gentiana rhodantha Franch. ex Hemsl. [77]. The di-C-glucosides 95 and 96 were isolated from Polygala tenuifolia [49] and P. glomerata Lour. (P. chinensis L.) [78], respectively. Di-C glucoside pseuduvarioside 97 was isolated from the leaves extract of Pseuduvaria fragrans Y.C.F.Su, Chaowasku and R.M.K.Saunders [79].
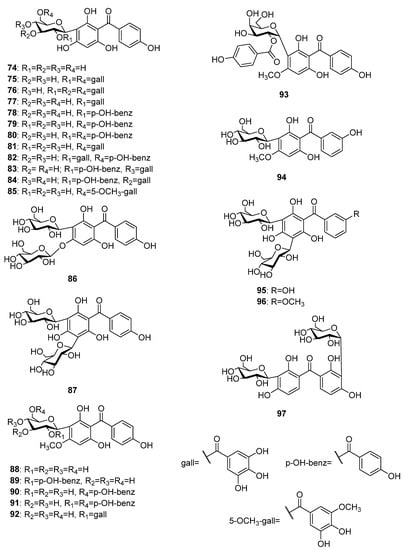
Figure 7.
The structures of C-glycosides of tetraoxygenated benzophenones.
Some C-glycosyl tetraoxygenated benzophenones (Figure 8) form rare spirocyclic systems. The first compound from this group was aquilarinoside A 98, which was isolated from hydroethanolic extract of Aquilaria sinensis leaves [80]. Compound 99, similar to 98 but with β-configuration of fructofuranose, was isolated from hydroethanolic extract of mango tree leaves [76]. In addition, 100 and 101 were found in leaf extract from the later plant [81,82][81][82]. Compounds 102 and 103 were isolated from the later plant source differed from 99 and 101, respectively, by the cyclization type of fructose unit [76].
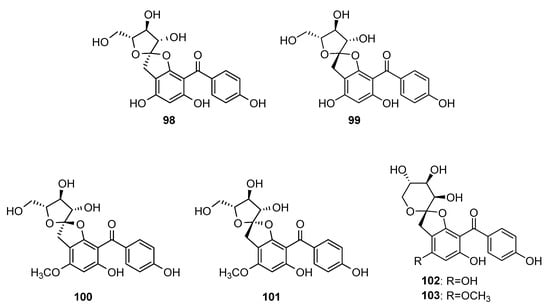
Figure 8.
The structures of spirocyclic C-glycosides of tetraoxygenated benzophenones.
More than twenty O-glycosides of tetraoxygenated benzophenones (Figure 9) have been isolated, so far. Most of these compounds (104–121) share iriflophenone 59 as their aglycone. The first O-glycoside of iriflophenone 104 was isolated from Coleogyne ramosissima Torr. [83]. Three acylated derivatives, 105–107, of 104 were found in the leaves of Planchonella obovata (R.Br.) Pierre [84]. Iriflophenone-2-O-α-L-rhamnoside 108 and its acylated derivatives 109–112 were reported for Aquilaria sinensis leaves and flower buds [75,85,86][75][85][86]. The methyl ester 113 of the later compound was isolated from the pericarps of A. yunnanensis S. C. Huang [87]. The presence of five biosides 114–118 of iriflophenone 59 in A. sinensis was established [85,88][85][88]. In addition, a 2-O-β-D-xylopyranoside 119 and a 2-O-α-L-arabinopyranoside 120 of 59 were reported for A. sinensis [86] and Mangifera indica [76], respectively. The only glycoside 121 with sugar connected to position 4 of iriflophenone was found in Davallia solida (G.Forst.) Sw. rhizomes [89]. Three compounds, 122–124, possess 2,4′,6-trihydroxy-4-methoxybenzophenone 60 as aglycone. The former compound 122 was isolated from the aerial parts of Gnidia involucrata Steud. ex A.Rich. [90]. The latter two compounds 123 and 124 were reported for nut shell of Phaleria macrocarpa (Scheff.) Boerl. fruits [91] and for roots of Vangueria agrestis (Schweinf. ex Hiern) Lantz [92], respectively. Mahkoside A 125 was isolated from the ethanolic extract of Phlaleria macrocarpa pits [93]. The tetraoxygenated benzophenone 61 was an aglycone of three glycosides, 126–128, that were isolated from Gentiana rhodantha Franch. ex Hemsl. [77], G. verna L. subsp. pontica (Soltok.) Hayek [94] and Garcinia mangostana fruit pericarp [95], respectively. The tetraoxygenated benzophenone O-glycosides 129–132 that possess unique aglycones were found in Gentiana verna subsp. pontica [94], Tripterospermum japonicum (Siebold and Zucc.) Maxim. [96], Cratoxylum formosum [97], and Anemarrhena asphodeloides [98], respectively.
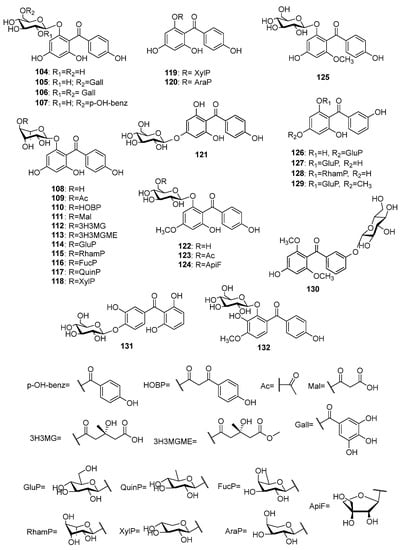
Figure 9.
The structures of O-glycosides of tetraoxygenated benzophenones.
6. Pentaoxygenated Benzophenones
More than 20 non-glycosylated (aglycones) naturally occurring pentaoxygenated benzophenones (Figure 10) representing seven oxygenation patterns have been reported, so far. Maclurin 133 was the firstly found pentaoxygenated benzophenone possessing 2,3′,4,4′,6-pentaoxygenation pattern. It was isolated in 1850 by Wagner from Maclura tinctoria (L.) Steud. [99,100][99][100] but its structure was finally established in 1895 by Ciamician and Silber [101]. Methoxy derivatives 134–138 of maclurin were found in Garcinia subelliptica Merr. [102], G. multiflora Champ. ex Benth. [103], G. mangostana [104], G. livingstonei T.Anderson [105], and G. mangostana [106], respectively. Compounds 139 and 140 were the only methylenedioxy-benzophenones with 2,3′,4,4′,6-pentaoxygenation pattern originally isolated from Aniba pseudocoto bark [20,22][20][22]. Compounds 141 and 142 were found in Garcinia subelliptica [107] and G. smeathmannii (Planch. and Triana) Oliv. [108], respectively, and shared a common 2,2′,3′,4,6-pentaoxygenation pattern. Compounds 143–148, possessing a common 2,3′,4,5′,6-pentaoxygenation pattern, were originally isolated from G. pedunculata Roxb. ex Buch.-Ham. [109], G. mangostana [106], G. hombroniana Pierre [110], G. speciosa Wall. [111], Hypericum annulatum Moris [8], and H. sampsonii [112], respectively. The rare compounds 149 and 150 share common ring A and differ in ring B; both were isolated from Dalbergia melanoxylon [59,113][59][113]. Compounds 151–153 possess a common 3,3′,4,5,5′-pentaoxygenation pattern. The former 151 and 152 were isolated from the twigs of Garcinia cantleyana var. cantleyana [65] while the later 153 was found in the pericarp of mangosteen (G. mangostana) [114]. The sole compound 154 with 2,3,3′,4,4′-pentaoxygenation pattern was isolated from the Securidaca diversifolia roots [38].
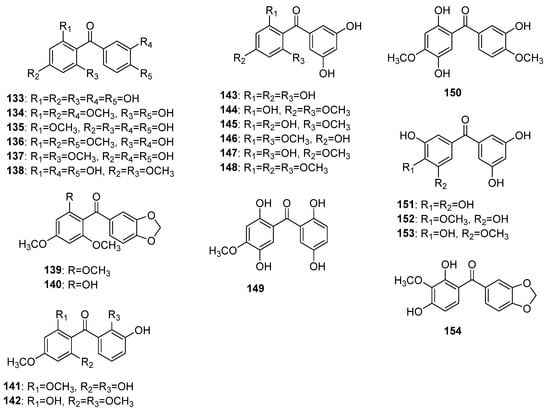
Figure 10.
The structures of hydroxy and methoxy pentaoxygenated benzophenones.
More than fifteen C-glycosylated derivatives of the pentaoxygenated benzophenones (Figure 11) have been found in plants, so far. Most of these compounds possess a 2,3′,4,4′,6-pentaoxygenation pattern. The first report for naturally occurring C-glycosylated pentaoxygenated benzophenones dated from 1984 when Tanaka and coworkers isolated maclurin-3-C-β-D-glucoside 155 along with its galloyl and p-hydroxybenzoyl esters 156–159 from the Mangifera indica leaves [70]. Further galloyl esters 160 and 161 [71] as well as methoxy derivatives 162 [81] and 163 [72] of 155 were found in the same plant source. Additional methoxy derivatives 164–166 of 155 were isolated from the aerial parts of Polygala telephioides Willd. [115,116][115][116]. The only di-C-glycoside 167 and C,O-glycoside 168 of pentaoxygenated benzophenones were isolated from an ethanolic extract of P. glomerata [78]. Compound 169 is the only benzophenone C-glycoside with 2,3′,4′5′,6-pentaoxygenation pattern that was isolated from the aerial parts of Hypericum humifusum L. ssp. austral Rouy et Foue [117]. C-glycosyl benzophenones 170 and 171 share common ring A with 2,3,5,6-oxygenation patters and differ in the hydroxyl place in ring B. The former was isolated from Gnidia involucrata [90] while the later was found in Polygala telephioides [115].
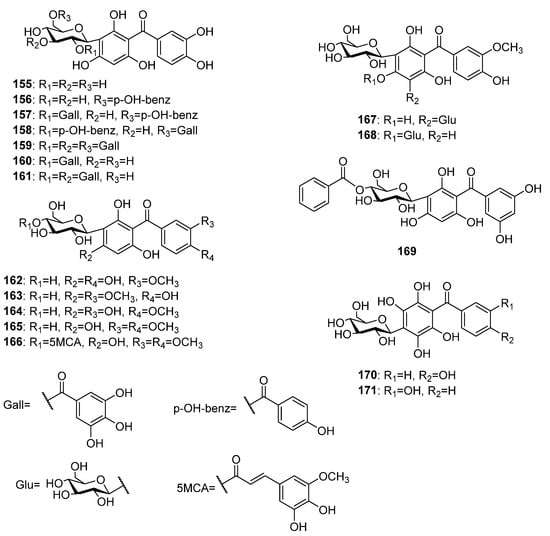
Figure 11.
The structures of C-glycosides of pentaoxygenated benzophenones.
Over 30 O-glycosides of pentaoxygenated benzophenones (Figure 12) have been reported to occur in plant species, so far. Contrary to C-glycosides, only six O-glycosides with 2,3′,4,4′,6-pentaoxygenation patterns 172–177 have been encountered. Maclurin-6-O-β-D-glucopyranoside 172 was simultaneously isolated from Gentiana verna subsp. pontica [94] and from G. rhodantha [77]. Compounds 173, 174, and 177 were isolated from Dobinea delavayi (Baill.) Baill. roots [118,119][118][119], while 175 and 176 were found in aerial parts of Polygala tenuifolia [48] and Garcinia livingstonei [105], respectively. The vast majority of O-glycosides (178–205) possess aglycones with 2,3′,4,5′,6-pentaoxygenation pattern and glycosylation at position 6(2), 4 or 3′(5′). The arabinofuranosides 178 and 179 were isolated from the aerial parts of Hypericum annulatum [120], while 180–183 were found in H. thasium Griseb. [121,122][121][122]. The 6(2)-O-rhamosides 187 and 188 were isolated from H. elegans [123] and H. pseudopetiolatum Keller, respectively. In addition, compounds 189–191 were also found in later species [124]. Further acylated rhamosides 192 and 193 of 143 were isolated from the aerial parts of H. seniawinii Maxim. [125]. The 6(2)-O-rhamnosides 194–196 of 145 were found in aerial parts of H. wightianum Wall. ex Wight et Arn. [126]. Only three O-xylopyranosides 197–199 of penataoxygenated benzophenones were originally isolated from H. thasium [121], while only two O-arabinopyranosides 200 and 201 were found in H. humifusum [117]. Compounds 202 and 203 isolated from the later plant [117] possess glycosylation at position 4, while 204 and 205 were found in H. sampsonii [112] and H. ellipticum Hook. [127], respectively, the sugar was attached at position 5′(3′) position. The compounds 206 and 207 share rare 2,2′,4,5′,6-pentaoxygenation patterns that were isolated from H. annulatum [8] and Garcinia mangostana [128], respectively.
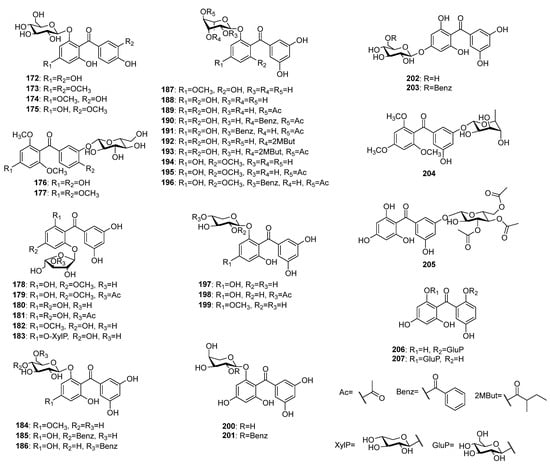
Figure 12.
The structures of O-glycosides of pentaoxygenated benzophenones.
7. Hexaxygenated Benzophenones
Hexaxygenated benzophenones were rarely found in nature. To date, only three compounds, 208–210 (Figure 13), have been isolated from Garcinia mangostana [128,129][128][129].
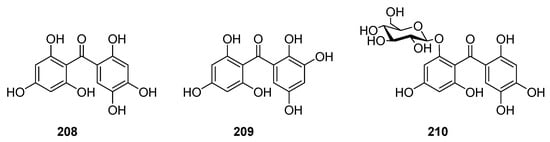
Figure 13.
The structures of hexaoxygenated benzophenones.
8. Uncategorized and Miscellaneous Benzophenones
In addition to the compounds shown so far, there are data on benzophenone derivatives (Figure 14) in which one or several protons are replaced by a methyl, alcohol, carboxyl, or acyl group, and dimeric compounds with xanthone have also been found. Some glycosides of this class benzophenones have been detected, as well. The first records of this type of benzophenone appeared in 1963 when 6-hydroxy-2,4-dimethoxy-3-methylbenzophenone 211 and its isomer 2-hydroxy-4,6-dimethoxy-3-methylbenzophenone 212 were isolated from the essential oil of Leptospermum luehmannii F.M.Bailey [130]. Eighteen years later, bis(2-hydroxy-4,6-dimethoxy-3-methylphenyl)methanone 213 was found in a benzene extract of the wood of Qualea lubouriauana [131].
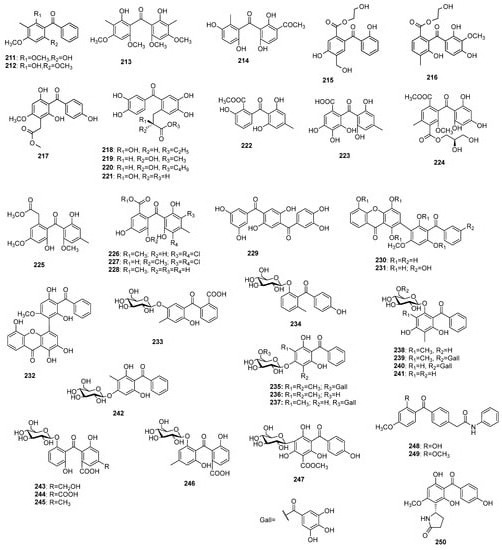
Figure 14.
The structures of uncategorized and miscellaneous benzophenones.
For the first time, beishouwubenzophenone 214 was isolated from root tubers of Cynanchum auriculatum Royle ex Wight [132]. Morintrifolin A 215 and B 216 were obtained from hydromethanolic extract of Morinda citrifolia L. roots [133]. Compound 217 was found in an extract of the Anemarrhena asphodeloides roots [134]. The adducts of 3,3′,4,4′-trihydroxybenzophenone, and 2-hydroxypropionic acid 218–221 were isolated from Ranunculus ssp. The former three compounds were found in R. ternatus DC. [135[135][136],136], while the later was obtained from R. muricatus Moench [137]. The benzophenones 222–228 have been isolated from fungi mostly belonging to Ascomycota group. Nidulalin B 222 was found in a dichloromethane extract of Emericella nidulans var. lata (Thom and Raper) Subram. [138], while cytosporaphenone A 223 was obtained from an ethyl acetate extract of Cytospora rhizophorae Kohlm. and E. Kohlm. [139]. Wentiphenone A 224 was discovered in the ethyl acetate extract of Aspergillus wentii Wehmer [140], while 225 was isolated from the fermentation broth of A. fumigatus Fresen. SWZ01 [141]. A halogenated benzophenone 226 was discovered in an extract of A. flavipes PJ03-11 [142], while 227 and 228 were found in A. terreus Thom [143] and A. wentii [144], respectively. Garcihombrianone 229, which was found in the roots of Garcinia hombroniana Pierre [145], can be viewed as two benzophenone molecules sharing a common benzene nucleus. Three benzophenone-xanthone dimers 230–232 were isolated from roots of G. dulcis (Roxb.) Kurz [146]. Benzophenone O-glucosides 233 and 234 were found in Mitracarpus villosus (Sw.) DC. [147] and Dobinea delavayi [119]. Seven benzophenone O-glucosides 235–241, some of which were galloyl esters, were isolated from the leaves or fruits of Psidium guajava [47,148[47][148][149][150],149,150], while 242 was found in P. littorale Raddi [151]. Compounds 243 and 244 were isolated from Cassia senna var. angustifolia L. [152], while 245 and 246 were found in C. abbreviata Oliv. [153]. The only C-glycoside 247 of this class of miscellaneous benzophenones was isolated from Aquilaria sinensis [154]. Two N-containing benzophenones 248 and 249 were found in the moss Pogonatum spinulosum Mitt. [155], while 250 was detected in Anemarrhena asphodeloides [62].
References
- Bukvički, D.; Novaković, M.; Ab Ghani, N.; Marin, P.D.; Asakawa, Y. Secondary Metabolites from Endemic Species Iris Adriatica Trinajstić Ex Mitić (Iridaceae). Nat. Prod. Res. 2018, 32, 1849–1852.
- Nandy, S.; Mukherjee, A.; Pandey, D.K.; Ray, P.; Dey, A. Indian Sarsaparilla (Hemidesmus Indicus): Recent Progress in Research on Ethnobotany, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2020, 254, 112609.
- Cuesta-Rubio, O.; Piccinelli, A.L.; Rastrelli, L. Chemistry and Biological Activity of Polyisoprenylated Benzophenone Derivatives. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 32, pp. 671–720. ISBN 1572-5995.
- Acuna, M.U.; Jancovski, N.; Kennelly, J.E. Polyisoprenylated Benzophenones from Clusiaceae: Potential Drugs and Lead Compounds. Curr. Top. Med. Chem. 2009, 9, 1560–1580.
- Wu, S.-B.; Long, C.; Kennelly, E.J. Structural Diversity and Bioactivities of Natural Benzophenones. Nat. Prod. Rep. 2014, 31, 1158–1174.
- Singh, P.I.; Bharate, S.B. Phloroglucinol Compounds of Natural Origin. Nat. Prod. Rep. 2006, 23, 558–591.
- Sultanbawa, M.U.S. Xanthonoids of Tropical Plants. Tetrahedron 1980, 36, 1465–1506.
- Kitanov, G.M.; Nedialkov, P.T. Benzophenone O-Glucoside, a Biogenic Precursor of 1,3,7-Trioxygenated Xanthones in Hypericum annulatum. Phytochemistry 2001, 57, 1237–1243.
- Mandal, S.; Das, P.C.; Joshi, P.C. Naturally Occurring Xanthones from Terrestrial Flora. J. Indian Chem. Soc. 1992, 69, 611–636.
- Heurung, A.R.; Raju, S.I.; Warshaw, E.M. Benzophenones. Dermatitis 2014, 25, 3–10.
- Pallares, E.S.; Hector, M.G. Study of Yoloxochitl. Arch. Inst. Cardiol. Mex. 1947, 17, 833–849.
- Sharma, M.L.; Nigam, M.C.; Hande, K.L. Essential Oil of Costus speciosus. Perfum. Essent. Oil Rec. 1963, 54, 579–580.
- Liu, L.-N.; Zhang, X.-W.; Hou, F.-J.; Duan, X.-H.; Zhao, J.-C.; Chen, Y.-L. Benzophenones from Endohydric Moss Polytrichastrum Formosum and Their Cytotoxic Activities. Chin. Tradit. Herb. Drugs 2022, 53, 667–670.
- Duan, X.-H.; He, P.; Qin, M.; Li, L.; Pei, L.; Zhao, J.-C. A New Benzophenone from Endohydric Moss Pogonatum Inflexum. Chin. Tradit. Herb. Drugs 2019, 50, 1291–1293.
- Duan, X.-H.; Zhao, J.-C.; Li, L.; Pei, L.; He, P.; Wang, R. Two New Benzophenones from Endohydric Moss Polytrichum Commune. Nat. Prod. Res. 2019, 33, 2750–2754.
- Duan, X.-H.; Zhang, X.-W.; Qin, M.; He, P.; Pei, L.; Zhao, J.-C.; Chen, Y.-L. Two New Benzophenones from the Endohydric Moss Polytrichastrum formosum. Nat. Prod. Commun. 2021, 16, 1934578X211002623.
- Gamiotea-Turro, D.; Cuesta-Rubio, O.; Prieto-González, S.; De Simone, F.; Passi, S.; Rastrelli, L. Antioxidative Constituents from the Leaves of Hypericum styphelioides. J. Nat. Prod. 2004, 67, 869–871.
- Rusby, H.H. New Species of Trees of Medical Interest from Bolivia. Bull. Torrey Bot. Club 1922, 49, 259–264.
- Jobst, J. Ueber Coto-Rinden Und Deren Krystallisirbare Bestandtheile. Berichte Der Dtsch. Chem. Ges. 1876, 9, 1633–1634.
- Jobst, J.; Hesse, O. Ueber Die Cotorinden Und Ihre Charakteristischen Bestandtheile. Justus Liebigs Ann. Der Chem. 1879, 199, 17–96.
- Ciamician, G. Ueber Die Constitution Des Cotoïns. Berichte Der Dtsch. Chem. Ges. 1894, 27, 409–426.
- Ciamician, G.; Silber, P. Ueber Das Hydrocotoïn, Einen Bestandtheil Der Cotorinde. Berichte Der Dtsch. Chem. Ges. 1891, 24, 299–301.
- Ciamician, G.; Silber, P. Synthese Des Benzophloroglucintrimethyläther. (Methylhydrocotoïn Oder Benzoylhydrocoton). Berichte Der Dtsch. Chem. Ges. 1894, 27, 1497–1501.
- Ciamician, G.; Silber, P. Ueber Die Constitution Einiger in Der Paracotorinde Enthaltenen Bestandtheile. Berichte Der Dtsch. Chem. Ges. 1892, 25, 1119–1138.
- Randriaminahy, M.; Proksch, P.; Witte, L.; Wray, V. Lipophilic Phenolic Constituents from Helichrysum Species Endemic to Madagascar. Z. Für Naturforschung C 1992, 47, 10–16.
- Bohlmann, F.; Suwita, A. Neue Phloroglucin-Derivate Aus Leontonyx-Arten Sowie Weitere Verbindungen Aus Vertretern Der Tribus Inuleae. Phytochemistry 1978, 17, 1929–1934.
- Jakupovic, J.; Zdero, C.; Grenz, M.; Tsichritzis, F.; Lehmann, L.; Hashemi-Nejad, S.M.; Bohlmann, F. Twenty-One Acylphloroglucinol Derivatives and Further Constituents from South African Helichrysum Species. Phytochemistry 1989, 28, 1119–1131.
- Don, M.-J.; Huang, Y.-J.; Huang, R.-L.; Lin, Y.-L. New Phenolic Principles from Hypericum sampsonii. Chem. Pharm. Bull. 2004, 52, 866–869.
- Zhu, H.; Chen, C.; Tan, D.; Li, D.; Guo, Y.; Wei, G.; Zhang, J.; Wang, J.; Luo, Z.; Xue, Y.; et al. Sampbenzophenones A–G, Prenylated Benzoylphloroglucinol Derivatives from Hypericum sampsonii. RSC Adv. 2016, 6, 86710–86716.
- Huang, C.-Y.; Chang, T.-C.; Wu, Y.-J.; Chen, Y.; Chen, J.-J. Benzophenone and Benzoylphloroglucinol Derivatives from Hypericum Sampsonii with Anti-Inflammatory Mechanism of Otogirinin A. Molecules 2020, 25, 4463.
- Wu, F.-S.; Hung, C.-J.; Lin, C.-L.; Huang, H.-Y.; Kuo, Y.-H.; Chang, T.-H.; Chen, C.-L.; Sung, P.-J.; Cheng, M.-J.; Kuo, C.-W.; et al. A New Benzophenone and Bioactive Constituents of Hypericum nokoense. Chem. Nat. Compd. 2021, 57, 645–649.
- Nedialkov, P.T.; Zheleva-Dimitrova, D.; Momekov, G.; Karlov, K.; Girreser, U.; Kitanov, G.M. Elegaphenone and 7-Epi-Clusianone, the Major Cytotoxic Constituents of Hypericum elegans. Nat. Prod. Res. 2011, 25, 1743–1750.
- Wu, F.-S.; Wang, I.-C.; Liaw, C.-C.; Huang, H.-Y.; Chang, T.-H.; Chen, C.-L.; Sung, P.-J.; Cheng, M.-J.; Kuo, C.-W.; Chen, J.-J. New Benzophenone and Bioactive Constituents from Hypericum Nagasawae. Chem. Nat. Compd. 2022, 58, 833–838.
- Ollis, W.D. Proceedings of the 6th Annual Symposium of the Plant Phenolics Group of North America, 1966; Mabry, T.J., Alston, R.E., Runeckles, V.C., Eds.; Recent Advances in Phytochemistry; Appleton–Century–Crofts: New York, NY, USA, 1968; Volume 1.
- Wang, W.; Weng, X.; Cheng, D. Antioxidant Activities of Natural Phenolic Components from Dalbergia odorifera T. Chen. Food Chem. 2000, 71, 45–49.
- Dibwe, D.F.; Awale, S.; Kadota, S.; Morita, H.; Tezuka, Y. Heptaoxygenated Xanthones as Anti-Austerity Agents from Securidaca longepedunculata. Bioorganic Med. Chem. 2013, 21, 7663–7668.
- Kang, W.-Y.; Wang, Z.-M.; Li, Z.-Q.; Xu, X.-J. Three New Compounds from Securidaca inappendiculata. Helv. Chim. Acta 2005, 88, 2771–2776.
- Casu, L.; Solinas, M.N.; Saba, A.R.; Cottiglia, F.; Caboni, P.; Floris, C.; Laconi, S.; Pompei, R.; Leonti, M. Benzophenones from the Roots of the Popoluca Amerindian Medicinal Plant Securidaca Diversifolia (L.) S.F. Blake. Phytochem. Lett. 2010, 3, 226–229.
- Triana, J.; López, M.; Pérez, F.J.; Platas, J.G.; Estévez, F.; León, J.F.; Hernández, J.C.; Brouard, I.; Bermejo, J. Chemical Constituents of Tolpis Species. Fitoterapia 2009, 80, 437–441.
- Wu, X.-D.; Cheng, J.-T.; He, J.; Zhang, X.-J.; Dong, L.-B.; Gong, X.; Song, L.-D.; Zheng, Y.-T.; Peng, L.-Y.; Zhao, Q.-S. Benzophenone Glycosides and Epicatechin Derivatives from Malania Oleifera. Fitoterapia 2012, 83, 1068–1071.
- Wu, Z.-J.; Ouyang, M.-A.; Yang, C.-R. Oligosaccharide Esters and Phenol Compounds from Polygala Arillata. Acta Bot. Yunnan. 2000, 22, 482–494.
- Huang, Y.-L.; Chen, C.-C.; Chen, Y.-J.; Huang, R.-L.; Shieh, B.-J. Three Xanthones and a Benzophenone from Garcinia mangostana. J. Nat. Prod. 2001, 64, 903–906.
- Li, J.; Jiang, Y.; Tu, P.-F. Xanthone O-Glycosides and Benzophenone O-Glycosides from the Roots of Polygala tricornis. J. Nat. Prod. 2005, 68, 1802–1804.
- Dai, Y.; He, X.-J.; Zhou, G.-X.; Kurihara, H.; Ye, W.-C.; Yao, X.-S. Acylphloroglucinol Glycosides from the Fruits of Pyracantha fortuneana. J. Asian Nat. Prod. Res. 2008, 10, 111–117.
- Shi, Y.-N.; Shi, Y.-M.; Yang, L.; Li, X.-C.; Zhao, J.-H.; Qu, Y.; Zhu, H.-T.; Wang, D.; Cheng, R.-R.; Yang, C.-R.; et al. Lignans and Aromatic Glycosides from Piper wallichii and Their Antithrombotic Activities. J. Ethnopharmacol. 2015, 162, 87–96.
- Fu, H.Z.; Yang, J.Z.; Li, C.J.; Zhang, D.M. A New Benzophenone Glycoside from the Leaves of Psidium Guajava L. Chin. Chem. Lett. 2011, 22, 178–180.
- Matsuzaki, K.; Ishii, R.; Kobiyama, K.; Kitanaka, S. New Benzophenone and Quercetin Galloyl Glycosides from Psidium guajava L. J. Nat. Med. 2010, 64, 252–256.
- Shi, T.-X.; Wang, S.; Zeng, K.-W.; Tu, P.-F.; Jiang, Y. Inhibitory Constituents from the Aerial Parts of Polygala Tenuifolia on LPS-Induced NO Production in BV2 Microglia Cells. Bioorganic Med. Chem. Lett. 2013, 23, 5904–5908.
- Jiang, Y.; Tu, P. Four New Phenones from the Cortexes of Polygala tenuifolia. Chem. Pharm. Bull. 2005, 53, 1164–1166.
- Zhou, L.-Y.; Wang, J.-M.; Huang, Y.-J.; Yu, X.-H.; Lu, B.; Hua, Y. Two New Glycosides Isolated from Polygala sibirica L. Var. megalopha Fr. Phytochem. Lett. 2016, 16, 174–177.
- An, H.; Thanh, L.N.; Khanh, L.Q.; Ryu, S.H.; Lee, S.; Yeon, S.W.; Lee, H.H.; Turk, A.; Lee, K.Y.; Hwang, B.Y.; et al. Characterization of Antioxidant and α-Glucosidase Inhibitory Compounds of Cratoxylum formosum ssp. pruniflorum and Optimization of Extraction Condition. Antioxidants 2023, 12, 511.
- Gottlieb, O.R.; Fineberg, M.; Salignac De Souza Guimaraes, I.; Taveira Magalhaes, M.; Ollis, W.D.; Eyton, W.B. The chemistry of the Brazilian leguminosae. VII. The Constituents of Machaerium scleroxylon. An. Acad. Bras. Cienc. 1964, 36, 33–34.
- Eyton, W.B.; Ollis, W.D.; Fineberg, M.; Gottlieb, O.R.; Salignac de Souza Guimarães, I.; Taveira Magalhães, M. The Neoflavanoid Group of Natural Products—II: The Examination of Machaerium Scleroxylon and Some Biogenetic Proposal Regarding the Neoflavanoids. Tetrahedron 1965, 21, 2697–2705.
- Ochora, D.O.; Kakudidi, E.; Namukobe, J.; Heydenreich, M.; Coghi, P.; Yang, L.J.; Mwakio, E.W.; Andagalu, B.; Roth, A.; Akala, H.M.; et al. A New Benzophenone, and the Antiplasmodial Activities of the Constituents of Securidaca longipedunculata Fresen (Polygalaceae). Nat. Prod. Res. 2022, 36, 2758–2766.
- Spada, A.; Cameroni, R.; Bernabei, M.T. The Pigments of Morus Alba. Gazz. Chim. Ital. 1956, 86, 46–55.
- De Barros Corrêa, D.; Gottlieb, O.R. Duckein, an Alkaloid from Aniba Duckei. Phytochemistry 1975, 14, 271–272.
- Atkinson, J.E.; Gupta, P.; Lewis, J.R. Some Phenolic Constituents of Gentiana Lutea. Tetrahedron 1969, 25, 1507–1511.
- Locksley, H.D.; Murray, I.G. Extractives from Guttiferae. Part XIX. The Isolation and Structure of Two Benzophenones, Six Xanthones and Two Biflavonoids from the Heartwood of Allanblackia floribunda Oliver. J. Chem. Soc. C 1971, 1332–1340.
- Donnelly, D.M.X.; O’Reilly, J.; Whalley, W.B. Neoflavanoids of Dalbergia melanoxylon. Phytochemistry 1975, 14, 2287–2290.
- Lin, S.; Liu, R.-H.; Ma, G.-Q.; Mei, D.-Y.; Shao, F.; Chen, L.-Y. Two New Compounds from the Heartwood of Dalbergia Melanoxylon. Nat. Prod. Res. 2020, 34, 2794–2801.
- Pathak, V.; Shirota, O.; Sekita, S.; Hirayama, Y.; Hakamata, Y.; Hayashi, T.; Yanagawa, T.; Satake, M. Antiandrogenic Phenolic Constituents from Dalbergia cochinchinensis. Phytochemistry 1997, 46, 1219–1223.
- Wu, D.-L.; Liao, Z.-D.; Chen, F.-F.; Zhang, W.; Ren, Y.-S.; Wang, C.-C.; Chen, X.-X.; Peng, D.-Y.; Kong, L.-Y. Benzophenones from Anemarrhena Asphodeloides Bge. Exhibit Anticancer Activity in HepG2 Cells via the NF-ΚB Signaling Pathway. Molecules 2019, 24, 2246.
- Smitha, C.; Udayan, P. GC-MS and HR-LCMS Fingerprinting of Various Parts of Oroxylum Indicum (L.) Vent. A Comparative Phytochemical Study Based on Plant Part Substitution Approach. J. Pharmacogn. Phytochem. 2020, 9, 1817–1824.
- Jiang, H.Z.; Quan, X.F.; Tian, W.X.; Hu, J.M.; Wang, P.C.; Huang, S.Z.; Cheng, Z.Q.; Liang, W.J.; Zhou, J.; Ma, X.F.; et al. Fatty Acid Synthase Inhibitors of Phenolic Constituents Isolated from Garcinia Mangostana. Bioorganic Med. Chem. Lett. 2010, 20, 6045–6047.
- Jantan, I.; Saputri, F.C. Benzophenones and Xanthones from Garcinia Cantleyana Var. Cantleyana and Their Inhibitory Activities on Human Low-Density Lipoprotein Oxidation and Platelet Aggregation. Phytochemistry 2012, 80, 58–63.
- Wabo, H.K.; Kowa, T.K.; Lonfouo, A.H.N.; Tchinda, A.T.; Tane, P.; Kikuchi, H.; Frédérich, M.; Oshima, Y. Phenolic Compounds and Terpenoids from Hypericum lanceolatum. Rec. Nat. Prod. 2012, 6, 94–100.
- Mian, J.V.Y.; Lian, E.G.C.; Aspollah, S.M.; Hin, T.-Y.Y.; Yen, K.H.; Yok, C.M.K. Benzophenone Constituents from the Roots of Garcinia eugenifolia. Res. J. Chem. Environ. 2012, 16, 36–39.
- Azlini, I.; Erlena, N.A.A.R.; Muhammad, N.O.; Wan, A.N.W.A. Antihypertensive Assay-Guided Fractionation of Syzygium Polyanthum Leaves and Phenolics Profile Analysis Using LCQTOF/ MS. Pharmacogn. J. 2020, 12, 1670–1692.
- Murakami, T.; Tanaka, N.; Wada, H.; Saiki, Y.; Chen, C.-M. Chemical and Chemotaxonomical Studies on Filices. LXIII. Yakugaku Zasshi 1986, 106, 378–382.
- Tanaka, T.; Sueyasu, T.; Nonaka, G.; Nishioka, I. Tannins and Related Compounds. XXI. Isolation and Characterization of Galloyl and p-Hydroxybenzoyl Esters of Benzophenone and Xanthone C-Glucosides from Mangifera indica L. Chem. Pharm. Bull. 1984, 32, 2676–2686.
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; de Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves, and Peel of Mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610.
- Zhang, Y.; Qian, Q.; Ge, D.; Li, Y.; Wang, X.; Chen, Q.; Gao, X.; Wang, T. Identification of Benzophenone C-Glucosides from Mango Tree Leaves and Their Inhibitory Effect on Triglyceride Accumulation in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2011, 59, 11526–11533.
- Zhang, Y.; Han, L.; Ge, D.; Liu, X.; Liu, E.; Wu, C.; Gao, X.; Wang, T. Isolation, Structural Elucidation, MS Profiling, and Evaluation of Triglyceride Accumulation Inhibitory Effects of Benzophenone C-Glucosides from Leaves of Mangifera indica L. J. Agric. Food Chem. 2013, 61, 1884–1895.
- Beelders, T.; Brand, D.J.; de Beer, D.; Malherbe, C.J.; Mazibuko, S.E.; Muller, C.J.F.; Joubert, E. Benzophenone C- and O-Glucosides from Cyclopia Genistoides (Honeybush) Inhibit Mammalian α-Glucosidase. J. Nat. Prod. 2014, 77, 2694–2699.
- Hara, H.; Ise, Y.; Morimoto, N.; Shimazawa, M.; Ichihashi, K.; Ohyama, M.; Iinuma, M. Laxative Effect of Agarwood Leaves and Its Mechanism. Biosci. Biotechnol. Biochem. 2008, 72, 335–345.
- Pan, J.; Yi, X.; Zhang, S.; Cheng, J.; Wang, Y.; Liu, C.; He, X. Bioactive Phenolics from Mango Leaves (Mangifera indica L.). Ind. Crops Prod. 2018, 111, 400–406.
- Xu, M.; Zhang, M.; Wang, D.; Yang, C.-R.; Zhang, Y.-J. Phenolic Compounds from the Whole Plants of Gentiana rhodantha (Gentianaceae). Chem. Biodivers. 2011, 8, 1891–1900.
- Li, C.-J.; Zhang, D.-M.; Yu, S.-S. Benzophenone C-Glucosides from Polygala glomerata Lour. J. Asian Nat. Prod. Res. 2008, 10, 293–297.
- Panidthananon, W.; Chaowasku, T.; Sritularak, B.; Likhitwitayawuid, K. A New Benzophenone C-Glucoside and Other Constituents of Pseuduvaria Fragrans and Their α-Glucosidase Inhibitory Activity. Molecules 2018, 23, 1600.
- Qi, J.; Lu, J.-J.; Liu, J.-H.; Yu, B.-Y. Flavonoid and a Rare Benzophenone Glycoside from the Leaves of Aquilaria sinensis. Chem. Pharm. Bull. 2009, 57, 134–137.
- Pan, J.; Yi, X.; Wang, Y.; Chen, G.; He, X. Benzophenones from Mango Leaves Exhibit α-Glucosidase and NO Inhibitory Activities. J. Agric. Food Chem. 2016, 64, 7475–7480.
- Gu, C.; Yang, M.; Zhou, Z.; Khan, A.; Cao, J.; Cheng, G. Purification and Characterization of Four Benzophenone Derivatives from Mangifera Indica L. Leaves and Their Antioxidant, Immunosuppressive and α-Glucosidase Inhibitory Activities. J. Funct. Foods 2019, 52, 709–714.
- Ito, H.; Nishitani, E.; Konoshima, T.; Takasaki, M.; Kozuka, M.; Yoshida, T. Flavonoid and Benzophenone Glycosides from Coleogyne ramosissima. Phytochemistry 2000, 54, 695–700.
- Lee, S.-S.; Tseng, C.-C.; Chen, C.-K. Three New Benzophenone Glucosides from the Leaves of Planchonella Obovata. Helv. Chim. Acta 2010, 93, 522–529.
- Sun, J.; Wang, S.; Xia, F.; Wang, K.-Y.; Chen, J.-M.; Tu, P.-F. Five New Benzophenone Glycosides from the Leaves of Aquilaria sinensis (Lour.) Gilg. Chin. Chem. Lett. 2014, 25, 1573–1576.
- Yuan, H.; Zhao, J.; Wang, M.; Khan, S.I.; Zhai, C.; Xu, Q.; Huang, J.; Peng, C.; Xiong, G.; Wang, W.; et al. Benzophenone Glycosides from the Flower Buds of Aquilaria sinensis. Fitoterapia 2017, 121, 170–174.
- Sun, H.; Zhang, Y.-F.; Huo, H.-X.; Guan, P.-W.; Wang, C.-C.; Yao, H.-N.; Zhao, Y.-F.; Tu, P.-F.; Li, J. Benzophenone Glycosides from the Pericarps of Aquilaria Yunnanensis S. C. Huang. Nat. Prod. Res. 2020, 34, 2030–2036.
- Feng, J.; Yang, X.-W.; Wang, R.-F. Bio-Assay Guided Isolation and Identification of α-Glucosidase Inhibitors from the Leaves of Aquilaria Sinensis. Phytochemistry 2011, 72, 242–247.
- Rancon, S.; Chaboud, A.; Darbour, N.; Comte, G.; Bayet, C.; Simon, P.-N.; Raynaud, J.; Di Pietro, A.; Cabalion, P.; Barron, D. Natural and Synthetic Benzophenones: Interaction with the Cytosolic Binding Domain of P-Glycoprotein. Phytochemistry 2001, 57, 553–557.
- Ferrari, J.; Terreaux, C.; Sahpaz, S.; Msonthi, J.D.; Wolfender, J.-L.; Hostettmann, K. Benzophenone Glycosides from Gnidia involucrata. Phytochemistry 2000, 54, 883–889.
- Zhang, S.-Y.; Zhang, Q.-H.; Zhao, W.; Zhang, X.; Zhang, Q.; Bi, Y.-F.; Zhang, Y.-B. Isolation, Characterization and Cytotoxic Activity of Benzophenone Glucopyranosides from Mahkota Dewa (Phaleria macrocarpa (Scheff.) Boerl). Bioorganic Med. Chem. Lett. 2012, 22, 6862–6866.
- Osman, A.G.; Ali, Z.; Fantoukh, O.; Raman, V.; Kamdem, R.S.T.; Khan, I. Glycosides of Ursane-Type Triterpenoid, Benzophenone, and Iridoid from Vangueria Agrestis (Fadogia Agrestis) and Their Anti-Infective Activities. Nat. Prod. Res. 2020, 34, 683–691.
- Zhang, Y.-B.; Xu, X.-J.; Liu, H.-M. Chemical Constituents from Mahkota Dewa. J. Asian Nat. Prod. Res. 2006, 8, 119–123.
- Kaya, D.; Yalçın, F.N.; Bedir, E.; Çalış, İ.; Steinhauser, L.; Albert, K.; Ersöz, T. New Benzophenone Glucosides from the Aerial Parts of Gentiana Verna L. Subsp. Pontica (Soltok.) Hayek. Phytochem. Lett. 2011, 4, 459–461.
- Mohamed, G.A.; Ibrahim, S.R.M. Garcixanthone E and Garcimangophenone C: New Metabolites from Garcinia Mangostana and Their Cytotoxic and Alpha Amylase Inhibitory Potential. Life 2022, 12, 1875.
- Otsuka, H.; Kijima, K. An Iridoid Gentiobioside, a Benzophenone Glucoside and Acylated Flavone C-Glycosides from Tripterospermum japonicum (SIEB. et ZUCC.) MAXIM. Chem. Pharm. Bull. 2001, 49, 699–702.
- Duan, Y.; Dai, Y.; Wang, G.; Chen, H.; Gao, H.; Chen, J.; Yao, X.; Zhang, X. Xanthone and Benzophenone Glycosides from the Stems of Cratoxylum formosum ssp. pruniflorum. Chem. Pharm. Bull. 2011, 59, 231–234.
- Jo, Y.H.; Kim, S.B.; Ahn, J.H.; Liu, Q.; Hwang, B.Y.; Lee, M.K. Inhibitory Activity of Benzophenones from Anemarrhena asphodeloides on Pancreatic Lipase. Nat. Prod. Commun. 2013, 8, 1934578X1300800419.
- Wagner, R. Ueber Die Farbstoffe Des Gelbholzes (Morus Tinctoria). J. Für Prakt. Chem. 1850, 51, 82–106.
- Hlasiwetz, H.; Pfaundler, L. Ueber Das Morin Und Die Moringerbsäure. Justus Liebigs Ann. Der Chem. 1863, 127, 351–361.
- Ciamician, G.; Silber, P. Ueber Die Constitution Des Maclurins Und Phloretins. Berichte Der Dtsch. Chem. Ges. 1895, 28, 1393–1398.
- Minami, H.; Kinoshita, M.; Fukuyama, Y.; Kodama, M.; Yoshizawa, T.; Sugiura, M.; Nakagawa, K.; Tago, H. Antioxidant Xanthones from Garcinia subelliptica. Phytochemistry 1994, 36, 501–506.
- Chiang, Y.-M.; Kuo, Y.-H.; Oota, S.; Fukuyama, Y. Xanthones and Benzophenones from the Stems of Garcinia multiflora. J. Nat. Prod. 2003, 66, 1070–1073.
- Nguyen, L.H.D.; Venkatraman, G.; Sim, K.Y.; Harrison, L.J. Xanthones and Benzophenones from Garcinia Griffithii and Garcinia Mangostana. Phytochemistry 2005, 66, 1718–1723.
- Muriithi, E.; Bojase-Moleta, G.; Majinda, R.R.T. Benzophenone Derivatives from Garcinia Livingstonei and Their Antioxidant Activities. Phytochem. Lett. 2016, 18, 29–34.
- Choodej, S.; Koopklang, K.; Raksat, A.; Chuaypen, N.; Pudhom, K. Bioactive Xanthones, Benzophenones and Biphenyls from Mangosteen Root with Potential Anti-Migration against Hepatocellular carcinoma Cells. Sci. Rep. 2022, 12, 8605.
- Minami, H.; Hamaguchi, K.; Kubo, M.; Fukuyama, Y. A Benzophenone and a Xanthone from Garcinia subelliptica. Phytochemistry 1998, 49, 1783–1785.
- Fouotsa, H.; Lannang, A.M.; Dzoyem, J.P.; Tatsimo, S.J.N.; Neumann, B.; Mbazoa, C.D.; Razakarivony, A.A.; Nkengfack, A.E.; Eloff, J.N.; Sewald, N. Antibacterial and Antioxidant Xanthones and Benzophenone from Garcinia smeathmannii. Planta Medica 2015, 81, 594–599.
- Rao, A.V.R.; Sarma, M.R.; Venkataraman, K.; Yemul, S.S. A Benzophenone and Xanthone with Unusual Hydroxylation Patterns from the Heartwood of Garcinia pedunculata. Phytochemistry 1974, 13, 1241–1244.
- Nargis, J.; Wong, K.-C.; Khairuddin, M.; Chantrapromma, S.; Fun, H.-K. (2,4-Dihydroxy-6-Methoxyphenyl)(3,5-Dihydroxyphenyl)Methanone Monohydrate. Acta Crystallogr. Sect. E 2011, 67, o2717–o2718.
- Pailee, P.; Kuhakarn, C.; Sangsuwan, C.; Hongthong, S.; Piyachaturawat, P.; Suksen, K.; Jariyawat, S.; Akkarawongsapat, R.; Limthongkul, J.; Napaswad, C.; et al. Anti-HIV and Cytotoxic Biphenyls, Benzophenones and Xanthones from Stems, Leaves and Twigs of Garcinia speciosa. Phytochemistry 2018, 147, 68–79.
- Nguyen Viet, D.; Le Ba, V.; Nguyen Duy, T.; Pham Thi, V.A.; Tran Thi, H.; Le Canh, V.C.; Bach Long, G.; Kim, Y.H.; Tuan Anh, H.L. Bioactive Compounds from the Aerial Parts of Hypericum sampsonii. Nat. Prod. Res. 2021, 35, 646–648.
- Wang, M.; Ma, G.; Shao, F.; Liu, R.; Chen, L.; Liu, Y.; Yang, L.; Meng, X. Neoflavonoids from the Heartwood of Dalbergia melanoxylon. Nat. Prod. Res. 2022, 36, 735–741.
- Yoshimura, M.; Ninomiya, K.; Tagashira, Y.; Maejima, K.; Yoshida, T.; Amakura, Y. Polyphenolic Constituents of the Pericarp of Mangosteen (Garcinia mangostana L.). J. Agric. Food Chem. 2015, 63, 7670–7674.
- Li, J.-C.; Nohara, T. Benzophenone C-Glucosides from Polygala telephioides. Chem. Pharm. Bull. 2000, 48, 1354–1355.
- Ma, T.-J.; Shi, X.-C.; Jia, C.-X. Telephenone D, A New Benzophenone C-Glycoside from Polygala telephioides. Chin. J. Nat. Med. 2010, 8, 9–11.
- Rouis, Z.; Abid, N.; Aouni, M.; Faiella, L.; Dal Piaz, F.; De Tommasi, N.; Braca, A. Benzophenone Glycosides from Hypericum Humifusum ssp. austral. J. Nat. Prod. 2013, 76, 979–982.
- Cheng, Z.-Q.; Yang, D.; Ma, Q.-Y.; Yi, X.-H.; Zhou, J.; Zhao, Y.-X. A New Benzophenone from Dobinea delavayi. Chem. Nat. Compd. 2013, 49, 46–48.
- Wu, X.-R.; Lang, L.-J.; Shen, Y.; Dong, X.; Xiao, C.-J.; Jiang, B. Four New Phenolic Glycosides from Dobinea delavayi. Nat. Prod. Res. 2023, 37, 1146–1153.
- Nedialkov, P.T.; Kitanov, G.M. Two Benzophenone O-Arabinosides and a Chromone from Hypericum annulatum. Phytochemistry 2002, 59, 867–871.
- Demirkiran, O.; Ahmed Mesaik, M.; Beynek, H.; Abbaskhan, A.; Iqbal Choudhary, M. Cellular Reactive Oxygen Species Inhibitory Constituents of Hypericum thasium Griseb. Phytochemistry 2009, 70, 244–249.
- Demirkiran, O. Three New Benzophenone Glycosides with MAO-A Inhibitory Activity from Hypericum thasium Griseb. Phytochem. Lett. 2012, 5, 700–704.
- Nedialkov, P.T.; Zheleva-Dimitrova, D.; Girreser, U.; Kitanov, G.M. Benzophenone O-Glycosides from Hypericum elegans. Nat. Prod. Res. 2009, 23, 1176–1180.
- Tanaka, N.; Kubota, T.; Kashiwada, Y.; Takaishi, Y.; Kobayashi, J. Petiolins F—I, Benzophenone Rhamnosides from Hypericum pseudopetiolatum var. kiusianum. Chem. Pharm. Bull. 2009, 57, 1171–1173.
- Xia, J.; Hu, B.; Qian, M.; Zhang, J.; Wu, L. Benzophenone Rhamnosides and Chromones from Hypericum Seniawinii Maxim. Molecules 2022, 27, 7056.
- Yang, L.; Wang, Z.-M.; Wang, Y.; Li, R.-S.; Wang, F.; Wang, K. Phenolic Constituents with Neuroprotective Activities from Hypericum wightianum. Phytochemistry 2019, 165, 112049.
- Petrunak, E.; Kester, A.C.; Liu, Y.; Bowen-Forbes, C.S.; Nair, M.G.; Henry, G.E. New Benzophenone O-Glucoside from Hypericum ellipticum. Nat. Prod. Commun. 2009, 4, 1934578X0900400412.
- Alhakamy, N.A.; Mohamed, G.A.; Fahmy, U.A.; Eid, B.G.; Ahmed, O.A.; Al-Rabia, M.W.; Khedr, A.I.; Nasrullah, M.Z.; Ibrahim, S.R. New Alpha-Amylase Inhibitory Metabolites from Pericarps of Garcinia mangostana. Life 2022, 12, 384.
- Mohamed, G.A.; Ibrahim, S.R.M. New Benzophenones and a Dihydroflavanonol from Garcinia Mangostana Pericarps and Their Antioxidant and Cytotoxic Activities. Phytochem. Lett. 2020, 39, 43–48.
- Powell, V.; Sutherland, M. Substituted Benzophenones from Leptospermum luehmannii (F. M. Bailey). Aust. J. Chem. 1963, 16, 282–284.
- de Corréa, D.B.; Guerra, L.F.B.; Gottlieb, O.R.; Maia, J.G.S. C-Methyl Phenolics from Qualea Species. Phytochemistry 1981, 20, 305–307.
- Gong, S.; Liu, C.; Liu, S.; Du, Y.; Kang, W.; Dong, X. Studies on constituents of the Chinese traditional drug baishouwu (Cynanchum auriculatum Royle ex Wight). Yao Xue Xue Bao 1988, 23, 276–280.
- Deng, Y.; Chin, Y.-W.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Anthraquinones with Quinone Reductase-Inducing Activity and Benzophenones from Morinda citrifolia (Noni) Roots. J. Nat. Prod. 2007, 70, 2049–2052.
- Liao, Z.-D.; Xu, F.-Q.; Wu, D.-L.; Zhang, W.; Huang, Q. A new benzophenone isolated from fibrous roots of Anemarrhena asphodeloides. Zhongguo Zhong Yao Za Zhi 2019, 44, 1392–1396.
- Xiong, Y.; Deng, K.Z.; Gao, W.Y.; Guo, Y.Q.; Zhang, T.J. A Novel Alkenoic Acid Ester and a New Benzophenone from Ranunculus ternatus. Chin. Chem. Lett. 2007, 18, 1364–1366.
- Deng, K.-Z.; Xiong, Y.; Zhou, B.; Guan, Y.-M.; Luo, Y.-M. Chemical Constituents from the Roots of Ranunculus Ternatus and Their Inhibitory Effects on Mycobacterium tuberculosis. Molecules 2013, 18, 11859–11865.
- Wu, B.-L.; Zou, H.-L.; Qin, F.-M.; Li, H.-Y.; Zhou, G.-X. New Ent-Kaurane-Type Diterpene Glycosides and Benzophenone from Ranunculus muricatus Linn. Molecules 2015, 20, 22445–22453.
- Kawahara, N.; Sekita, S.; Satake, M.; Udagawa, S.I.; Kawai, K.I. Structures of a New Dihydroxanthone Derivative, Nidulalin A, and a New Benzophenone Derivative, Nidulalin B, from Emericella nidulans. Chem. Pharm. Bull. 1994, 42, 1720–1723.
- Liu, H.-X.; Tan, H.-B.; Liu, Y.; Chen, Y.-C.; Li, S.-N.; Sun, Z.-H.; Li, H.-H.; Qiu, S.-X.; Zhang, W.-M. Three New Highly-Oxygenated Metabolites from the Endophytic Fungus Cytospora rhizophorae A761. Fitoterapia 2017, 117, 1–5.
- Form, I.C.; Bonus, M.; Gohlke, H.; Lin, W.; Daletos, G.; Proksch, P. Xanthone, Benzophenone and Bianthrone Derivatives from the Hypersaline Lake-Derived Fungus Aspergillus wentii. Bioorganic Med. Chem. 2019, 27, 115005.
- Liu, B.; Chen, N.; Chen, Y.; Shen, J.; Xu, Y.; Ji, Y. A New Benzophenone with Biological Activities Purified from Aspergillus fumigatus SWZ01. Nat. Prod. Res. 2021, 35, 5710–5719.
- Zhang, L.-H.; Feng, B.-M.; Zhao, Y.-Q.; Sun, Y.; Liu, B.; Liu, F.; Chen, G.; Bai, J.; Hua, H.-M.; Wang, H.-F.; et al. Polyketide Butenolide, Diphenyl Ether, and Benzophenone Derivatives from the Fungus Aspergillus flavipes PJ03-11. Bioorganic Med. Chem. Lett. 2016, 26, 346–350.
- Liao, W.-Y.; Shen, C.-N.; Lin, L.-H.; Yang, Y.-L.; Han, H.-Y.; Chen, J.-W.; Kuo, S.-C.; Wu, S.-H.; Liaw, C.-C. Asperjinone, a Nor-Neolignan, and Terrein, a Suppressor of ABCG2-Expressing Breast Cancer Cells, from Thermophilic aspergillus Terreus. J. Nat. Prod. 2012, 75, 630–635.
- Assante, G.; Camarda, L.; Nasini, G. Secondary Mould Metabolites. IX. Structure of a New Bianthrone and of Three New Secoanthraquinones from Asperguillus wentii Wehmer. Gazz. Chim. Ital. 1980, 110, 629–631.
- Salleh, W.M.N.H.W.; On, S.; Ahmad, F.; Sirat, H.M.; Taher, M.; Sarker, S.D.; Nahar, L. A New Xanthone and a New Benzophenone from the Roots of Garcinia hombroniana. Phytochem. Lett. 2020, 35, 216–219.
- Iinuma, M.; Tosa, H.; Ito, T.; Tanaka, T.; Riswan, S. Three New Benzophenone-Xanthone Dimers from the Root of Garcinia dulcis. Chem. Pharm. Bull. 1996, 44, 1744–1747.
- Ngwoke, K.G.; Orame, N.; Liu, S.; Okoye, F.B.C.; Daletos, G.; Proksch, P. A New Benzophenone Glycoside from the Leaves of Mitracarpus villosus. Nat. Prod. Res. 2017, 31, 2354–2360.
- Shu, J.; Chou, G.; Wang, Z. Two New Benzophenone Glycosides from the Fruit of Psidium guajava L. Fitoterapia 2010, 81, 532–535.
- Park, B.-J.; Matsuta, T.; Kanazawa, T.; Chang, K.-J.; Park, C.-H.; Onjo, M. Phenolic Compounds from the Leaves of Psidium Guajava. I. Hydrolysable Tannins and Benzophenone glycosides. Chem. Nat. Compd. 2011, 47, 632.
- Ukwueze, S.E.; Osadebe, P.O.; Okoye, F.B.C. A New Antibacterial Benzophenone Glycoside from Psidium guajava (Linn.) Leaves. Nat. Prod. Res. 2015, 29, 1728–1734.
- Shu, J.-C.; Peng, C.-Y.; Liu, J.-Q.; Zhang, R. New Benzophenone and Diphenylmethane Glycosides from Psidium Littorale. Chem. Nat. Compd. 2015, 51, 865–869.
- Terreaux, C.; Wang, Q.; Ioset, J.-R.; Ndjoko, K. Grimminger, Wolf; Hostettmann, Kurt Complete LC/MS Analysis of a Tinnevelli Senna Pod Extract and Subsequent Isolation and Identification of Two New Benzophenone glucosides. Planta Medica 2002, 68, 349–354.
- Munsimbwe, L.; Suganuma, K.; Ishikawa, Y.; Choongo, K.; Kikuchi, T.; Shirakura, I.; Murata, T. Benzophenone Glucosides and B-Type Proanthocyanidin Dimers from Zambian Cassia Abbreviata and Their Trypanocidal Activities. J. Nat. Prod. 2022, 85, 91–104.
- Wu, Y.; Li, E.; Li, Y.; Wu, Q.; Tian, W.; Liu, K.; Niu, Y.; Wang, D.; Liu, J.-G.; Hu, Y. Iriflophenone Glycosides from Aquilaria sinensis. Chem. Nat. Compd. 2016, 52, 834–837.
- Duan, W.-B.; Peng, A.-T.; Yuan, S.-N.; Wang, S.-N.; Li, B.-W.; Duan, X.-H. Two New Benzophenones from the Moss Pogonatum spinulosum. Nat. Prod. Res. 2023, 1–6.
More
