Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Kaiyang Li and Version 2 by Rita Xu.
Under the combination of certain corrosive ions and stress, Cu-Ni alloys may experience severe stress corrosion cracking (SCC), which causes premature failure and hinders their further applications as crucial construction materials in various engineering fields.
- stress corrosion cracking
- Cu-Ni alloys
- corrosion
1. Introduction
Cu-Ni alloys (Cupronickel) are the important branch of Cu alloys with several outstanding properties, such as good mechanical properties at both low and elevated temperatures, high thermal/electric conductivity, wear resistance, high corrosion resistance, biofouling resistance, tarnish resistance, weldability, and good processing characteristics. Because of these attractive features, Cu-Ni alloys were developed and widely used in marine, electrical, chemical, and petroleum fields as heat changers, shipbuilding materials, electrical resistors, pumps, valves, frames, fittings, lines/tubes, coinage, and so on. Typical Cu-Ni alloys include Cu-10Ni (B10, C70600) and Cu-30Ni (B30, C71500) ones. Ni has unlimited solubility in Cu, and it is added as the major alloying element to improve corrosion resistance and mechanical strength. Additionally, small amounts of Fe and Mn are also introduced in Cu-Ni alloys for the optimization of microstructures and the improvement in properties such as mechanical strength and corrosion resistance. Table 1 presents the typical chemical compositions of Cu-10Ni and Cu-30Ni alloys. Given that Cu and Ni are adjacent to each other in the periodic table, they share a lot of similarities in atomic properties. Therefore, Cu and Ni are completely miscible in both liquid and solid conditions, and the formed Cu-Ni alloys have an FCC structure with no heterogeneous structure over the whole concentration range.
Table 1. Chemical compositions of Cu-10Ni and Cu-30Ni alloys based on ASTM B-111 standard (in wt.%) [1].
| Cu | Ni | Fe | Mn | Sn | C | Pb | S | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| Cu-10Ni | Bal. | 9.0–11.0 | 1.2–2.0 | 0.5–1.0 | <0.02 | <0.05 | <0.03 | <0.05 | <0.5 |
| Cu-90Ni | Bal. | 29.0–32.0 | 0.4–1.0 | 0.5–1.5 | <0.02 | <0.06 | <0.03 | <0.06 | <0.5 |
During their industrial service, Cu-Ni alloys inevitably experience environment-induced degradation. Previous studies systemically investigated the corrosion behaviors in various environments [2]. For instance, the effect of Cl− content on the corrosion behavior of Cu-10Ni in H2SO4 solution was investigated by several electrochemical techniques [3]. In another study, the corrosion performance of Cu-10Ni in natural seawater was investigated in a one-year field exposure test in the North Atlantic Ocean [4]. In general, Cu-Ni alloys show acceptable performance in industrial environments, and they are believed to be Cl-resistant, especially when compared with other commonly used Cu alloys such as Cu-Sn and Cu-Zn alloys [5]. However, when serving as structural components, Cu-Ni alloys may experience stress corrosion cracking (SCC) when subject to the synergistic effects of stress and corrosion [6][7][6,7]. When served as heat exchangers, valves, compressor blades, etc., or other load-bearing components in corrosive environments, Cu-Ni alloys are prone to the gradual growth of cracks [8]. They become especially susceptible to corrosion in ammonia-, sulfide-, and nitrate-containing environments. For example, in the simultaneous presence of NH4+, S2−, and seawater, SCC cracks were found on Cu-Ni alloys within a short testing period [9]. These SCC issues will cause irreparable losses and sudden failure of Cu-Ni alloys as structural components, thus making them a threat to facility operation, fortune, or even human safety. Therefore, more attention should be paid to the SCC of Cu-Ni alloys, and it is of great importance to elucidate how the SCC of Cu-Ni alloys takes place and how it is affected by different factors.
As schematically shown in Figure 1, SCC is a combination of three factors, namely stress, corrosive environment, and susceptible material. To elucidate the SCC behavior of Cu-Ni alloys, the specific effects of different factors should be thoroughly screened. Unfortunately, the related review articles reporting SCC of Cu-Ni alloys are still insufficient. Some previous works try to make a summarization, yet they fail to cover all the crucial factors [8]. How different factors affect the SCC of Cu-Ni needs further clarification. It should be confirmed in which environment the SCC becomes more pronounced. The related corrosion mechanisms in different environments are still unclear, which makes the setting up a corrosion mitigation strategy difficult. Therefore, this article tries to conduct a comprehensive literature survey and make a systematic summarization. First, basic information on Cu-Ni alloys is provided. Second, the general corrosion behaviors of Cu-Ni alloys are summarized based on corrosion products and the effects of different corrosive ions. Then, the research progress on the SCC of Cu-Ni alloys is reviewed regarding the corrosion features of various environments and SCC mitigation strategies. More importantly, future research directions regarding the SCC of Cu-Ni alloys are proposed at the end of this article. It is anticipated to provide a summary of how different factors affect the SCC performance of Cu-Ni alloys, to make a theoretical guideline of SCC mitigation, and finally, to bring an economic benefit by reducing the maintenance cost and increasing the service life of these Cu-Ni components during their industrial applications.
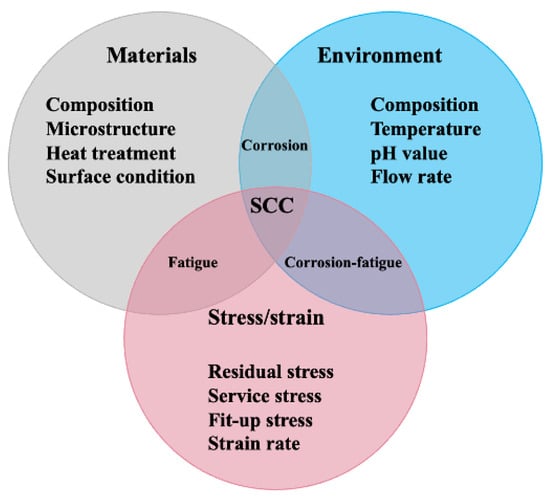
Figure 1. The relationship between materials, environment, and stress/strain.
2. SCC Studies of Cu-Ni Alloys
The study of stress corrosion cracking (SCC) is significant for Cu-Ni alloys. In practical applications, the stress applied on Cu-Ni alloys includes residual stress during machining, sudden opening and closing of the valve, fluid pressure, and so on [10][17]. As for the SCC resistance, it is widely recognized that Cu-Ni alloys are typically SCC-resistant in seawater or related marine environments [11][12][18,19]. Nonetheless, in Cl− environments containing ammonia and/or sulfide, the SCC of Cu-Ni alloys becomes notably apparent.
2.1. SCC Testing Methods
2.1.1. Slow Strain Rate Test
Slows strain rate test (SSRT), as one can tell from its name, is an SCC testing method that makes the sample subjected to a constant yet slow strain rate (usually at 10−5~10−7/s). Once the fracture takes place, the SCC susceptibility of samples can be evaluated by the fracture time/elongation, yield/ultimate tensile strength, and fracture morphology. The specific sample size can be referred to as per ASTM G129 standard [13][20]. It is one of the most commonly used methods for SCC sensitivity evaluation.
2.1.2. Constant Loading Method
This method carries out SCC tests by machining the material into a cylinder or rectangular bar, fixing one end, and then applying a constant static load (via springs or weights) on the other end when the whole bar is immersed in the testing solution. Analyses of the time to failure as a function of applied stress will help to evaluate the SCC susceptibility.
2.1.3. Constant Strain Method
In this method, the tested sample is pre-deformed by stretching or bending to a certain strain value and then exposed to testing environments. The commonly strained samples include three/four-point bending ones, U-bended ones, and C-ring ones. The surface corrosion products and cracking conditions will be examined after certain periods. This method is greatly popular for the advantages of simpleness, low cost, and good adaptability for experiments in isolated containers.
2.2. SCC Performance
2.2.1. Sulfide
S2− can react with Cu and Ni to form non-protective corrosion products. With the assistance of external force, S2− can also increase the SCC-susceptibility of Cu-Ni alloys, especially at higher concentrations. A. El Domiaty and J. N. Alhajji reported the SCC behavior of Cu-10Ni alloy in sulfide-polluted seawater by slow strain rate tests (SSRT) at 20 °C [14][16]. It was found that the severity of SCC increases with the increasing concentration of sulfide S2− (from 0 to 1000 ppm) in the seawater. The selective dissolution of copper resulted in the accelerated degradation of Cu-10Ni alloys in low (<100 ppm) and high (>1000 ppm) sulfide solutions. Two SCC mechanisms were proposed according to the damaging effect of stress on the protective layer, namely sulfide stress cracking associated with the anodic dissolution and hydrogen embrittlement due to the synergism of sulfide and stress in the low and high sulfide concentration ranges, respectively. With the sulfide concentration rising to 3120 ppm, both the Cu-10Ni and Cu-30Ni alloys exhibited susceptibility to SCC. The most severe SCC was observed in Cu-30Ni alloys at 25 °C, which also reflects the influence of Ni content [15][21]. With sulfide concentrations reaching up to 3120 ppm, both the Cu-10Ni and Cu-30Ni alloys are susceptible to SCC. The most severe SCC was observed in the Cu-30Ni alloys at 25 °C, which also highlights the Ni content. With the sulfide concentration diluted to 0.002–0.03 M, the Cu-Ni alloys were no longer sensitive to SCC [16][17][22,23].
2.2.2. Ammonium
NH4+ is a well-known SCC inducer on Cu alloys. T. R. Pinchback et al. [18][24] documented the intergranular SCC of Cu-40Ni alloys, which was induced by exposing stressed C-ring specimens to ammoniacal vapor [18][24]. Results show that increasing ammonia concentration enhanced the corrosion rates of vapor-exposed specimens, reducing the strength of SCC samples and thus causing more severe SCC failure. The SCC mechanism was attributed to the depletion of copper from the material at the crack tip. Furthermore, the exposure time governed the depth of corrosion penetration, which was independent of the ammonia concentration [19][25]. Upon sufficient exposure, the crack walls developed as a corrosion layer consisting of two phases, namely an inner nickel-rich phase adjacent to the alloy substrate and an outer copper-rich phase. M. H. Johar et al. [20][26] observed the intergranular brittle SCC of Cu-27Ni alloy in Mattsson’s solution (containing 1M NH4+ and 0.05 M Cu2+). Figure 2 presents the fracture morphology, with Figure 2a,b,d,e from the top view and Figure 2c,f from the side view. Intergranular fracture presented in Figure 2b illustrates the crack propagation features. It indicates that intergranular corrosion is the failure mechanism in such an NH4+-containing environment.

Figure 2. SEM fracture images of both front and side views of Cu-27Ni after SSRT tests in different environments: (a–c) Mattsson’s solution; (d–f) Mattsson’s solution with 500 ppm of Benzotriazole. (a,b,d,e): top view; (c,f): side view.

SCC typically occurs beyond the elastic limit, namely in the plastic region. However, it was observed to occur below the elastic limit on the Cu-5.37Ni alloy when subjected to superimposed cyclic stress. During the cyclic stress test, a static load of 180 MN/m2 was loaded, followed by a periodic cyclic load of 18 MN/m2 with a low frequency (15–30 Hz) [19][25]. The accelerative influence of superimposed cyclic stresses on ammonia-induced brittle SCC failures was also reported by D.C. Agarwal et al. [21][22][27,28]. As shown in Figure 3, the Cu-30Ni experiences pitting corrosion upon exposure to seawater and seawater + NH4+. With the further application of stress, cracks developed on the surface [23][13]. Note that Figure 3 has a lower magnification than Figure 3a,b to show the full morphology of the formed crack. Ammonia initiates an SCC attack at the surface via an intergranular mechanism, and crack propagation may transition to a transgranular mode [19][21][22][25,27,28]. Once the crack is initiated, it can propagate to failure unless MgCl2 is added and plays a mitigating effect.

Figure 3. Optical images of Cu-30Ni alloy surfaces after corrosion and corrosion product removal in different environments: (a) seawater, (b) seawater with NH4+, and (c) seawater with NH4+ under stress.
H. Y. Xing et al. [23][13] reported the synergistic effects of stress and NH4+ on the pitting corrosion of the Cu-30Ni alloy. NH4+ destroys the oxide film on the surface of the Cu-30Ni alloy, hindering the restoration process of the oxide film, and thereby diminishing the quantity of protective Cu2O and NiO in the corrosion products of the specimen. After the application of load stress, the pitting depth of the specimen intensifies, indicating the onset of stress corrosion cracking.
2.2.3. Sulfide + Ammonium−
H. Ardy et al. [24][29] studied the corrosion behavior of Cu-10Ni alloy in a solution containing a mixture of both ammonia and sulfide. No SCC behavior was observed, as the specimens were still in the initial stage of SCC where the slip dissolution mechanism occurs, accompanied by pitting and intergranular corrosion [24][29]. Further studies are still needed to confirm the synergistic effects of NH4+ and S2− on SCC.
2.2.4. Strain Rate
SCC is also found to be associated with the tensile strain rate. A. A. Nazeer et al. [25][30] investigated SCC behaviors of Cu-10Ni alloys at room temperature in a 3.5% NaCl solution containing sulfide ions at various strain rates during the SSRT tests. The study suggested that similar SCC behavior may be observed when using strain rates of 1.8 × 10−6, 2.5 × 10−6, and 5 × 10−6 s−1, with the most severe SCC occurring at a strain rate of 5 × 10−6 s−1 and a concentration of 20 ppm S2−.
2.2.5. Temperature
It is also noted that the testing temperature played a significant role in influencing the SCC of Cu-Ni alloys. K. Habib et al. [26][31] evaluated the SCC of Cu-10Ni and Cu-30Ni in natural seawater polluted with sulfides at temperatures ranging from 25 to 70 °C. The most severe SCC deterioration of the Cu-10Ni specimens at 39% was in polluted seawater containing 200 ppm sulfide at 70 °C. In contrast, the Cu-30Ni specimen exhibited a maximum deterioration percentage of 27% in polluted seawater with 3120 ppm sulfide at 70 °C. H. M. Shalaby et al. [27][32] also reported similar findings, indicating that Cu-30Ni was more susceptible to transgranular SCC at 90 °C than 60 °C in 18% monoethanolamine solutions. This cracking phenomenon was attributed to the stress-assisted dissolution of copper from the alloy matrix.
2.3. SCC Mechanisms
As can be found in Reaction 2, the cathodic reaction during Cu-Ni corrosion is oxygen adsorption rather than hydrogen evolution. This feature is decided by the high reduction potential of H on the Cu surface. Therefore, the hydrogen embrittlement theory, which is popular for other metals such as Fe- and Mg-based alloys is not applicable to Cu-Ni alloys. Actually, there are two widely accepted SCC failure mechanisms of Cu-Ni alloys: the film-rupture theory and the dealloying theory.
Based on the film rupture theory, the Cu-Ni alloys develop a corrosion product upon exposure to aqueous environments. Under the synergistic effect of tensile loading, cracks are prone to develop within the film, thus providing a short-circuit path for the inwardly diffused corrosive solution. The exposed underlying substrate at the crack tip experiences severe corrosion; however, repassivation is inhibited due to the presence of external stress. In this way, the SCC crack develops at a fast speed.
As for the dealloying theory, selective dissolution of certain elements at the crack tip is the main contributor to SCC. For instance, in S2−-polluted seawater, Cu-10Ni experienced severe SCC due to the selective dissolution of Cu induced by S2− [14][16]. Theoretical tools such as Pourbaix diagrams and the solubility database should be used to predict the tendency of dealloying or selective dissolution of certain metallic elements in the presence of other corrosive ions, thus evaluating the SCC susceptibility in various aqueous environments [8].
