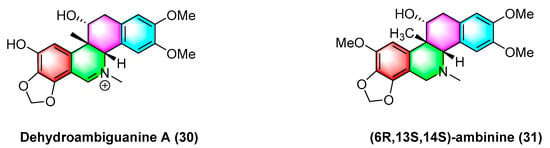2. Antitumor Activities of Benzophenanthridine Alkaloids from Papaveraceae Plants
2.1. Antitumor Activities of Benzophenanthridine Alkaloids from Papaver SPP
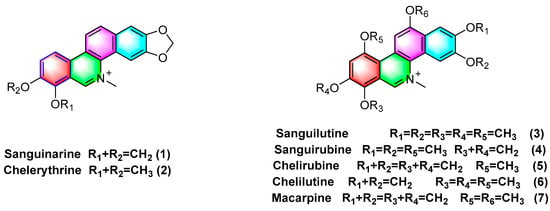
Quaternary benzophenanthridine alkaloids (QBAs) primarily originate from plants of the genus
Papaver. Representative compounds comprise sanguinarine (
1), chelerythrine (
2), sanguilutine (
3), sanguirubine (
4), chelirubine (
5), chelilutine (
6), and macarpine (
7) (
Figure 2).
Figure 2. Benzophenanthridine alkaloids from Papaver spp.
The presence of moderate or high levels of ROS in tumor cells affects the initiation and proliferation of cancer to a certain extent. Benzophenanthridine natural products are capable of inducing, or activating different molecular signal transduction, activating proteins of related pathways, or making them apoptosis by interfering with the signal pathway of ROS, and treating and eliminating tumor cells by regulating dysfunctional proteins
[9]. Sanguinarine (
1), one of the most famous benzophenanthridine alkaloids, can act on ROS-dependent mitochondria to induce autophagy and apoptosis, or inhibit the mitosis of cancer cells by changing the acidic conditions of lysosomes and interfering with the formation of autophagosomes lysosomes, such that liver cancer
[10] and MDA-MB-231 human breast cancer
[11] can be inhibited. Sanguinarine (
1) is capable of significantly targeting ephrin type-B receptor 4 (EphB4) and hypoxia inducible factor-1α (HIF-1α) in breast cancer, inhibiting the activation of the downstream protein signal transducer and activator of transcription 3 (STAT3) in cells, blocking hypoxia-induced HIF-1α or STAT3 interacts, and downregulating the mRNA levels of its target genes, thus inhibiting breast cancer cell hyperplasia
[12]. In addition, in the breast cancer model, sanguinarine (
1) has been proven to have the effects of inhibiting the metastasis of breast cancer and anti epithelial mesenchymal transformation (EMT)
[13]. A recent review article also showed that sanguinarine (
1) is a very promising therapeutic option for breast cancer
[14]. Sanguinarine (
1) facilitates apoptosis in HeLa cells as a treatment for cervical cancer with an IC
50 value of 3.5 μM
[15]. Sanguinarine (
1) exhibits anti-microtubule activity while inhibiting the binding of colchicine and podophyllotoxin to tubulin with IC
50 values of 32 μM and 46 μM. The IC
50 values for chelerythrine (
2) have been obtained as 55 μM and 60 μM
[16]. Sanguinarine (
1) induces apoptosis in HeLa cells by upregulating the expression of the proapoptotic protein Bax and inhibiting the antiapoptotic protein BcI-2, and 0.5 μM sanguinarine treatment leads to a significantly reduced number of colonies formed by HeLa cells
[15]. Sanguinarine (
1) is cytotoxic to different resistant cancer cell lines, and the main mechanisms of action are the inhibition of P-glycoprotein transporters, NF-
kB activation, and so forth. For CCRF-CEM, CEM/ADR5000, U87MG, U87ΔEGFR, MDA231, MDA-BCRP, p53
+/+, p53
−/−, HEK293, and HEK293/ABCB5 cell lines are significantly inhibited with IC
50 values of 0.3–4.1 μM
[17].
Moreover, sanguinarine (
1) exhibits strong cytotoxicity against non-small cell lung cancer (NSCLC) with an IC
50 value of 2.19 μM. Its mechanism of action is likely to be correlated with blocking NF-κB, and Akt and ERK1 signaling pathways have a correlation with the inhibition of cancer cell migration
[18]. Sanguinarine (
1) inhibits the proliferation of BGC-823 gastric cancer cells by downregulating the expression of miR-96-5p and miR-29c-3p and upregulating the expression of MAP4K4, pMEK4, and pJNK1 protein in gastric cancer cells BGC-823
[19]. Sanguinarine (
1) inhibits human prostate cancer cells by inducing ROS-dependent Par-4 cleavage and increasing ROS concentrations in cancer cells. Moreover, it induces growth arrest and apoptosis of human prostate cancer cells PC3 and DU145 with active caspases, which are activated at 2 μM concentration, such that their colony formation can be inhibited
[20]. The existing research has also suggested that long-term treatment with sanguinarine (
1) causes telomere attrition and cell growth retardation, such that cancer cells become senescent. The main mechanisms are associated with the downregulation of the reverse transcriptase
hTERT gene expression and inhibition of telomerase activity
[21]. Sanguinarine (
1) induces apoptosis in human HT-29 cells, demonstrating potential therapeutic applications in the treatment of colon cancer
[22].
Sanguinarine (
1) overexpresses inducing long non-coding RNA casc2 in SKOV3 cells or inhibits the NF-
kB signaling pathway, thus inhibiting SKOV3 cell growth, proliferation, migration, invasion, and so forth, while ultimately facilitating apoptosis
[23]. Moreover, it enhances the sensitivity of cisplatin-resistant cells’ ovarian cancer A2780 to cisplatin
[24]. Sanguinarine (
1) also has potential therapeutic effects against several cell lines with leukemia (e.g., HL-60, drug-resistant HL-60/MX1, drug-resistant HL-60/MX2 (acute promyelocytic leukemia), J45.01 (acute T cell leukemia), U266B1 (myeloma), CCRF/CEM, and CEM/C1 (acute lymphoblastic leukemia)). To be specific, the most potent activity is observed against drug-resistant HL-60/MX2 with IC
50 values of 0.10 ± 0.05
[25]. Sanguinarine (
1) can inhibit the migration of 786-O cells in vitro and in vivo, and reverse the epithelial−mesenchymal transition with IC
50 values of 0.5959 μM
[26].
Moreover, recent research has confirmed that sanguinarine (
1) is capable of inducing H
2O
2-dependent cellular ferroptosis in human cervical cancer (Hela) based on a major mechanism that is correlated with the downregulation of SLC7A11 and the depletion of GSH
[6]. Besides the above cancer cells, existing studies have found that sanguinarine (
1), which specifically targets lung cancer stem cells, is a natural anti-lung cancer drug-resistant compound. Furthermore, sanguinarine (
1) inhibits pancreatic cancer stem cells by inhibiting the sonic hedgehog signaling pathway
[27]. The therapeutic effect of sanguinarine on various tumors has been verified at the animal level
[28][29][30][28,29,30]. Overall, sanguinarine (
1) has anticancer potential and is expected to become a leading compound of anticancer natural products
[31].
Extensive research has confirmed that chelerythrine (
2) is capable of affecting estrogen signaling pathways (e.g., the estrogen receptor ER- α 36, ER- α 66, ER-β1, and Src expression), inhibiting gastric cancer cell (AGS) growth proliferation, and facilitating their apoptosis
[32]. Chelerythrine (
2) can act on tgfb1-erk1/2/Smad2/3-snail/ZEB1 signaling to inhibit the progression of cell lines U251 and T98G of glioblastoma (GBM) (e.g., proliferation, migration, stemness, and invasion
[33]). In addition, chelerythrine (
2) also promotes Drp1 mitochondrial translocation to enhance glioma cell lines necroptosis
[34].
In human hepatocellular carcinoma (HCC), chelerythrine (
2) can inhibit human hepatocellular carcinoma Hep3B cells by downregulating the expression of p-FAK and MMP-2/9. Moreover, the main mechanism of action is correlated with the alteration of phosphoinositide 3-kinase (PI3K), Akt, and the mammalian target of rapamycin (mTOR) signaling pathways
[35]. Chelerythrine (
2) has exhibited anticancer activity in vivo and in vitro, and considerable existing research has confirmed that chelerythrine can act on different pathways (e.g., DNA, MAPK, apoptosis, ROS, cell cycle, autophagy, tumor metastasis, and PKC) to inhibit or facilitate apoptosis in a variety of cancer cells (e.g., non-small cell lung cancer
[36], prostate cancer
[37], lung adenocarcinoma
[38], renal cancer
[39], and melanoma cells
[40], colorectal cancer
[41]), thus suggesting that the benzophenanthridine alkaloids exhibit high anticancer activity. Previous studies have proven that chelerythrine (
2) exhibits antitumor stem cell properties, which are mediated by the downregulation of β- Catenin expression, thus inhibiting non-small cell lung cancer stem cells
[42]. Both sanguinarine (
1) and chelerythrine (
2) have anticancer activities on human breast cancer cells, but sanguinarine (
1) has more potential
[43]. However, chelerythrine (
2) has been reported as a promoter that can regulate c-MYC oncogenes, which has become a new strategy to develop anticancer molecules
[44].
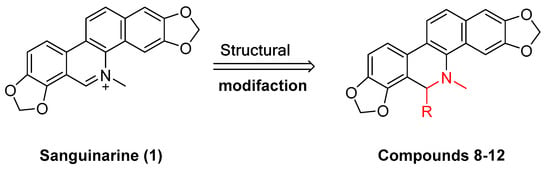
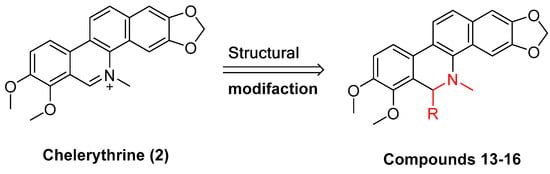
Existing research has confirmed that the hydroxymethyl group at the C-6 position of benzophenanthridine alkaloids takes on a critical significance to cellular activity, and the introduction of different groups at the C-6 position can change their activity (
Figure 3 and
Figure 4)
[45].
Figure 3. Study on structure–activity relationship of sanguinarine.
Figure 4. Study on structure–activity relationship of chelerythrine.
The compounds chelerythrine (
2), structurally modified to yield compounds
13–
16, sanguinarine (
1), and derivatives
8–
12, exhibit potent activity against Jurkat clone e6-1 and THP-1 leukemia cell lines, with IC
50 values from 0.18 to 7.94 μM. Notably, most of the activities of the above compounds are higher than those of chelerythrine (
2) and sanguinarine (
1)’s original activities, in which the IC
50 values of compound
12 against the above two leukemia cell lines reach 0.53 ± 0.05 and 0.18 ± 0.03 μM, respectively
[46].
Sanguinarine (
1), chelerythrine (
2), sanguilutine (
3), sanguirubine (
4), chelirubine (
5), and macarpine (
7) (
Figure 1) exhibit antitumor activity against various cancer cell lines, including human leukemia (e.g., HL-60, THP-1, MT-4, CEM, and U937), human prostate cancer (e.g., DU-145, LNCaP, and PC3), human epidermoid carcinoma (e.g., A431, nheks,), human pancreatic cancer (e.g., AsPC-1 and BXPC-3), human melanoma (e.g., M4Beu, A372, OCM-1), human non-small cell lung cancer (A549), human breast cancer (e.g., MCF-7 and MDA-MB-231), human ovarian adenocarcinoma (OVCAR-3), cervical cancer (e.g., hen-16-2 and HeLa), human colon cancer (e.g., HCT-116, SW480), and human gastric cancer cells (BGC-823) with IC
50 values < 10 μM
[47].
Sanguinarine (
1), chelerythrine (
2), chelirubine (
5), macarpine (
7), and sanguirubine (
4) inhibit HL-60, KF-II, A431, and HeLa activity with IC
50 values ≤ 0.7 (μg/mL). Of these, macarpine (
7) shows the optimal activity, with IC
50 values against four cell lines (μg/mL) followed by 0.012, 0.013, 0.024, and 0.015
[48].
Sanguinaria canadensis L. can extract and purify sanguilutine (
3). Some research has suggested that the antiproliferative activities of sanguilutine (
3) and chelilutine (
6) are related to the induction of oxidative stress. As indicated by the results, against three different cancer cell lines, HeLa, A2780, and HL-60, the IC
50 values of sanguilutine range from 0.04 to 0.46 (μg/mL), and the IC
50 values of chelilutine (μg/mL) range from 0.16 to 0.84
[49].
Hammerová, Jindřiš Ka et al.
[50] further elucidated the mechanism of sanguilutine (
3) in inducing apoptosis in melanoma cells. As a result, sanguinarine caused a decrease in the mitochondrial membrane potential and levels of antiapoptotic proteins of the bcl-2 protein family, BCL XL, and myeloid cell leukemia protein 1 (Mcl-1), as well as downregulated levels of the X-linked inhibitor of apoptosis protein (XIAP) to facilitate melanoma cell apoptosis.
2.2. Antitumor Activities of Benzophenanthridine Alkaloids from Corydalis saxicola Bunting
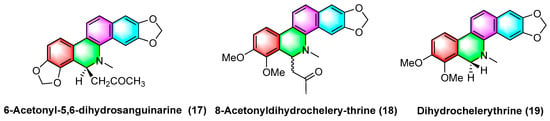
The 6-acetyl-5,6-dihydrosanguinarine (
17), 8-acetyldihydrochelerythrine (
18), and dihydrochelerythrine (
19) (
Figure 5) are isolated from
Corydalis saxicola Bunting, and the above alkaloids have some antitumor effects on squamous cell carcinoma, lung cancer, and liver cancer of the tongue. To be specific, the mechanism of action against human tongue squamous cell carcinoma may be inhibiting NF-κB activation, downregulating BcI-2 protein expression on mRNA, and reducing telomerase activity; inhibiting non-small cell lung cancer A549 cell proliferation, migration, and inducing apoptosis; inhibiting proliferation and migration and upregulating the intracellular NF-κB p65 expression of the subunit
[51].
Figure 5. Benzophenanthridine alkaloids from Corydalis saxicola Bunting.
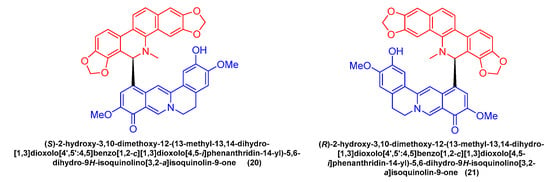
Feng Qin et al.
[52] extracted and isolated two novel dimeric benzophenanthridine alkaloids (
20,
21) (
Figure 6) from
Corydalis saxicola, which comprised a mixture of benzophenanthridine and protoberberine passing between the 6, 12 C-C σ Bond direct coupling generation. Compounds
20 and
21 inhibited T24 cells with IC
50 values of 13.26 μM and 9.45 μM.
Figure 6. Benzophenanthridine alkaloids from Corydalis saxicola.
2.3. Antitumor Activities of Benzophenanthridine Alkaloids from Chelidonium
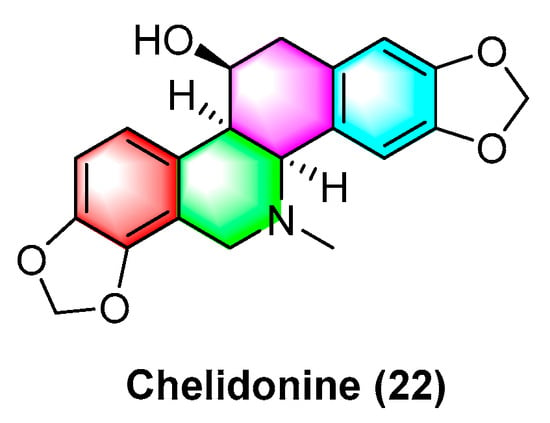
Sakineh Kazemi noureini et al.
[53] have indicated that chelidonine (
22) (
Figure 7) can inhibit MCF-7 in a dose-dependent manner, including cell senescence, apoptosis of autophagic syncytial cells by inhibiting telomerase activity, and chelidonine (
22) at a 0.05 μM concentration, thus inducing senescence in MCF-7 cells with an LD
50 value of 8 μM. Chelidonine (
22) is capable of inhibiting the pharmacological activity of the NRAS activator stk19 kinase. It inhibits arginine, lysine, and leucine (Q61R, Q61K, and Q61L) in NRAS mutant melanoma at glutamate 61 (Q61), thus inhibiting the downstream signaling pathways of RAS proteins (e.g., Raf/MEK and P13K-AKT). As a result, cellular senescence and apoptosis are caused. Chelidonine inhibits STK19 kinase with an IC
50 value of 123.5 ± 19.3 Nm
[54]. Csomós, I., et al.
[55] have indicated that chelidonine (
22) arrests the G2/S phase of melanoma cells by inhibiting the phosphorylation of complexine and serine in the STAT3 signaling pathway in human melanoma cells, thus inhibiting melanoma. The inhibition effect is significant at 1 μg/mL concentration. It has also been documented that chelidonine (
22) can inhibit growth, invasion, angiogenesis, and suppress gene expression in head and neck cancer cell lines. At 10 μg/mL, it significantly inhibits FADU, HLaC78, HlaC79, and HLaC79-Tax cell lines
[56]. Radim havelek et al.
[57] indicated that chelidonine (
22) can inhibit the cell cycle of leukemic T cells in different p53 states while suppressing tubulin polymerization in A549 cells. The IC
50 value of different tumor suppressor proteins of the
p53 gene for MOLT-4, HL-60, U-937, Raji, Jurkat, and others ranges from 2.2 to 5.0 μM. For non-small cell lung cancer cells (NSCLC), chelidonine (
22) has strong inhibitory effects, which are achieved primarily through the ability to selectively inhibit the EGFR phosphorylation and inhibit mitochondrial function in EGFR double mutant cells. The IC
50 of chelidonine after 72 h treatment of various NSCLC cell lines (e.g., H1975, PC9, H460, and H358) ranges from 2.58 to 12.77 μM. Against A549, CCD19 is less active with IC
50 value > 20 μM
[58]. Chelidonine (
22) can induce cell death in T98G cells through two apoptotic pathways: caspase-dependent and caspase-independent. As a result, cell mitosis is arrested, thus causing cell death, and inhibiting human glioblastoma. 0.6 μM of chelidonine (
22) can significantly inhibit the G2/M phase of mitosis in T98G cells
[59]. Lenvatinib is capable of enhancing the apoptosis of HCC cells by chelidonine (
22), thus inhibiting the epithelial mesenchymal transition (EMT)-related factor of HCC cells based on the possible mechanism. Moreover, chelidonine inhibits HCC cells MHCC97-H and LM-3 with IC
50 values of 7.72 μM and 6.34 μM
[60]. Chelidonine (
22) can inhibit the cell cycle of leukemic T cells in different p53 states while suppressing tubulin polymerization in A549 cells, and the IC
50 values of different tumor suppressor proteins’
p53 gene for MOLT-4, HL-60, U-937, and Raji range from 4.8 to 8.3 μM
[51].
Figure 7. Benzophenanthridine alkaloid from Chelidonium.
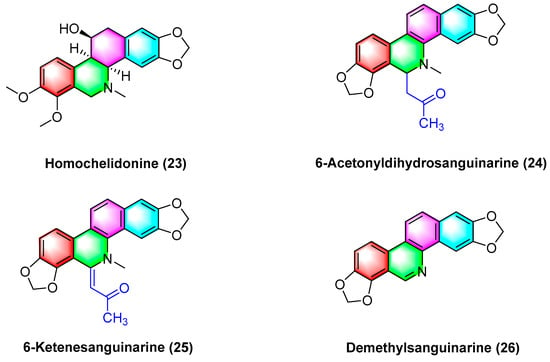
Havelek R. et al.
[61] have indicated that homochelidonine (
23) (
Figure 8) induces apoptosis and arrests the G2 phase mitotic cell cycle in cancer cells, and 20 µM homochelidonine (
23) inhibits the cell growth of SK-BR-3, HepG2, and MCF-7 by over 50%.
Figure 8. Benzophenanthridine alkaloids from Corydalis bungeana Turcz.
Acetyldihydrosanguinarine (
24), 6-ketenesanguinarine (
25), and demethylsanguinarine (
26) (
Figure 8) are isolated from
Corydalis bungeana Turcz. Xiyun Ye et al.
[62] determined the cytotoxic activity of the above two compounds against A549, HT-29, kb16, and P-388 cell lines, respectively, and their ED
50 (μg/mL) values reach 1.840, 1.600, 0.340, and 0.051, respectively.
2.4. Antitumor Activities of Benzophenanthridine Alkaloids from Corydalis
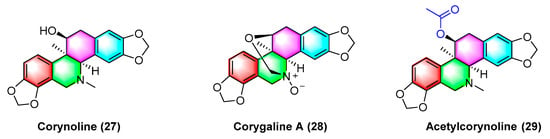
Corynoline (
27) (
Figure 9) is a natural product derived from the traditional Chinese medicine
Corydalis. It significantly inhibits the cell cycle and induces apoptosis in melanoma cells B16F10 and A375 in vivo, with an IC
50 value of 6.16 μM. The IC
50 value of A375 reaches 5.56 μM. The mechanism between them is correlated with the upregulated gene expression of
Bax and cleavage of Caspase-3
[63].
Figure 9. Benzophenanthridine alkaloids from corydalis.
Corygaline A (
28) (
Figure 9), isolated from
Corydalis bungeana Turcz, refers to a hexahydrobenzophenanthridine alkaloid with an unusual carbon skeleton. Corygaline A (
28) is capable of inhibiting the NO production in LPS-activated RAW264.7 macrophages with an IC
50 value of 2.9 μM. Moreover, it is independent of dose
[64].
Acetylcorynoline (
29) (
Figure 9), originating from the rhizome of the natural plant
Corydalis incisa, inhibits the mitotic process of cancer cells by affecting chromosomes, spindles, and the cytoplasm during mitosis, which eventually arrests the mitotic process and induces apoptosis. The mitotic process of the cells is significantly inhibited by 10 μM acetylcorynoline. Acetylcorynoline (
29) potently inhibits human colon carcinoma HCT-116, lung adenocarcinoma cell NCI-H23, lung carcinoma H460, as well as cervical carcinoma TuWi with EC
50 values < 20 μg/mL
[65].
As shown in
Figure 10, dehydroambiguanine A (
30) and (6
R, 13
S, 14
S)-ambinine (
31) are extracted from
Corydalis ambigua subsp.
Amurensis. As indicated by the result, both alkaloids can inhibit the proliferation of tumor cells during activity tests. They exhibit strong activity against the human colon cancer cell line HCT-116, dehydroambiguanine A (
30) with an IC
50 value of 49.8 ± 4.79 μM. Furthermore, (6
R, 13
S, 14
S)-ambinine (
31) is less active (IC
50 values > 200 μM)
[66].
Figure 10. Benzophenanthridine alkaloids from Corydalis ambigua subsp. Amurensis.
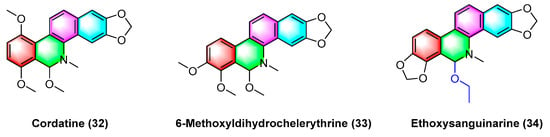
2.5. Antitumor Activities of Benzophenanthridine Alkaloids from Macleaya cordata
The benzophenanthridine alkaloids cordatine (
32) and 6-methoxyldihydrochelerythrine (
33) in
Figure 11 are extracted from the fruits of
Macleaya cordata. Hui Liang Zou et al.
[67] determined the cytotoxic activity of the above two benzophenanthridine alkaloids against MCF-7 and SF-268 through the MTT assay. As revealed by the results, the IC
50 value of cordatine (
32) against MCF-7 cells is 34.78 mM, and that against SF-268 cells reaches 11.79 mM. Furthermore, the IC
50 value of 6-methoxydihydrochelerythrine (
33) reaches 21.45 mM and 4.28 mM, respectively.
Figure 11. Benzophenanthridine alkaloids from the fruits of Macleaya cordata.
Ethoxysanguinarine (
34) is derived from
Macleaya cordata (
Willd)
r. br., and ethoxysanguinarine (
34) inhibits the anchorage-dependent and anchorage-independent growth of breast cancer cells by inducing cell autophagy by upregulating the activity of AMP-activated protein kinase (AMPK). Ethoxysanguinarine (
34) exhibits strong activity against seven breast cancer cell lines (including MCF-7, sk-br3, MDA-MB-231, MDA-MB-436, MDA-MB-468, MDA-MB-453, and MDA-MB-435S) with IC
50 values from 2.63 to 9.15 μM
[68]. Recently, a study showed that it inhibits the viability of MCF-7 and MDA-MB-231 human breast cancer cells and induces apoptosis via a mechanism related to a Hakai-related signaling pathway
[69].
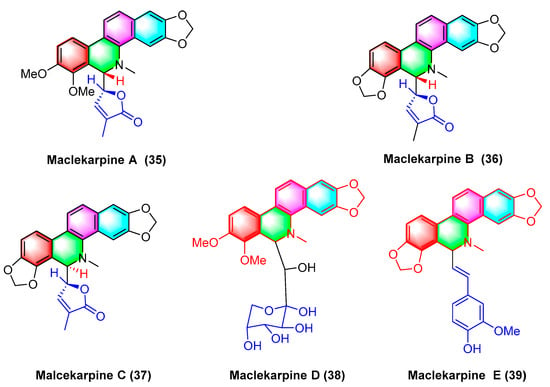
Five dihydrodibenzophenanthridine alkaloids, termed maclekarpine A–E (
35–
39) (
Figure 12), are isolated from the fall back roots of
Macleaya cordata. The alkaloids maclekarpine A–E (
35–
39) exhibit high antitumor activity and have an inhibitory effect on several human cancer cells (e.g., human colon cancer cell line HCT-8, human hepatoma cell line BEL-7402, human gastric cancer cell line BGC-823, human ovarian cancer cell line A2780, as well as human lung cancer cell line A549). Maclekarpine A (
35) exhibits excellent activity against BGC-823 with an IC
50 value of 0.7 μM, except that maclekarpine B (
36) is inactive. The IC
50 value of maclekarpine C (
37) ranges from 1.6 to 3.4 μM. Maclekarpine D (
38) IC
50 values range from 0.2 to 2.0 μM. Maclekarpine E (
39) has a significant inhibitory activity against BGC-823 with the IC
50 value of 0.1 μM
[70].
Figure 12. Benzophenanthridine alkaloids from the fall back roots of Macleaya cordata.
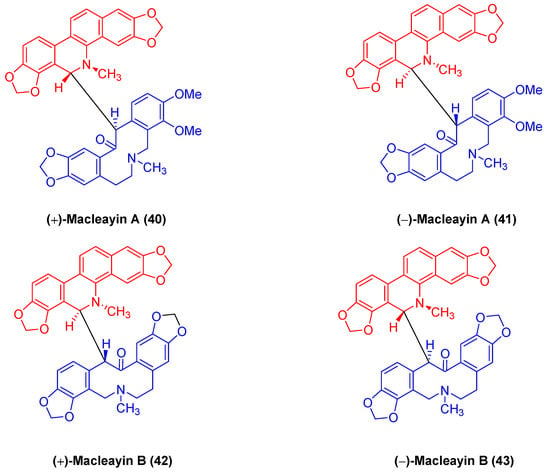
(±)-macleayin A (
40,
41) and (±)-macleayin B (
42,
43) in
Figure 13 originating from
Macleaya cordata (
Willd.)
r. br. are enantiomeric natural dimeric alkaloids. (±)-macleayin A (
40,
41) are prepared by coupling dihydrosanguinarine with allocryptamine through the 6,13′-C-C bond, and (±)-macleayin B (
42,
43) are synthesized by coupling dihydrosanguinarine with proto opioid through a 6,13′-C-C bond. In the in vitro activity test, (±)-macleayin A and (±)-macleayin B have significant inhibitory effects on cancer cell HL-60 with IC
50 values from 3.51 to 9.64 μM. Macleayin A exhibits the optimal activity, while the IC
50 values of sanguinarine and allocryptophylline reach 7.71 and 7.18 μM, respectively
[71].
Figure 13. Benzophenanthridine alkaloids from Macleaya cordata (Willd.) r. br.