Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ligeng Ma and Version 2 by Peter Tang.
Gene expression can be regulated through transcriptional and post-transcriptional mechanisms. Transcription in eukaryotes produces pre-mRNA molecules, which are processed and spliced post-transcriptionally to create translatable mRNAs. More than one mRNA may be produced from a single pre-mRNA by alternative splicing (AS); thus, AS serves to diversify an organism’s transcriptome and proteome.
- gene expression
- alternative splicing
- transcriptional regulation
- environmental fitness
- plant
1. Introduction
Nearly 90% of the protein-coding genes in plants are interrupted genes; that is, the coding region is divided by introns. Therefore, an essential step in gene expression is the removal of introns through the splicing of precursor mRNA transcripts (pre-mRNAs) [1][2][1,2]. Splicing is performed by the spliceosome, a large ribonucleoprotein (RNP) complex that assembles around splice sites in the introns of a pre-mRNA molecule and then removes the introns catalytically through sequential phosphodiester transfer reactions [3][4][5][6][3,4,5,6]. During splicing, 5′ and 3′ splice sites, which mark the beginning and end of each intron in a pre-mRNA, together with the branch site (a consensus sequence-containing region located near the 3′ splice site) are recognized by the uridine (U)-rich small nuclear RNPs (snRNPs) U1, U2, U4, U5, and U6, and a multitude of non-snRNP splicing factors, including U2AF65, U2AF35, and serine/arginine-rich (SR) proteins [7]. Together, these factors form one of the most complex machines in cells, the spliceosome, which performs the two transesterification reactions that are necessary to excise introns and join together the selected exons [8]. Spliceosome assembly at an intron is a highly ordered and dynamic process that is guided by consensus sequences [9].
Genome-wide transcriptome mapping has revealed that the extent of alternative splicing (AS) in plants ranges from 42% to 61% [10][11][12][10,11,12]. In AS, a single pre-mRNA can produce more than one mRNA through the use of alternative splice sites. AS is a regulated process that increases the diversity of an organism’s transcriptome and proteome. It can regulate transcript levels by producing new unstable mRNA isoforms, which can be degraded by nonsense-mediated decay (NMD) [13][14][13,14]. AS can also produce alternate functional mRNAs encoding protein isoforms with differences in subcellular localization, stability, or function by changing or completely removing functional domains via the introduction of a premature termination codon (PTC), intron retention, or alternative 3′ or 5′ splice site selection [15]. New isoforms of transcripts, proteins, or even polypeptides may act as dominant-negative inhibitors of the authentic proteins by means of interactions with dimerization partners [16].
2. Ambient Temperature and Vernalization-Mediated Flowering
Higher plants go through numerous developmental transitions during their life cycle. Among these transitions, the floral transition is the best studied. Analyses of the regulation of flowering in Arabidopsis have demonstrated how environmental signals and endogenous cues may be integrated to create a developmental switch in plants [17]. In recent decades, key regulators of flowering time and their targets have been described in Arabidopsis. To determine the right moment for flowering, plants utilize many environmental signals, including photoperiod (day length), light quality, biotic/abiotic stresses, and temperature. Temperature in particular has been shown to have tremendous effects on the timing of flowering; in the vernalization response of Arabidopsis thaliana, plants quantitatively sense long-term cold exposure and epigenetically store this information to regulate flowering time [18]. In contrast, theour knowledge of the genetic and molecular mechanisms that regulate flowering time in response to small changes in ambient temperature (i.e., the thermosensory pathway) is limited. Evidence indicates that AS plays an important role in the ability of plants to measure ambient temperatures and to integrate this external information with endogenous signals to regulate flowering [19][20][19,20]. In A. thaliana, the central mechanism of accelerated flowering in response to prolonged cold exposure is repression of the transcription of FLOWERING LOCUS C (FLC), which encodes a MADS-box transcription factor and negative regulator of flowering. MADS-domain transcription factors have important roles in the development of plants throughout their life cycle, including flowering [21]. FLC expression is regulated by several pathways: the FRIGIDA pathway, which up-regulates FLC expression; the autonomous pathway, which down-regulates FLC expression; the vernalization pathway, a cold-induced epigenetically silenced FLC expression during winter [22]. COOLAIR is a non-coding RNA, which fully encompasses FLC in the antisense direction [23]. The level of FLC expression is associated with the level of COOLAIR splicing isoforms. Class I COOLAIR variants, which links with low level of FLC expression, uses a small intron and polyadenylation at a proximal region. Class II COOLAIR variants, linking with high level of FLC expression, is associated with a large intron and polyadenylation at a distal region [24][25][24,25]. PRP8 is a conserved and central component of the spliceosome, mutation in PRP8 influences the splicing efficiency of COOLAIR introns, which reduces COOLAIR proximal poly(A) site usage [25]. Furthermore, the splicing of COOLAIR plays an important role in the adaptive evolution of flowering time in Arabidopsis thaliana accessions. SNP259, a single natural intronic polymorphism located in FLC, can significantly change COOLAIR splicing, further affecting FLC expression and flowering time. SNP259 is a major contributor to the natural variation in FLC haplotypes [26]. In addition, long noncoding RNA not only acts as AS target, but is also involved in regulating AS. AS competitor long noncoding RNA (ASCO-lncRNA) can hijack nuclear speckle RNA-binding protein (NSR) to affect the splicing patterns of several NSR-regulated mRNA targets that specifically promote lateral root growth in Arabidopsis [27]. SHORT VEGETATIVE PHASE (SVP) and FLOWERING LOCUS M (FLM) also belong to MADS-box proteins. FLM is subject to temperature-dependent AS. Two splice variants, FLM-β and FLM-δ, which differ in the incorporation of either the second or third cassette exon and can be translated into two protein isoforms, have been detected in the Columbia-0 accession [28]. Interestingly, the ratio of FLM-β to FLM-δ changes in response to deviations in ambient temperature [29]. FLM-β is down-regulated in response to increases in temperature, suggesting that FLM-β and FLM-δ have different roles in the control of flowering by ambient temperature [30]. However, the biological functions of FLM-β/δ are unclear. Recent discoveries have revealed a combinatorial role for FLM and SVP in flowering, with ambient temperature modulating both factors in different ways. FLM-β and FLM-δ encode proteins that interact antagonistically with SVP. FLM-β was demonstrated to bind DNA only in the presence of SVP in vitro; however, FLM-δ could not bind DNA in vitro, thus, it prevented SVP from binding the target DNA in a dose-dependent manner [19][20][19,20]. These results indicate that FLM-β and -δ protein levels mirror those of their corresponding mRNAs, which are regulated by ambient temperature changes. These results suggest a working model in which FLM-δ acts as a dominant-negative isoform of FLM that renders the SVP-FLM-β complex inactive, thereby indirectly promoting the flowering transition at elevated temperatures [19][20][19,20]. Similar to FLM/MAF1, the MADS AFFECTING FLOWERING 2 (MAF2) gene, which is closely related to FLM, is subjected to temperature-dependent AS to produce two isoforms, var1 and var2. Only MAF2 var1 encodes a functional full-length MIKC-type MADS-domain transcription factor that is sufficient to repress flowering when overexpressed in A. thaliana accession Ll-2, which does not express MAF1–MAF4 [31][32][31,32]. Similar to FLM, the repressive isoform var1 is regulated by temperature-dependent AS. In addition, plants overexpressing MAF2 var2 are not early flowering, suggesting that var2 does not act as a dominant-negative protein, as is the case for FLM-δ. In summary, AS functions as a “thermometer” in plants, measuring moderate changes in ambient temperature (Figure 1). Although the effects of different splice variants (e.g., FLM-β/δ and MAF2 var1/2) on flowering are clear, the exact mechanism by which temperature-dependent AS modulates the onset of flowering is poorly understood. Furthermore, ambient temperature-responsive splicing factors that regulate AS have not yet been discovered.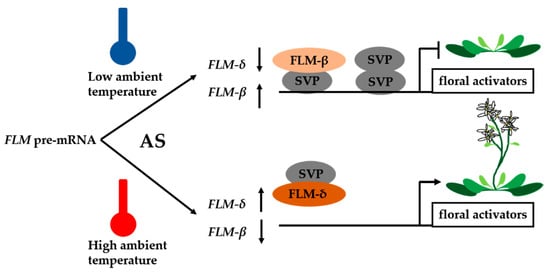
Figure 1. Model of the temperature-dependent FLOWERING LOCUS M (FLM) function. At low temperature, SHORT VEGETATIVE PHASE (SVP) can form homodimer or interact with FLM-β to form heterodimer to repress floral activators. When the temperature increased, the alternative splicing of FLM pre-mRNA increased the level of FLM-δ. FLM-δ proteins compete with the FLM-β to form FLM-δ-SVP complex and repress the transcription of floral activator genes. AS, alternative splicing.
3. The Circadian Clock in Plants
The circadian clock, an endogenous timekeeper that generates rhythms with an approximately 24 h period, plays critical roles in diverse aspects of plant growth and development, and coordination of biological processes with daily environmental cycles [33][34][33,34]. It was reported that the expression of about 30% of the genes in Arabidopsis genome is controlled under the circadian clock [35][36][37][35,36,37]. The circadian clock is composed of several interlocking regulatory feedback loops in Arabidopsis [38]. CIRCADIAN CLOCK-ASSOCIATED1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) repress the expression of TIMING OF CAB EXPRESSION 1 (TOC1) through direct binding to its promoter [39][40][39,40]. Recent studies have shown that TOC1 binds directly to specific morning elements in the promoters of CCA1 and LHY as a transcriptional repressor and suppresses CCA1/LHY accumulation [41][42][43][41,42,43]. In the morning loop, CCA1 and LHY directly activate the expression of PSEUDO RESPONSE REGULATOR7 (PRR7) and PRR9, two homologs of TOC1; in turn, PRR7 and PRR9 suppress the expression of CCA1 and LHY [44][45][44,45]. In the evening loop, TOC1 represses the expression of GIGANTEA (GI), while the expression of TOC1 is up-regulated by GI [46]. The proteins encoded by EARLY FLOWERING (ELF)3, ELF4, and LUX ARRHYTHMO form the so-called evening complex (EC), which suppresses the expression of TOC1, GI, and PRR9 [47][48][47,48]. Together, these interlocking central, morning, and evening loops form the basis of the circadian clock in Arabidopsis. To date, most studies of the regulation of the circadian clock in both animals and plants have focused on the transcriptional and post-translational regulatory mechanisms. The roles of AS in the regulation of the circadian clock have been uncovered recently. Through whole-genome sequencing and RT-PCR analysis, two CCA1 transcripts, CCA1α and CCA1β, have been reported [49][50][49,50]. CCA1β, an alternatively spliced variant of CCA1 that retains the fourth intron, is conserved in the dicot tree Populus and the monocot grasses Oryza and Brachypodium, suggesting its functional importance; in addition, CCA1β is accumulated under high light conditions but decreased in cold conditions. The roles of these variants in regulating the circadian clock have been recently reported [50]. CCA1β has a dimerization domain, like CCA1α, but lacks the N-terminal DNA-binding MYB motif [50][51][50,51]. Homodimerization and heterodimerization of CCA1α and LHY are required for these proteins to regulate circadian rhythms [52]. The splice variant CCA1β, as a dominant regulator, represses CCA1α/LHY heterodimerization by competing with CCA1α and LHY to form nonfunctional CCA1α/CCA1β and CCA1β/LHY heterodimers, revealing the regulatory role of the AS of CCA1 in circadian rhythms [50]. Recent research showed that SR45 bound specifically to the retained CCA1 intron in vitro, suggesting that SR45 is involved in the regulation of intron splicing [53]. Protein arginine methyltransferase 5 (AtPRMT5) has been shown to regulate circadian period in Arabidopsis. PRMT5, a type II protein arginine methyltransferase, catalyzes the methylation of diverse non-histone proteins, including components of the spliceosome (e.g., AtSmD1 and AtLSm4); the reduction in the methylation levels of SmD1 and LSm4 causes the splicing defects in genes involved in multiple biological processes, including the circadian clock, probably by regulating 5′ splice site recognition [54][55][54,55]. The circadian period is lengthened by mutations in AtPRMT5 [56]. Atprmt5 exhibits defects in the AS of PRR7 and PRR9, resulting in lengthening of the circadian period; thus, AS regulates the circadian clock at the post-transcriptional level [54]. LSM4 and LSM5, which encode core components of the spliceosomal U6 snRNP complex, can be methylated by PRMT5. lsm4 and lsm5 plants display a long period phenotype as well as aberrant splicing of several clock genes, including CCA1 and TOC1, but not PRR9, suggesting that the effect of PRMT5 on the circadian clock is not simply due to its effect on LSM4. Besides PRMT5, which indirectly affects the splicing of clock genes, the involvement of splicing factors in the circadian clock control has been demonstrated [57][58][57,58]. The Ski-interacting protein (SKIP) is the first splicing factor that is required for the regulation of circadian clock. SKIP is a conserved component of the precursor RNA processing (Prp)19 complex, a sub-complex of spliceosome complexes B and C, which are required for catalysis of the first and second steps in pre-mRNA splicing in yeast and human cells [59]. The mutation of SKIP has dramatic effects on the circadian clock in Arabidopsis. For example, the skip-1 mutant exhibits a temperature-sensitive long period phenotype and changes in light input and clock sensitivity to resetting by light [57]. Consistent with the role of SKIP in mammals (SKIP) and yeast (Prp45), AtSKIP encodes a conserved SNW domain-containing protein. AtSKIP co-localizes in nuclear speckles with the spliceosome components U1-70k [60] and SR45 [61], and it associates with the pre-mRNAs of clock genes, including PRR7 and PRR9 [57][62][57,62]. Defects in the AS of PRR7 and PRR9 partially contribute to the lengthened period of the clock in the skip-1 mutant [57][62][57,62]. Similarly, the RNA-binding protein SPLICEOSOME TIMEKEEPER LOCUS 1 (STIPL1) is homologous to the spliceosomal proteins Ntr1p in Saccharomyces cerevisiae and TFP11 in humans, which are required for spliceosome disassembly. The mutation of STIPL1 increased levels of the intron-retained variants of CCA1, LHY1, TOC1, PRR9, and GI, and this may contribute to the observed clock phenotype [58], indicating the requirement of STIPL1 for the correct splicing of clock genes. The spliceosomal snRNP assembly factor GEM NUCLEAR ORGANELLE ASSOCIATED PROTEIN 2 (GEMIN2) plays an important role in modulating the effect of low temperatures on the splicing of certain pre-mRNAs (e.g., TOC1 and PRRs), and it attenuates the effects of temperature on the period length of the circadian clock [63]. Recently, another regulator, SICKLE (SIC), a conserved proline/serine-rich protein found in nuclear foci, emerged as a link between the circadian clock, temperature compensation, and AS. The sic-3 mutant exhibits defects in the clock genes, such as arrhythmic or low-amplitude expression of several core circadian clock genes under cool ambient temperature cycles, but no defect was observed under light-dark entrainment. Additionally, sic exhibits increased levels of splice variants of LHY, ELF3, CCA1, and PRR7. Further, compared to wild type, sic has a broader range of temperature conditions under which these splice variants occur, particularly at cool temperatures [64]. The above mentioned results indicated that AS is essential for normal functioning of the circadian clock in Arabidopsis (Figure 2). The circadian oscillator plays an important role in the interaction between plants and their environment by synchronizing endogenous biological activities, biochemical processes, and behavior with daily environmental changes in the day-night cycle. Interlocking transcription-translation feedback loops form the basis for regulation of the clock. Recent studies also showed that AS is required for the regulation of the circadian clock in Drosophila, and Neurospora, suggesting that AS, a key step in post-transcriptional gene expression regulation, is a general mechanism of clock regulation. However, the detailed molecular mechanisms of AS that regulate the circadian clock, how the circadian clock regulates AS in response to external environment changes, and how endogenous developmental processes affect the circadian clock via AS are far from clear. The spliceosome has been described as a sea of proteins; indeed, more than 200 components have been purified from the mammalian spliceosome [65]. However, only a small proportion of them have been functionally characterized. Future studies should identify the roles of splicing factors or AS in the regulatory network of the circadian clock.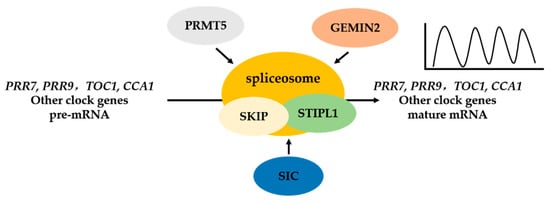
Figure 2. Components of spliceosome and splicing regulators mediate alternative splicing of the circadian clock genes in Arabidopsis. The Ski-interacting protein (SKIP), SPLICEOSOME TIMEKEEPER LOCUS 1 (STIPL1) are components of the spliceosome. Protein arginine methyltransferase 5 (PRMT5), GEM NUCLEAR ORGANELLE ASSOCIATED PROTEIN 2 (GEMIN2), SICKLE (SIC) are regulators of spliceosome. These splicing factors are involved in regulating the circadian clock through alternative splicing of clock genes pre-mRNAs, such as PRR7, PRR9, TOC1, CCA1. These studies suggest that pre-mRNA splicing, a post-transcriptional gene expression regulation, is a new means of circadian clock regulation.
4. Abiotic Stress Responses
Given their sessile nature, plants are dependent on their immediate environment for survival, and the growth and development of plants are heavily affected by environmental cues [66][67][68][66,67,68]. AS in response to abiotic/biotic stresses has a wide range of effects on plants, and most genes involved in plant stress responses are said to be regulated by AS. Heat shock (HS) transcription factors (Hsfs) are key regulators of the response of plants to heat stress; thus, HS-induced transcriptional regulation has been extensively studied [69]. Recently, AS has been shown to be critical for the HS-inducible expression of HsfA2. HsfA2 contains a single 324-nucleotide intron that is fully spliced at 22 °C to generate the full-length HsfA2 transcript [70]. Moderate heat (37 °C) activates a 31-nucleotide cryptic miniexon within the HsfA2 intron to generate a splice variant HsfA2-II. HsfA2-II contains a PTC within the miniexon, thus is degraded by NMD. The third splice variant HsfA2-III is generated through the cryptic 5′ splice site in the intron, which is activated by extreme heat (42–45 °C), while HsfA2-II decreases [71]. HsfA2-III encodes a truncated protein, S-HsfA2, which localizes to the nucleus; it contains an Hsf helix-turn-helix DNA binding motif, and can bind to HS elements in the HsfA2 promoter, generating a positive autoregulatory loop that controls HsfA2 expression through AS. The maize gene ZmrbohB is required for the production of reactive oxygen species (ROS) in response to avirulent pathogens and several abiotic stresses. ROS levels need to be finely tuned to prevent toxicity, and to support their activity as signaling molecules [72][73][72,73]. ZmrbohB has two alternatively spliced isoforms, ZmrbohB-δ and ZmrbohB-β (which retains intron 11); these isoforms carry a PTC that probably leads to NMD in response to several abiotic stimuli, including cold, heat, ultraviolet radiation, and salt stress [74]. The splicing of some transcription factor genes also undergo AS under stressful conditions. For example, two splice variants of OsDREB2B are differentially expressed in response to heat and drought stresses in rice [75]. The transcript of OsDREB2B1 is more abundant under normal growth conditions; it contains a 53-base pair exon 2 insertion in the mature mRNA that introduces an open reading frame shift. In plants exposed to high temperature stress, OsDREB2B2, in which exon 2 is spliced out in the mature mRNA, dominates. Through AS, rice can produce dehydration-responsive element-binding protein2 (DREB2B) rapidly, independentally of transcriptional activation. A similar mechanism of AS has been described for WDREB2 in wheat, and for its orthologs HvDRF1 in barley and ZmDREB2A in maize [76][77][78][76,77,78]. The splicing of non-protein coding RNA transcripts is an emerging area of study, specifically in plant systems. The first example of environmental changing-induced AS is from the expression of miR400. miR400 is located in the intron of a protein-coding gene. Heat stress-induced AS of a transcript that contains this intron results in decreased production of the mature miRNA by affecting miRNA processing. The altered miR400 level in turn changes the level of its host transcript [79]. A number of RNA processing factors is associated with plant responses to abscisic acid (ABA). RNA binding motif protein 25 (RBM25) binds to the last intron in HAB1 pre-mRNA and regulates its AS to produce two splice variants: HAB1.1 and HAB1.2. HAB1.1 contains four exons and encodes a protein that interacts with and inhibits the kinase activity of SnRK2.6/OST1, switching ABA signaling off. HAB1.2 contains four exons and the last intron; it encodes a truncated protein lacking 105 amino acids at the C-terminal end. HAB1.2 is able to interact with SnRK2/OST1, but it cannot inhibit the protein’s kinase activity; thus, ABA signaling remains on [80][81][80,81]. Therefore, the alternative splicing of HAB1 pre-mRNA results in the production of two variants of HAB1 mRNA, and translates to two functional antagonistic proteins in ABA signaling pathway. Thus, AS for genes encoding the components of ABA signaling pathway is critical for ABA function. In addition to the induction of AS by environmental stress, evidence shows that spliceosomal proteins play crucial roles in the proper function of abiotic stress response pathways in Arabidopsis. SR proteins bind splicing signal sites and intronic and exonic splicing enhancer/silencer sequences through interactions with multicomponent splicing factors to determine splice site selection and where the spliceosome assembles. SR45 is an SR-like protein with two SR domains. The mutation of SR45 in Arabidopsis produces pleiotropic developmental defects, including altered leaf and flower morphology, delayed root growth, late flowering, [82], and defects in ABA and glucose signaling [83], resulting in dramatic genome-wide changes in the AS of pre-mRNAs [84]. Interestingly, AS generates two SR45 transcripts. SR45.1 contains a 21-nucleotide sequence that is absent from SR45.2 due to the selection of an alternative 3′ splice site in intron 6 in SR45.1 [85][86][85,86]. These two splice variants encode similar proteins, differing by only eight amino acids, which include several putative phosphorylation sites. Remarkably, complementation studies showed that SR45.1 rescued the floral but not the root phenotype in sr45-1, while SR45.2 could complement the root growth defect observed in sr45-1 [86]. By contrast, AS of SR45 does not likely play a role in sugar signaling, as both splice forms were able to rescue the glucose hypersensitivity of the sr45-1 [84].5. Biotic Stress Responses
Plants are often attacked by a variety of pathogens, including fungi, viruses, and bacteria, as well as by insects and nematodes. Thus, plants have developed a number of defense mechanisms against these pathogens during evolution. Resistance (R) proteins are crucial proteins for plant defense against pathogens. The AS of R genes plays crucial roles in the regulation of plant defense responses at the post-transcriptional level [87][88][89][87,88,89]. Most R genes in plants encode the nucleotide-binding site (NBS) leucine-rich repeat (LRR) proteins which are characterized by containing NBS and LRR domains [90][91][92][93][94][90,91,92,93,94]. The coding region of most TIR-NBS-LRR genes contains three or four extrons. Among them, the first exon encodes the TIR domain, the second exon encodes the NBS domain, and the remaining exons encode the LRR region. Alternative isoforms have been observed from many TIR-NBS-LRR genes, including tobacco (Nicotiana tabacum) N and Bs4, Arabidopsis RAC1, RPS4, RPS6, RPP5, and SNC1 [95][96][97][98][99][100][101][95,96,97,98,99,100,101]. The tobacco N gene confers resistance to the tobacco mosaic virus (TMV). AS produces two transcript variants: a short NS transcript encoding the functional N protein and a long NL transcript containing an alternative 70-nucleotide exon within the third intron that leads to a frame shift and PTC. Thus, the long NL transcript encodes a truncated protein that lacks most of the LRRs. Before infection, NS is prevalent. At 4–8 h after TMV infection, however, the level of NL is 60-fold higher than that of NS. Plants overexpressing only NS show little resistance to TMV. Thus, the alternative exon in intron 3 is required for full resistance to TMV in tobacco [102]. Similar to the tobacco N gene, Arabidopsis RPS4 confers resistance to Pseudomonas syringae pv. tomato strain DC3000 (DC3000) expressing AvrRps4; however, this resistance is regulated by AS. Due to premature stop codons, the alternatively spliced isoforms encode no or a reduced number of LRRs. Transformation of the genomic sequence lacking intron 2 or 3 driven by the RPS4 promoter into rps4 revealed that the deletion any of the introns was sufficient to abolish the function of RPS4. Therefore, AS of RPS4 is required for Arabidopsis to resistance to DC3000 [99][103][99,103]. AS produces different transcript isoforms, and thus produces protein variants which may contain a combination of the TIR and NBS domains or only the TIR domain [104]. Screening for suppressors of the gain-of-function mutant suppressor of npr1-1 constitutive1 (snc1) in Arabidopsis identified a series of modifier Of snc1 (MOS) genes, some of which encode subunits of a splicing-associated protein complex, including modifier Of snc1, 4 (MOS4), cell division cycle 5 (CDC5), and pleiotropic regulatory locus 1 (PRL1) [105]. Interestingly, their homologs in humans and yeast are components of the Prp19 complex, which is essential for catalytic activation of the spliceosome [106]. MOS4-associated complex (MAC)3A and MAC3B, two closely related proteins with sequence homology to Prp19, were identified by immunoprecipitation followed by mass spectrometry. Reported defects in the AS of SNC1 in mos4, cdc5, and mac3a mac3b demonstrate that MAC mediates the AS of R genes and influences plant defenses [107]. MAC5A is also involved in pathogen defense [108]. The MAC5A counterpart in humans is RNA-binding motif protein 22, which interacts with U6 small nuclear RNA and pre-mRNA and participates in splicing. This suggests that MAC5A is also involved in pre-mRNA splicing in Arabidopsis [109]. All the results above suggest that AS is required for plants in response to biotic stresses.6. Splicing Factors or Transcriptional Co-Regulators in Plants
Many splicing factors are not only components of the spliceosome that participate in pre-mRNA splicing, they can also interact with other proteins to form complexes that regulate different biological processes. Recent research has demonstrated that some plant splicing factors play an important role in transcriptional regulation. SKIP is a spliceosome component that interacts with the pre-mRNAs of circadian clock genes and is essential for regulating their AS [57]. Still, SKIP is not involved in the splicing of sense or anti-sense FLC pre-mRNA. However, the levels of mature and unspliced FLC mRNAs were found to be obviously repressed in skip-1. Yeast two-hybrid screening showed that SKIP interacts with ELF7, a component of the Paf1 complex (Paf1c), which is conserved from yeasts to humans and plants [110][111][110,111]. The Paf1c is required for the recruitment of histone modification factors and chromatin remodeling factors, and for small RNA-mediated gene silencing [112][113][112,113]. In Arabidopsis, the Paf1c represses the floral transition by activating FLC transcription [110]. The Paf1c modulates the expression of FLC clade genes at the transcriptional level by binding to and mediating H2B mono-ubiquitination (H2Bub1) and the tri-methylation of lysine 4 on histone H3 (H3K4me3) of FLC clade gene chromatin in Arabidopsis [111]. The results of a chromatin immunoprecipitation assay suggested that SKIP and the Paf1c are required for the H2Bub1 and H3K4me3 of FLC clade gene chromatin [111], supporting the proposed interaction between SKIP and the Paf1c in the regulation of flowering in Arabidopsis. Therefore, SKIP appears to be involved in two complexes. It can function as a splicing factor that ensures the accurate splicing of pre-mRNAs on a genome-wide scale by interacting with other components of the spliceosome, and as a transcriptional activator that interacts with other transcriptional regulators (e.g., the Paf1c) to regulate the expression of specific genes at the transcriptional level [62][111][62,111] (Figure 3). However, whether the function of these two complexes is independent or coupled through SKIP is unknown [111].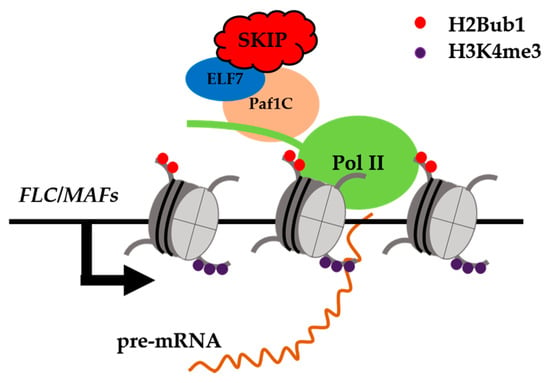
Figure 3. SKIP interacts directly with EARLY FLOWERING 7 (ELF7) to promote FLC/MADS AFFECTING FLOWERING (MAFs) transcription. SKIP is a transcriptional activator that interacts with the ELF7, a component of the Pol II associated factor 1 complex (Paf1c), to regulate the levels of H2B monoubiquitination (H2Bub1) and H3K4 trimethylation (H3K4me3) in FLC clade gene chromatin. SKIP represses the floral transition mainly by activating the transcription of FLOWERING LOCUS C (FLC) clade genes in Arabidopsis.
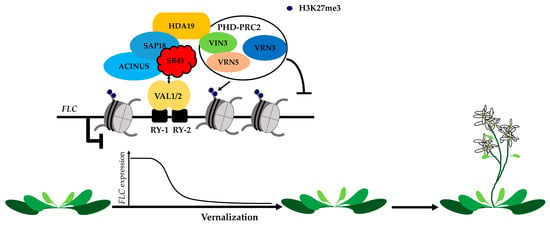
Figure 4. Apoptosis- and splicing-associated protein (ASAP) complex is involved in Polycomb silencing of FLC expression during vernalization. During vernalization, VAL1/2 bind the RY-1/2 cis-elements in the first intron of FLC, interact with components of the conserved ASAP complex, which consists of SIN3-associated protein 18 (SAP18), ACINUS and SR45, and further recruit PHD-PRC2. This transcriptional repression complex triggers histone deacetylation in FLC chromatin and leads to FLC transcriptional silencing in a sequence specific manner during vernalization. HDA: histone deacetylase. PRC2: polycomb repressive complex 2. VIN3: vernalization insensitive 3. VRN: vernalization. VAL: viviparous1/ABI3-like.
