Despite the relatively wide availability of the numerous, above-listed conservative management options, surgical procedures still remain the basic treatment form for uterine fibroids. The spectacular development of laparoscopic technology has led to the vast majority of leiomyoma open operations being carried out via laparoscopy. This change in surgical approach is supported by the well-known advantages of laparoscopy, such as minimal traumatizing of the abdominal wall, excellent cosmetic effect, and a quick return to health and full physical activity. Except for cases of total laparoscopic hysterectomy followed by transvaginal removal of the uterus, all the remaining laparoscopic operations related to leiomyomas include sharp fragmentation of the specimen. This technique, called power morcellation, was introduced by Steiner and first described in 1993
[17]. Since then, many types of morcellators have been developed and have become an integral element of the laparoscopic instrumentarium. Recently, mechanical intraperitoneal morcellation has become the subject of heated discussion because of the risk of intraabdominal dissemination of preoperatively undiagnosed malignant tumors
[18][19][18,19]. The true incidence of uterine sarcoma developing from fibroma remains unknown. In myomectomy or hysterectomy specimens, sarcomas were detected in 0.02–0.8% of women
[20][21][22][20,21,22], while within morcellated specimens, they were detected in 0.02% of cases
[23]. The probability of morcellation of occult neoplastic tissue during laparoscopic myomectomy or hysterectomy varies from 0.28 to 0.52%
[24][25][26][24,25,26]. However, the accurate evaluation of the risk of an accidental morcellation of the leiomyosarcoma is challenging because of the paucity of research
[27][28][29][30][31][27,28,29,30,31]. It is equally difficult to assess the real impact of morcellation on a patient’s prognosis. The prospective randomized trials necessary for this purpose are impossible to conduct for bioethical reasons. Nonetheless, the scientific data from previously published retrospective cohort studies unequivocally show that morcellation of stage I occult sarcoma involves a very high risk of its intraabdominal dissemination
[23][27][28][23,27,28]. F
2. State of the Art
Currently, the most frequently used are the second-generation containers, called two-port or dual opening bags. They are usually made of polyurethane or polyurethane-coated nylon fabric
[32][33][34][62,64,65]. This material is inflatable, impermeable for cells and water, not inflammable, and resistant to thermal effects caused by cold light exposure. The majority of the bags are transparent, which increases the safety of the procedure. Transparency allows simultaneous visual control of the bag content and the surrounding abdominal structures. Thanks to this bag wall property, the operating surgeon can continue the morcellation while being perfectly aware of the neighboring tissues and maintaining the appropriate protective distance from them
[32][62]. The contemporary bags are rectangular or stomach shaped.
The typical dual opening bag has two separate ports (
Figure 1).
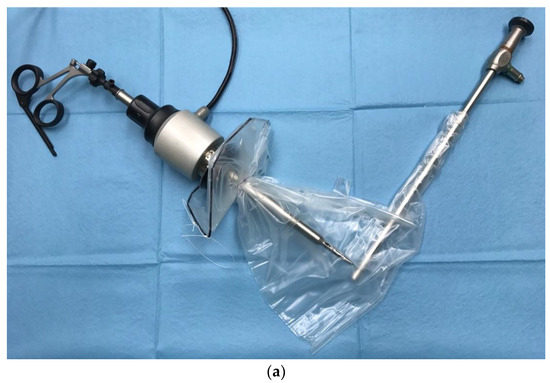
Figure 1. Exemplary bags for contained morcellation: (a) More-Cell-Safe A.M.I. GmbH, Feldkirch, Austria; (b) EMP200ECO-TMF-6, Fannin Ltd., Somerset, UK.
The diameter of the main opening varies between 13 and 17 cm, depending on the size of the bag and the manufacturer. This opening is used to put a specimen into the bag, which might be myoma/myomas, corpus uteri, the entire uterus, etc., according to conducted procedure. The large opening also serves as morcellator access. The second port (opening) is designed for the insertion of the optic trocar at the umbilical site. It is sleeve-shaped, narrow (about 15–17 mm), and long (10–19 cm). In some of the systems, the second opening (second sleeve) is double-layered
[32][35][62,63]. After the morcellation is completed and the telescope removed, the sleeve is turned inside out and closed by tying a double knot. This means that the potentially contaminated part of the sleeve that comes into contact with the abdominal organs during removal of the bag remains oncologically “clean”. After the surgical phase of the procedure is completed, the bag is inserted into the abdominal cavity through a left lateral or suprapubic trocar with a 12–15 mm diameter. Once inside the peritoneal cavity, the bag is unrolled and opened by blunt graspers to prevent any damage to its integrity. In some systems, the bag is rolled up beforehand and placed in a specially designed cover, which can ease and shorten this phase of the procedure. Other manufacturers offer folded bags which allow for self-opening. After opening the large mouth, the specimen is placed into the bag (
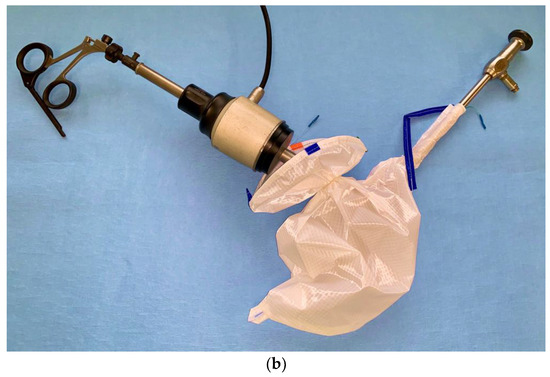
Figure 2) with the help of grasping forceps using the laterals or alternatively suprapubic and right trocars.
Figure 2.
Uterine specimen in the bag inside the peritoneal cavity.
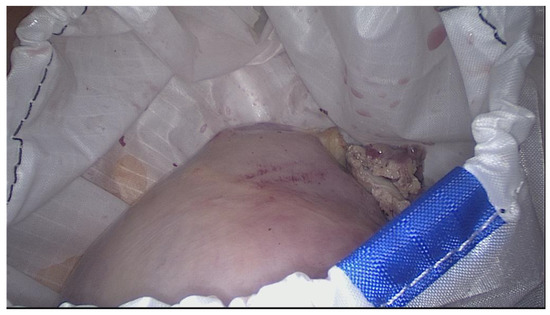
Once the specimen is positioned, the large opening is closed by pulling the drawstring and extracted outside the abdominal wall (
Figure 3).
Figure 3.
The large opening of the retrieval bag exteriorized for morcellator access.
The second opening is pulled out through the umbilical access while the previously inserted optic trocar is removed. At this point, the recently removed optic trocar is reinserted into the bag via the small tubular opening and so-called pseudoperitoneum is established by inflating with CO
2. Next, the morcellator is entered bluntly into the bag through its large opening. The power morcellation proceeds in a contained space, but its technique remains unchanged. Once the morcellation is completed, the electro-morcellator, as well as the optic, are removed from the bag. Small pieces of specimen and a certain volume of blood remain in the bag. Depending on the study, the median weight of the bag content varies from 12 to 29 g
[36][37][82,83]. This amount of residual fluid with tissue fragments does not hinder the correct bag removal. After securing the small opening, the bag is slowly extracted through the left lateral or suprapubic access. A very important step is checking the bag walls in terms of their tightness by thorough visual control and fluid filling. For final inspection of the operating field in terms of bleeding or potential organ complications, the pneumoperitoneum should be reestablished, and the optic trocar reinserted.
Recent years have brought a new solution in the field of closed morcellation, the so-called third-generation bag, which is a multiport retrieval container. Apart from a large opening, it is equipped with three working ports, through which instruments of a diameter up to 15 mm can be inserted, for example graspers or irrigator. The large mouth is self-opening. Sealable access points allow safe retrieval of the bag. Multiport bags are not yet widely spread, and publications relating to their use are lacking.
3. Impact of the Bag Use on the Course of the Operation
Introducing the new bag systems to the routine surgical practice was preceded by thorough preclinical evaluation of its ability to diminish the risk of fluid and cell dissemination during the surgical procedures. The properties of the bag material were first tested in vitro, where the morcellation of animal tissue in a laparoscopy training set was performed with the usage of typical laparoscopic instrumentarium [32][62].
To objectively assess the practical utility of a retrieval bag and the impact of contained morcellation on the entire course of the operation, numerous aspects of the procedure and its specific phases must be taken into consideration. They involve in particular:
-
Insertion of the retrieval bag into the peritoneal cavity;
-
Deployment of the bag;
-
Positioning of the bag inside the abdominal cavity;
-
Positioning the specimen into the bag;
-
Large-opening exterioration;
-
Exterioration of the optic sleeve;
-
Insertion of the umbilical trocar;
-
Establishing the pseudoperitoneum;
-
Introduction of the telescope into the inflated bag;
-
Initial and continuous in-bag visualization;
-
Insertion of the morcellation device;
-
Manipulation of the specimen within the bag to find the optimal position for morcellation;
-
Course of the in-bag morcellation;
-
Morcellation-associated complications;
-
Uncontaminated closing of the optic sleeve;
-
Removal of the bag;
-
Damage of the containment bag;
-
Occurrence of any organ damage related to bag use;
-
Other intraoperative complications;
-
Time necessary to conduct particular steps of the procedure;
-
Overall satisfaction of the operating doctor.
Generally, numerous publications have confirmed in-bag morcellation to be feasible and satisfactory
[33][34][36][38][39][64,65,66,82,84]. A pilot study conducted by Rimbach et al. revealed that from the technical point of view, the use of the dual-opening contained bag was successful in the vast majority of procedures. Only in one case did the intended use of the bag fail, because the size of the specimen turned out to surpass the diameter of the bag mouth
[35][63]. An additional total time related to bag use ranged from 8.5 to 26.5 min (median 14 min). Introduction of the bag and its deployment lasted 1.5 ± 1 min, placement of the specimen 4.7 ± 5.7 min, extraction of the bag’s large opening 2.7 ± 1 min, entering the optic trocar while establishing the pseudoperitoneum 5.6 ± 4.3 min, inserting the morcellation device 1.3 ± 0,7 min, and closing and removing the containment bag 1.9 ± 0.7 min
[35][63]. Subsequent studies on contained morcellation with the application of dual-opening systems reported the time related to bag use as ranging from 7 to 22 min
[39][40][41][42][84,85,86,87].
In-bag morcellation, as any other new operating technique, has its own learning curve. Observations demonstrate that systematic training allows for a reduction in the median total time related to bag use from 14 to 10 min. Another positive aspect of the learning curve is the growth in the technical success rate, which is reported to increase from 85.7% to 93.9%
[35][36][63,82].
As shown above, the laparoscopic contained morcellation technique requires additional time for bag use. However, this approach also saves time, because the meticulous collection of tissue remnants and extensive washing of the abdominal cavity, typical for uncontained morcellation, is not necessary.
Another advantage of using the retrieval bag is the walls of the inflated bag pushing up intestinal slings. This makes the operating space more comfortable for the surgeon and simultaneously safer for the patient.
Despite the relatively high success rate, difficulties with handling of the bag happen in about 16% of cases. A too small umbilical fascia incision may cause problems with optic trocar placement into the optic sleeve, which leads to repeated attempts of insertion or additional enlarging of the fascia opening. Sometimes the bag remains twisted after insertion and requires removal with subsequent replacement
[36][82].
The risk of bag damage with subsequent tissue dissemination depends on the type of tissue extraction system, applied surgical technique, experience of the operating team, and the size of the specimen. Early studies reported damage to the bag in about 6% of cases
[36][82]. This happened mainly during optic trocar insertion, through handling of morcellator forceps, or during forced extraction of the bag containing bigger tissue remnants
[36][82]. Gaining experience results in reducing the bag lesions rate to zero
[43][59].
4. Conclusions
Minimally invasive approaches, especially the laparoscopic technique, have significantly changed contemporary gynecology and are currently considered the “gold standard” in uterine fibroids surgery. Power morcellators were developed as a response to the requirement for special techniques to remove the specimen from the abdominal cavity inherent in the use of minimally invasive access. A 2014 FDA safety communication discouraged the routine use of open morcellation, given concerns for iatrogenic dissemination of a previously undiagnosed malignancy. Despite the unresolved issue of the occurrence and significance of this phenomenon, and even existing doubts whether a myoma can develop or transform into a sarcoma, all possible measures must be taken to optimize patient safety. Contained morcellation is considered to be one such solution that reduces the potential intraabdominal spread of undetected malignancy and myometrial cells, while simultaneously maintaining the benefits of a minimally invasive surgical approach.