The urgent need for efficient energy storage devices (supercapacitors and batteries) has attracted ample interest from scientists and researchers in developing materials with excellent electrochemical properties. Electrode material based on carbon, transition metal oxides, and conducting polymers (CPs) has been used. Among these materials, carbon has gained wide attention in Electrochemical double-layer capacitors (EDLC) due to its variable morphology of pores and structural properties as well as its remarkable electrical and mechanical properties. In this context, the present work summarizes the history of supercapacitors, the type of carbon electrode materials, and the different strategies to improve the performance of these devices. In addition, different approaches to studying the charging mechanism of these devices through different electrochemical techniques are presented, including advantages and challenges. Since a deeper understanding of the interfacial charge storage mechanisms is also crucial in the elaboration and performance of the electrode material.
- carbon materials
- supercapacitors
- ac-electrogravimetry
- EQCM (Electrochemical Quartz Crystal Microbalance)
- NMR (Nuclear Magnetic Resonance)
1. Introduction
One of the strategies to deal with climate change is to reduce the consumption of fossil fuels and develop renewable and sustainable energy sources. In this way, more efficient electrical energy conversion and storage devices are required [1][2]. Batteries and supercapacitors are the most used energy storage technologies. Batteries store energy through faradaic redox reactions providing a high-energy supplement, with energy densities of a few hundreds of W h kg−1. However, these battery-type faradaic reactions undergo slow kinetics leading to limited energy yield and lifetime [3]. In contrast, supercapacitors store the charge on reversible electroadsorption of electrolyte ions toward the surface of electrodes [4]. Although featuring lower energy density, supercapacitors can provide a high power delivery in a relatively short time and can operate for a high number of charge/discharge cycles and a longer lifetime than batteries [2][5], as shown in the Ragone Plot. These fast and highly reversible storage mechanisms make supercapacitors promising candidates for energy storage devices, which are presently used in a broad range of applications ranging from small devices (watches, sensors, mobile, headphones, and others) [6] to large-size cells for automotive transportation such as electric car and buses and their charging stations [7][8].
2. History
Becker of GEC (General Electric Company) patented the first supercapacitor in 1957. Electrodes composed of porous carbon and an aqueous electrolyte based on sulfuric acid were used by Becker. It formed an electric double layer at the electrode/electrolyte surface [9]. A few years later, a device using graphite was patented by SOHIO (Standard Oil of Ohio). In an experiment with organic electrolytes, SOHIO observed a higher operating voltage than those obtained in aqueous electrolytes. In 1971, NEC (Nippon Electric Company) licensed the SOHIO patent under the name of “supercapacitors”. NEC successfully introduced these new devices on the market as backup memories for electronics [10][11][12]. Following the success of NEC, several companies began to produce and develop supercapacitors. For instance, in 1982, the first high-power supercapacitor intended for military applications was designed by Pinnacle Research Institute [9][13].
Presently, there are different types of supercapacitors in terms of charge-storing mechanisms . The two main types are the Electrochemical double-layer capacitors (EDLC) and the so-called Pseudocapacitors. In EDLC, the charge is stored by electrostatic interaction between electrolyte ions and the surface of electrodes, typically using carbon materials as electrodes. In pseudocapacitors, the charge is stored by fast and reversible faradaic redox reactions between the electrolyte and electroactive species on the surface of the electrode, generally using conducting polymers (CPs) and transition metal oxide materials as electrodes [5]. Finally, another type of supercapacitor is the so-called hybrid capacitor, where the charging mechanism is due to electrostatic interactions and faradaic reactions [14] (See Figure 12).
Supercapacitors can be classified as symmetric and asymmetric. Symmetric is when both electrodes have the same design and mass loading, while in asymmetric, both electrodes are different [15].
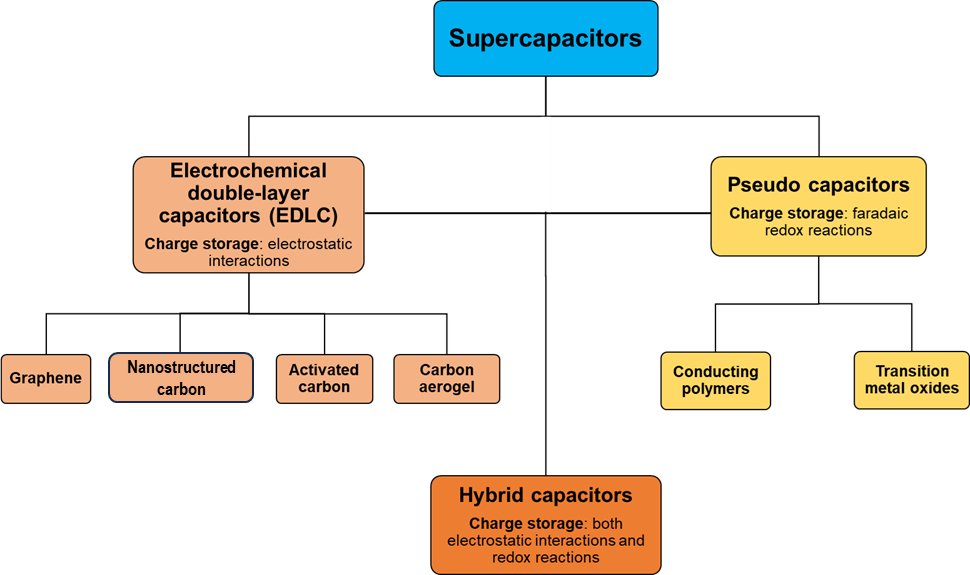
Figure 12. Categorization of supercapacitors: Electrochemical double-layer capacitors (EDLC), Pseudocapacitors and Hybrid capacitors.
3. Proposed Strategies for Higher Performance in Supercapacitors: (Nano)Structuring, Electrolyte Composition, Pseudocapacitance, and Hybrid and Composite Electrodes
Research on (i) nanostructuration, (ii) electrolyte composition, (iii) pseudocapacitance behavior, and (iv) hybrid and (v) composite electrodes has been proposed to improve the performance of supercapacitors. These strategies are detailed below.
-
Nanostructurationas 1018Mysyk, R.; Raymundo-Piñero, E.; Béguin, F. Saturation of subnanometer pores in an electric double-layer ca
The performance of supercapacitors can be affected by the physical and chemical properties of carbon materials, e.g., pure CNTs. It includes size, purity, shape, defects, annealing, and functionalization. Furthermore, composites such as CNT/oxide and CNT/polymer have shown an increase in capacitance and supercapacitor stability. It has been achieved through optimal engineering of composition, particle size, and coverage [16]. For example, high values of specific capacitance (350 F/g), power density (4.8 kW/kg), and energy density (3.3 kJ/kg) have been shown in pyrrole-treated functionalized SWCNTs [17]. Moreover, 73 wt.% PANI deposited onto SWCNTs have shown high values of specific capacitance (485 F/g), specific power (228 Wh/kg), and specific energy (2250 W/kg) [18].
-
Electrolyte Composition
According to the literature, an ideal electrolyte for EDLC must have the following characteristics: a wide electrochemical window (>4), specific conductance of >75 mS.cm−1 at room temperature, thermal stability up to 300 °C, and low toxicity. However, the ionic conductivity is low at room temperature in the case of ionic liquids [19].
Lin, R [11] has studied the influence of the type of electrolyte vs. energy density at different voltage windows. The author confirmed that ionic liquids (ILs) are the most important electrolytes in terms of energy density compared to organic and aqueous electrolytes. ILs operated in a wider voltage window. Lin, R also mentioned that the combination of anions and cations influences the composition and associated properties of ILs. Millions of different structures can form an IL. The number of cation and anion combinations can be as high as 1018. However, Aprotic, Protic, and Zwinteerionic are the main classes used in different types of applications [20]. Aprotic ILs are most commonly used for EDLC applications as they allow cell voltage to be increased above 3 V. Therefore, ILs are well known for their high electrochemical stability and thermal stability and are largely studied in energy storage devices [21].
-
Pseudocapacitance
The pseudocapacitors are another type of technology that provides an increase in the specific capacitance and the energy density. In this technology, charge storage is achieved through a reversible redox reaction within the electrode surface [22][23][24].
Metal oxides, such as RuO2 [25] and MnO2 [26], and conducting polymers are the main class of electrode materials used for charge storage in pseudocapacitors [27][28]. However, the redox reactions can affect the cycling stability due to aging.
-
Hybrid Electrodes
An ideal storage device that has the high-power density of a supercapacitor and the high energy density of a battery is the primary goal of a hybrid technology. Some examples of asymmetrically structured hybrid supercapacitors have been reported in the literature. The combination of a carbon electrode (of supercapacitor type) with a faradaic electrode (of battery type) is achieved in this kind of technology. However, these hybrid systems have intermediate characteristics. In these systems, the charge/discharge speed is slower than the classic supercapacitor due to the influence of the redox reactions at the faradaic electrode. A shorter lifetime is also caused by the chemical reactions that are due to the consummation of the active material. Considering these characteristics, hybrid supercapacitors have been the subject of numerous studies [29][30][31]. For instance, JSR Micro and JM Energy Corporation proposed two Li-ion hybrid supercapacitors called the 2300F and 3300F prototypes. Their energy density is about 10 Wh∙kg−1 [32].
-
Nanocomposite electrodes
Nanocomposite electrodes have been reported in the literature [33], to improve the performance of supercapacitors. Binary-composite or ternary-composite films have been evaluated, employing various methods of preparation. It has been revealed that high specific capacitances were reached with PANI-CNT [34] or PEDOT-CNT [35][36] composites. It was either enhancing the robustness of the flexible PPy-CNTs [37] or improving the capacitive properties of PTh-CNTs films [38]. Ternary nanocomposites of Mn3O4, TiO2, and reduced graphene oxide (RGO) electrodes have been also studied for supercapacitor applications [39]. This material has achieved a specific capacitance of 356 F∙g−1 in 6 M KOH aqueous electrolyte and respectable cycling performance, making it suitable for supercapacitors.
In an overview, the specific capacitance of the representative EDLCs and pseudocapacitors systems based on various active materials are shown in Figure 23.
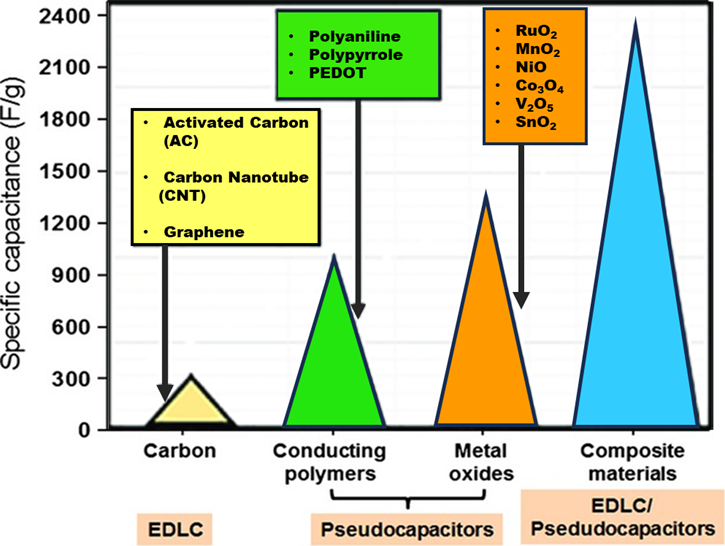
Figure 23. Comparison of various materials (electrodes) according to their specific capacitance for different supercapacitor applications. Adapted from Ref. [40] with permission from Elsevier (copyright 2020) and Ref. [41] open access.
Furthermore, Table 1 shows a comparison between the different types of supercapacitors. Some advantages and disadvantages are also included.
Table 1. Comparison between EDLCs, pseudocapacitors, and hybrid capacitors. Modified from Ref. [42] open access.
|
Parameters |
EDLC |
Pseudocapacitor |
Hybrid Capacitor |
|
Material |
Carbon-based materials, e.g., activated carbon, carbon nanotubes |
Metal oxides, conducting polymers, e.g., NiO, MgO, PANI |
Metal oxide/carbon-based materials, conducting polymer/carbon-based materials, e.g., Ni(OH)2/rGO, PANI/rGO |
|
Storage mechanism |
Non-faradic/electrostatic, electrical charge stored at the metal/electrolyte interface |
Faradic, reversible redox reaction |
Both faradic and non-faradic |
|
Specific capacitance |
Lower |
Higher |
Higher |
|
Energy density |
Low |
High |
High |
|
Cycle Life/stability |
High |
Low |
High |
|
Voltage operation |
High voltage and high-power operation |
Low-voltage functioning is restricted by electrochemistry and the solvent’s solvent decomposition voltage |
Increased cell voltage |
|
Charge/discharge speed |
Faster |
--- |
Slower |
4. Diagnostic Tools for Electrode Materials in Energy Storage
To improve the performances of supercapacitors, it is necessary to use appropriate tools to examine the electrochemical behavior of the different electrode materials. Electrochemical techniques, commonly used to explain the charge storage mechanisms in electrode materials and covered in this research, include NMR spectroscopy, EQCM, as well as non-classical EQCM based method, i.e., ac-electrogravimetry. Computer Simulations have also been shown to provide a significant understanding of the charge storage phenomena. Advantages and challenges are detailed in Figure 34.
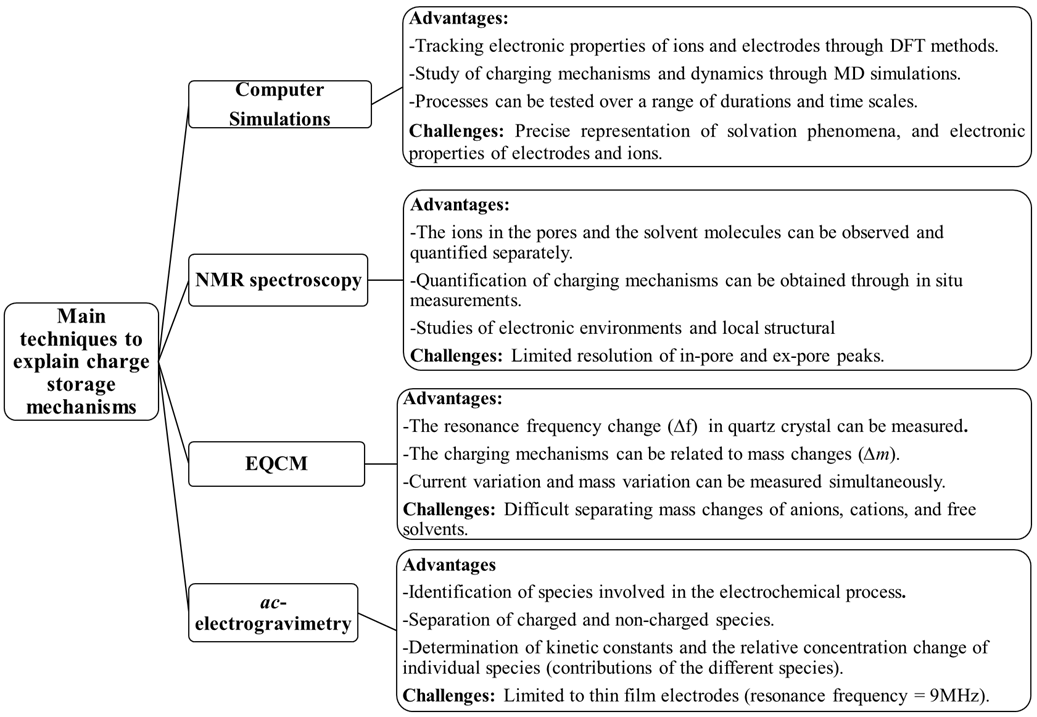
Figure 34. Advantages and challenges of main techniques to explain charge storage mechanisms.
5. Summary and Perspectives
Carbon electrode materials have been extensively studied for electrochemical double-layer capacitors (EDLC). The relationship between the pore size and the ion size is an important factor to consider in order to maximize the specific capacity [43]. The questions related to charging mechanisms are experimentally difficult since there are no appropriate electrochemical or physicochemical techniques that allow direct access to such information. The electrochemical quartz crystal microbalance (EQCM) technique has begun to address these questions. The EQCM technique was also combined with electronic conductance studies or with nuclear magnetic resonance (NMR) to access a complete description of the electrical double layer [44][45]. Furthermore, in situ SAS techniques and other techniques, such as in situ XRD, in situ infrared spectroelectrochemical, etc., have been found to be useful for investigating ion adsorption on nanoporous carbon [3].
However, the dynamic aspects of ion electroadsorption within carbon electrode materials remain a partially unresolved problem with these techniques. Consequently, some research groups have used coupled methods/techniques to better understand the charge storage mechanisms. For instance, the ac-electrogravimetry (coupling between fast QCM and EIS) technique has been largely used to study the capacitive behavior in carbon materials since this technique provides relevant information concerning the identification and separation of charged and non-charged species, the determination of kinetic constants and relative concentration/contribution of individual species [4][5][43][46][47]. In situ or operando techniques with simulation or modeling are another powerful tool for extracting additional information, such as the distribution and population of ions on carbon nanopores [3].
The choice of type of material and electrolyte-ion to improve the performance of the supercapacitor electrodes has been widely studied in the literature. However, the choice of a characterization technique that allows us a deeper understanding of the interfacial charge storage mechanisms is also crucial in the elaboration and performance of the electrode material.
In this way, this research focused on the study of charge storage mechanisms using electrochemical techniques as well as strategies that improve the performance of supercapacitors.
References
- M.J.B. Kabeyi, O.A. Olanrewaju, Sustainable Energy Transition for Renewable and Low Carbon Grid Electricity Generation and Supply, Frontiers in Energy Research 9 (2022).
- P. Simon, Y. Gogotsi, Materials for electrochemical capacitors, Nat Mater 7 (2008) 845-854.
- H. Shao, Y.-C. Wu, Z. Lin, P.-L. Taberna, P. Simon, Nanoporous carbon for electrochemical capacitive energy storage, Chemical Society Reviews 49 (2020) 3005-3039.
- F. Escobar-Teran, H. Perrot, O. Sel, Ion Dynamics at the Carbon Electrode/Electrolyte Interface: Influence of Carbon Nanotubes Types, Materials 15 (2022) 1867.
- F. Escobar-Teran, H. Perrot, O. Sel, Single Wall Carbon Nanotubes/Polypyrrole Composite Thin Film Electrodes: Investigation of Interfacial Ion Exchange Behavior, Journal of Composites Science 5 (2021) 25.
- S. Banerjee, B. De, P. Sinha, J. Cherusseri, K.K. Kar, Applications of Supercapacitors, in: K.K. Kar (Ed.), Handbook of Nanocomposite Supercapacitor Materials I: Characteristics, Springer International Publishing, Cham, 2020, pp. 341-350.
- S.B. Devi, V. Vignesh, P.V. Kumar, M.S. Oh, R. Navamathavan, Chapter 19 - Transport supercapacitors, in: C.M. Hussain, M.B. Ahamed (Eds.), Smart Supercapacitors, Elsevier2023, pp. 503-534.
- B. Evanko, S.W. Boettcher, S.J. Yoo, G.D. Stucky, Redox-Enhanced Electrochemical Capacitors: Status, Opportunity, and Best Practices for Performance Evaluation, ACS Energy Letters 2 (2017) 2581-2590.
- B.K. Kim, S. Sy, A. Yu, J. Zhang, Electrochemical Supercapacitors for Energy Storage and Conversion, Handbook of Clean Energy Systems, John Wiley & Sons, Ltd2015.
- Z. Zhai, L. Zhang, T. Du, B. Ren, Y. Xu, S. Wang, J. Miao, Z. Liu, A review of carbon materials for supercapacitors, Materials & Design 221 (2022) 111017.
- R. Lin, FORMULATION OF ELECTROLYTES BASED ON IONIC LIQUIDS FOR SUPERCAPACITOR APPLICATIONS. Ph. D. Thesis., Sciences de la Matiére, Université de Toulouse, 2012, pp. 202.
- L. BENHADDAD, Elaboration et caractérisation de poudres nanostructurées de MnO2 et de polypyrrole : Application comme materiaux delectrodes dans des dispositifs de stockage de lenergie. Ph.D. Thesis., Chimie Physique et Chimie Analytique de Paris-Centre, UNIVERSITE PIERRE ET MARIE CURIE, 2014, pp. 217.
- T. Shiferaw, Investigation of the interfaces of solid electrolyte based supercapacitors and batteries. Ph.D. Thesis., Technischen Fakultät der Christian-Albrechts, Universität zu Kiel, 2008.
- M.A.A. Mohd Abdah, N.H.N. Azman, S. Kulandaivalu, Y. Sulaiman, Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors, Materials & Design 186 (2020) 108199.
- A.G. Olabi, Q. Abbas, M.A. Abdelkareem, A.H. Alami, M. Mirzaeian, E.T. Sayed, Carbon-Based Materials for Supercapacitors: Recent Progress, Challenges and Barriers, Batteries 9 (2023) 19.
- H. Pan, J. Li, Y. Feng, Carbon Nanotubes for Supercapacitor, Nanoscale Research Letters 5 (2010) 654 - 668.
- C. Zhou, S. Kumar, C.D. Doyle, J.M. Tour, Functionalized Single Wall Carbon Nanotubes Treated with Pyrrole for Electrochemical Supercapacitor Membranes, Chemistry of Materials 17 (2005) 1997-2002.
- Y.K. Zhou, B.L. He, W.J. Zhou, H.L. Li, Preparation and Electrochemistry of SWNT/PANI composite films for electrochemical capacitors, Journal of the Electrochemical Society 151 (2004) A1052-A1057.
- F. Béguin, V. Presser, A. Balducci, E. Frackowiak, Carbons and Electrolytes for Advanced Supercapacitors, Advanced Materials 26 (2014) 2219-2251.
- M. Armand, F. Endres, D.R. MacFarlane, H. Ohno, B. Scrosati, Ionic-liquid materials for the electrochemical challenges of the future, Nat Mater 8 (2009) 621-629.
- R. Lin, P.-L. Taberna, S. Fantini, V. Presser, C.R. Pérez, F. Malbosc, N.L. Rupesinghe, K.B.K. Teo, Y. Gogotsi, P. Simon, Capacitive Energy Storage from −50 to 100 °C Using an Ionic Liquid Electrolyte, The Journal of Physical Chemistry Letters 2 (2011) 2396-2401.
- B. Alresheedi, SUPERCAPACITORS BASED ON CARBON NANOTUBE FUZZY FABRIC STRUCTURAL COMPOSITES. Ph.D. Thesis., School of Engineering, UNIVERSITY OF DAYTON, 2012.
- J. SEGALINI, Etude de l’adsorption des ions dans des carbones microporeux ; application aux supercondensateurs. Ph.D. Thesis., Sciences de la Matiére Université de Toulouse 2012.
- F. Yao, D.T. Pham, Y.H. Lee, Carbon-Based Materials for Lithium-Ion Batteries, Electrochemical Capacitors, and Their Hybrid Devices, ChemSusChem (2015) n/a-n/a.
- D. Majumdar, T. Maiyalagan, Z. Jiang, Recent Progress in Ruthenium Oxide-Based Composites for Supercapacitor Applications, ChemElectroChem 6 (2019) 4343-4372.
- C.R. Arias, C. Debiemme-Chouvy, C. Gabrielli, C. Laberty-Robert, A. Pailleret, H. Perrot, O. Sel, New Insights into Pseudocapacitive Charge-Storage Mechanisms in Li-Birnessite Type MnO2Monitored by Fast Quartz Crystal Microbalance Methods, The Journal of Physical Chemistry C 118 (2014) 26551-26559.
- A. Laforgue, P. Simon, J.F. Fauvarque, Chemical synthesis and characterization of fluorinated polyphenylthiophenes: application to energy storage, Synth. Met. 123 (2001) 311-319.
- K. Naoi, S. Suematsu, A. Manago, Electrochemistry of poly(1,5-diaminoanthraquinone) and its application in electrochemical capacitor materials, J. Electrochem. Soc. 147 (2000) 420-426.
- M. Conte, Supercapacitors Technical Requirements for New Applications, Fuel Cells 10 (2010) 806-818.
- M.S.E. Halper, James C., Supercapacitors: A Brief Overview, The MITRE Corporation, McLean, Virginia, USA., 2006.
- C. Merlet, Modelisation de ladsorption des ions dans les carbones nanoporeux. Ph.D. Thesis., Theoretical and physical chemistry, Université Pierre et Marie Curie, 2013.
- P. Odru, Le stockage de lenergie, Paris, France, 2013.
- Z. Cao, B. Wei, A perspective: carbon nanotube macro-films for energy storage, Energy & Environmental Science 6 (2013) 3183-3201.
- A. Imani, G. Farzi, Facile route for multi-walled carbon nanotube coating with polyaniline: tubular morphology nanocomposites for supercapacitor applications, Journal of Materials Science: Materials in Electronics 26 (2015) 7438-7444.
- Y. Zhou, H. Xu, N. Lachman, M. Ghaffari, S. Wu, Y. Liu, A. Ugur, K.K. Gleason, B.L. Wardle, Q.M. Zhang, Advanced asymmetric supercapacitor based on conducting polymer and aligned carbon nanotubes with controlled nanomorphology, Nano Energy 9 (2014) 176-185.
- M. Tahir, L. He, W.A. Haider, W. Yang, X. Hong, Y. Guo, X. Pan, H. Tang, Y. Li, L. Mai, Co-Electrodeposited porous PEDOT–CNT microelectrodes for integrated micro-supercapacitors with high energy density, high rate capability, and long cycling life, Nanoscale 11 (2019) 7761-7770.
- Y. Chen, L. Du, P. Yang, P. Sun, X. Yu, W. Mai, Significantly enhanced robustness and electrochemical performance of flexible carbon nanotube-based supercapacitors by electrodepositing polypyrrole, Journal of Power Sources 287 (2015) 68-74.
- C. Fu, H. Zhou, R. Liu, Z. Huang, J. Chen, Y. Kuang, Supercapacitor based on electropolymerized polythiophene and multi-walled carbon nanotubes composites, Materials Chemistry and Physics 132 (2012) 596-600.
- M. El-Shahat, M. Mochtar, M.M. Rashad, M.A. Mousa, Single and ternary nanocomposite electrodes of Mn3O4/TiO2/rGO for supercapacitors, Journal of Solid State Electrochemistry 25 (2021) 803-819.
- S. Satpathy, S. Debbarma, B. Kumar Bhattacharyya, An integration of the review of electrode’s materials and a new gamma function-based charging methodology of supercapacitor for high current applications, Materials Today: Proceedings 26 (2020) 2151-2156.
- I. Shown, A. Ganguly, L.-C. Chen, K.-H. Chen, Conducting polymer-based flexible supercapacitor, Energy Science & Engineering 3 (2015) 2-26.
- D. Lemian, F. Bode, Battery-Supercapacitor Energy Storage Systems for Electrical Vehicles: A Review, Energies 15 (2022) 5683.
- F. Escobar-Teran, A. Arnau, J.V. Garcia, Y. Jiménez, H. Perrot, O. Sel, Gravimetric and dynamic deconvolution of global EQCM response of carbon nanotube based electrodes by Ac-electrogravimetry, Electrochemistry Communications 70 (2016) 73-77.
- S. Sigalov, M.D. Levi, G. Salitra, D. Aurbach, J. Maier, EQCM as a unique tool for determination of ionic fluxes in microporous carbons as a function of surface charge distribution, Electrochemistry Communications 12 (2010) 1718-1721.
- J.M. Griffin, A.C. Forse, W.-Y. Tsai, P.-L. Taberna, P. Simon, C.P. Grey, In situ NMR and electrochemical quartz crystal microbalance techniques reveal the structure of the electrical double layer in supercapacitors, Nat Mater advance online publication (2015).
- F. Escobar-Teran, H. Perrot, O. Sel, Ion Dynamics at the Single Wall Carbon Nanotube Based Composite Electrode/Electrolyte Interface: Influence of the Cation Size and the Electrolyte pH, The Journal of Physical Chemistry C (2019).
- H. Goubaa, F. Escobar-Teran, I. Ressam, W. Gao, A. El Kadib, I.T. Lucas, M. Raihane, M. Lahcini, H. Perrot, O. Sel, Dynamic Resolution of Ion Transfer in Electrochemically Reduced Graphene Oxides Revealed by Electrogravimetric Impedance, The Journal of Physical Chemistry C 121 (2017) 9370-9380.
