Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Carlos Alexandre Henrique Fernandes and Version 2 by Lindsay Dong.
Single-particle cryo-electron microscopy (cryo-EM SPA) has recently emerged as an exceptionally well-suited technique for determining the structure of membrane proteins (MPs). Indeed, in recent years, huge increase in the number of MPs solved via cryo-EM SPA at a resolution better than 3.0 Å in the Protein Data Bank (PDB) has been observed. However, sample preparation remains a significant challenge in the field.
- membrane proteins
- cryo-electron microscopy
- detergents
- nanodiscs
- amphipols
- structural biology
1. Introduction
Membrane proteins (MPs) play a crucial role in various essential functions in the cells, including the regulation of molecule transport across the membrane, cell–cell recognition, signal transduction, cell adhesion, and control of membrane lipid composition [1]. MPs, when dysfunctional, are also involved in several diseases, including cancers, viral infections, channelopathies and neurodegenerative disorders, being the target of more than 50% of currently marketed drugs [2][3][2,3]. The obtention of high-resolution structures of MPs is essential to a comprehensive understanding of their mechanisms of action and for the successful design of new drugs [4].
Over the past three decades, X-ray crystallography has been the prevailing technique for determining high-resolution structures of MPs, as well as other macromolecules and complexes. However, this technique faces a significant challenge in obtaining well-ordered 3D crystals, which require large amounts of samples. This challenge is particularly pronounced for MPs due to their low yield per liter of cell culture in heterologous expression systems [1]. Furthermore, the surfactants employed to extract MPs from the host cell membrane have a detrimental effect on protein stability, impairing the crystallization process [5].
In this context, single-particle cryo-electron microscopy (cryo-EM SPA) has emerged as an exceptionally suitable technique for determining the structure of MPs. Unlike X-ray crystallography, cryo-EM SPA eliminates the need for crystal formation, requires much smaller sample quantities, and allows the use of a wide variety of hydrophobic environments [6][7][6,7]. The impact of cryo-EM SPA on the field of studying the structural biology of MPs can be illustrated by some numbers since the first atomic structures of a MP were determined through the use of this technique in 2013 [8][9][8,9]. While the use of X-ray crystallography has yielded a total of 5,828 deposits of MP structures in the Protein Data Bank (PDB) in all history, cryo-EM SPA has led to 3,556 deposits of MP structures in just the last ten years (2013–2022).
Cryo-EM SPA is a technique that involves the application of purified macromolecules onto cryo-EM grids, which are then rapidly frozen to embed the molecules in a thin layer of vitreous ice (vitrification step). The ice layer thickness must be thin enough to support all orientations of the vitrified molecule, as thicker layers can lead to a significant increase in noise in the high-resolution information [10]. Then, the cryo-EM grid is transferred to a transmission electron microscope (TEM), where images are acquired under cryogenic conditions [6][11][6,11]. The image processing pipeline to obtain the final 3D cryo-EM map of a target molecule encompasses several steps [12]. Briefly, each captured image consists of a movie composed of dose-fractionated frames, which require motion estimation and correction. The corrected images, known as micrographs, are then used to estimate the contrast transfer function (CTF) parameters, which describe how aberrations in the TEM affect the image of the samples. Additionally, micrographs are utilized to locate and select single particles. The particles are extracted from the micrographs and subjected to iterative classifications to sort out suitable ones for constructing the 3D cryo-EM map. Finally, the atomic structure of the molecule is built within the 3D cryo-EM map, resulting in the final structure of the target molecule [12].
The significant increase in the popularity of cryo-EM SPA among structural biologists in the last ten years is a result of several technical advancements, often referred to as the ‘resolution revolution’ [13]. One of the most significant technical advancements was the introduction of complementary metal–oxide–semiconductor (CMOS) direct detector device (DDD) cameras, which improved the detective quantum efficiency (DQE) compared to charged-coupled device (CCD) and other detectors [14]. Moreover, DDD cameras have a high frame rate that allows the data to be captured as dose-fractionated image stacks rather than a single micrograph. This approach enables the correction of motion induced by stage or beam movements, leading to a substantial improvement in image quality by recovering the high-resolution signal that may have been compromised by such motion [15][16][15,16].
In view of these great advancements, sample preparation arises as the most important challenge for cryo-EM SPA, especially for MPs, which require their extraction and purification from the membrane and their stabilization in an aqueous environment. This is typically achieved by solubilizing the MPs in surfactants that can be later replaced with more suitable surfactant or amphiphile molecules prior to the vitrification step.
23. Sample Preparation
2.1. Amphiphiles Used for Membrane Protein Extraction
3.1. Amphiphiles Used for Membrane Protein Extraction
MPs are characterized by their dual hydrophobic–hydrophilic nature, which arises from the presence of a highly hydrophobic transmembrane domain, along with an hydrophilic extracellular and cytoplasmic domains within their structures. Consequently, maintaining MPs in a soluble and stable state outside the lipid biological bilayer poses a considerable challenge, as they have a heightened tendency to aggregate or denature in an aqueous medium [17][25]. Therefore, upon the expression of MPs in heterologous systems, it becomes necessary to extract them from the lipid bilayer and replace it with another amphiphilic system for subsequent structural and functional studies. Various surfactants have been developed to provide a suitable environment for MP extraction from the membrane, with detergents being the historically first ones used for this purpose [18][26]. Detergents are amphipathic molecules consisting of a hydrophilic head group (typically polar, sometimes charged) and a lipophilic or hydrophobic (apolar) tail. This architecture allows detergents to insert their lipophilic tails into the lipidic membrane, thereby disrupting it and extracting the MP from the membrane as the detergent concentration increases. As a result of the hydrophilic–lipophilic balance of detergent molecules, they form spontaneously micelles, which are pseudo-spherical assemblies. Micelle formation occurs once the critical micelle concentration (CMC) is reached and when the sample is above critical micellar temperature. Through micelle formation, an MP becomes part of the detergent–protein complex, sometimes leading to the complete loss of surrounding lipids [19][27]. Among the MP structures solved by using cryo-EM SPA at near-atomic resolution (better than 3.0 Å resolution), almost all molecules employed for membrane protein extraction are detergents (301 of 306 reports). The only exceptions were the five cases where styrene–maleic acid copolymer (SMA) was used. This is a free detergent system where SMA copolymers solubilize MPs directly from the membrane, keeping the native lipid environment from forming polymer-bounded nanodiscs, often called native nanodiscs [20][28]. This approach addresses the challenge of the transient protein destabilization caused by detergents by dissolving integral membrane proteins from biological membranes into nanosized discs. Within these nanoparticles, proteins are embedded in a patch of their native lipid bilayer, which is stabilized in solution by the amphipathic polymer that envelops the disc [20][28]. This detergent-free approach for membrane protein solubilization offers significant simplifications in terms of the purification and manipulation of the samples. However, it poses some considerable difficulties for the conjugation of functional groups to the membrane proteins, which is often required for their biochemical and biophysical characterization [21][29]. The four most common detergents used for membrane protein extraction are, in order of frequency of use (Figure 3A):- (1)
-
Detergents with a single maltose-based polar moiety and single alkyl chain (DDM, UDM, DM): 127 of 306 reports (41%). In this group, the most widely used detergent is DDM, which is present in 117 of 127 reports of this detergent class. DM is present in 9 of 131 reports and UDM in 1 of 131 reports;
- (2)
-
Detergents belonging to the maltoside–neopentyl glycol (MNG) family (MNG, LMNG and DMNG): 104 of 306 reports (34%). In this group, the most widely used detergent is LMNG, which is present in 101 of 104 reports of this detergent class. MNG is present in 2 of 104 reports and DMNG is present in 1 of 107 reports;
- (3)
-
Glyco-diosgenin (GDN): 18 of 306 reports (6%);
- (4)
-
Digitonin: 16 of 306 reports (5%).
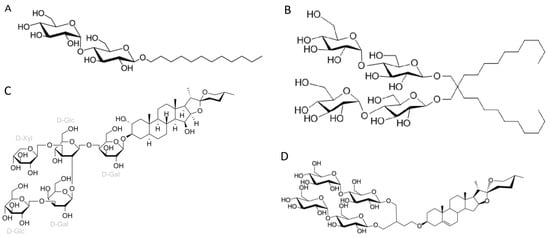 Figure 14. Chemical structures of the four most common detergents used for membrane protein extraction step of the membrane protein structures solved using cryo-EM single-particle analysis in the last two years (2021–2022) available in the Protein Data Bank. (A) n-Dodecyl-β-D-maltopyranoside (DDM); (B) Lauryl maltose neopentyl glycol (LMNG); (C) Digitonin; (D) Glyco-diosgenin (GDN).LMNG (lauryl maltose neopentyl glycol) is a detergent that belongs to the maltoside–neopentyl glycol (MNG) family. This detergent class is characterized as having a maltoside head group and two alkyl chains and two carbohydrate head groups connected with each other via a neopentyl glycol unit in the central region, which result in a dimeric architecture compared with detergents from alkyl maltoside class. LMNG, for example, is a dimer of DDM, thus presenting two maltoside units in their hydrophilic domain and two alkyl chains (Figure 14B). This molecular rearrangement of LMNG results in a CMC value even lower than DDM (0.01 mM rather than 0.17 mM), although it has been reported to form larger micelles [18][22][23][26,30,31].Although DDM is still the most widely used detergent for membrane protein extraction, even for cryo-EM studies, its once clear dominance seems to have slightly decreased in recent years. In comparison to a previous report [24][22], the analysis focusing on high-resolution cryo-EM structures, indicates a slight decrease in DDM usage from membrane protein extraction (~41% compared to ~43% from the previous report) and a significant rise in the utilization of detergents from the MNG family, particularly LMNG (~34% compared to ~22% from the previous report). Originally employed with remarkable success for the solubilization and stabilization of GPCRs (G protein-coupled receptors) in comparison to DDM [22][30], the utilization of LMNG has extended to numerous other classes of membrane proteins, as shown by the existing literature [18][26].Two other detergents that rank among the top four most commonly used surfactants for membrane protein extraction identified here are the steroidal detergents GDN and digitonin (6% and 5% of the reports, respectively). Digitonin is a natural steroidal saponin produced by the purple foxglove plant Digitalis purpurea. Its commercial form is, in fact, a mixture of about five different glycosides, with digitonin and digalonin, another saponin of similar structure, as the main components [25][34]. GDN is a recently developed synthetic variant of digitonin with some modifications. In digitonin, the head group comprises two galactoses, two glucoses, and a xylose, whereas GDN features a branched maltoside head group (Figure 14C and Figure 14D, respectively).
Figure 14. Chemical structures of the four most common detergents used for membrane protein extraction step of the membrane protein structures solved using cryo-EM single-particle analysis in the last two years (2021–2022) available in the Protein Data Bank. (A) n-Dodecyl-β-D-maltopyranoside (DDM); (B) Lauryl maltose neopentyl glycol (LMNG); (C) Digitonin; (D) Glyco-diosgenin (GDN).LMNG (lauryl maltose neopentyl glycol) is a detergent that belongs to the maltoside–neopentyl glycol (MNG) family. This detergent class is characterized as having a maltoside head group and two alkyl chains and two carbohydrate head groups connected with each other via a neopentyl glycol unit in the central region, which result in a dimeric architecture compared with detergents from alkyl maltoside class. LMNG, for example, is a dimer of DDM, thus presenting two maltoside units in their hydrophilic domain and two alkyl chains (Figure 14B). This molecular rearrangement of LMNG results in a CMC value even lower than DDM (0.01 mM rather than 0.17 mM), although it has been reported to form larger micelles [18][22][23][26,30,31].Although DDM is still the most widely used detergent for membrane protein extraction, even for cryo-EM studies, its once clear dominance seems to have slightly decreased in recent years. In comparison to a previous report [24][22], the analysis focusing on high-resolution cryo-EM structures, indicates a slight decrease in DDM usage from membrane protein extraction (~41% compared to ~43% from the previous report) and a significant rise in the utilization of detergents from the MNG family, particularly LMNG (~34% compared to ~22% from the previous report). Originally employed with remarkable success for the solubilization and stabilization of GPCRs (G protein-coupled receptors) in comparison to DDM [22][30], the utilization of LMNG has extended to numerous other classes of membrane proteins, as shown by the existing literature [18][26].Two other detergents that rank among the top four most commonly used surfactants for membrane protein extraction identified here are the steroidal detergents GDN and digitonin (6% and 5% of the reports, respectively). Digitonin is a natural steroidal saponin produced by the purple foxglove plant Digitalis purpurea. Its commercial form is, in fact, a mixture of about five different glycosides, with digitonin and digalonin, another saponin of similar structure, as the main components [25][34]. GDN is a recently developed synthetic variant of digitonin with some modifications. In digitonin, the head group comprises two galactoses, two glucoses, and a xylose, whereas GDN features a branched maltoside head group (Figure 14C and Figure 14D, respectively).2.2. Amphiphiles and Other Molecules Used in the Vitrification Step
3.2. Amphiphiles and Other Molecules Used in the Vitrification Step
The six most classes of amphiphiles (or other types of molecules) present at the vitrification step of MP structures solved by using cryo-EM SPA at near-atomic resolution (better than 3.0 Å resolution) are shown in order of frequency of use:- (1)
-
Mixed detergents: 63 of 312 reports (20%). In this group, the most widely used association of detergents was the combination of LMNG and GDN, which is present in 47 of 63 reports.
- (2)
-
Nanodiscs: 62 of 312 reports (20%). In this group, the most widely used type is the membrane scaffold proteins (MSP)-based nanodiscs, which is present in 50 of 62 reports.
- (3)
-
Detergents with a single maltose-based polar moiety and single alkyl chain (DDM, UDM, DM): 50 of 312 reports (16%). In this group, the most widely used detergent is DDM, which is present in 42 of the 50 reports of this detergent class. DM is responsible for the eight remaining reports;
- (4)
-
Glyco-diosgenin (GDN): 50 of 312 reports (16%).
- (5)
-
Detergents belonging to the maltoside–neopentyl glycol (MNG) family (MNG and LMNG): 40 of 312 reports (13%). In this group, the most widely used detergent is LMNG, which is present in 38 of the 40 reports of this detergent class. MNG is responsible for the two remaining reports.
- (6)
-
Digitonin: 29 of 312 reports (9%).These data indicate that a step of surfactant exchange or reconstitution into nanodiscs is often performed after extraction from the membrane and prior to vitrification of the membrane protein. While there is a clear predominance of DDM and LMNG usage during the protein membrane extraction step, no particular surfactant or other type of molecule exhibits distinct dominance during the vitrification step. There is a similar frequency of use between the mixed detergents, nanodiscs, DDM and GDN (20%, 20%, 16% and 16% of the reports, respectively).The combination of different detergents can be useful to explore their stabilizing properties of detergent–protein and detergent–detergent interactions and increase the effectiveness of the surfactant power obtained from this association without denaturing the membrane proteins. Strong detergent–protein interactions enhance membrane protein stabilization, and strong detergent–detergent interactions prevent protein aggregation and may minimize protein denaturation [18][26]. However, it is important to note that excessively strong detergent–protein interactions can lead to protein denaturation [26][35]. In the case of association between LMNG and GDN, LMNG interacts more strongly with the hydrophobic surface of MPs than DDM, GDN and digitonin due to its two alkyl chains, providing a strong detergent–protein interaction but without causing protein denaturation [18][26]. On the other hand, GDN has similar effects on protein–detergent interactions than DDM; however, the rigid hydrophobic group, resulting from the presence of a steroidal unit, promotes enhanced detergent–detergent interactions than LMNG [18][26].The selection of detergent and its concentration significantly impacts the structure, stability, and functionality of MPs. When detergent concentration is too low, MPs tend to aggregate and precipitate, whereas an excessive amount of detergent can lead to denaturation or dissociation of protein complexes [27][36]. In addressing these concerns, Size Exclusion Chromatography Multi-Angle Light Scattering (SEC-MALS) emerges as a highly suitable technique for assessing the molecular mass and the physicochemical heterogeneity of purified membrane protein–detergent complexes [28][29][37,38]. SEC-MALS integrates a SEC column that is connected in-line to detectors for ultraviolet (UV), light scattering (LS), and refractive index (RI) [28][37].Nanodiscs offer detergent-free environments that represent another prominent type of preparation present in the vitrification step (20% of the reports), where the protein is reconstituted in a lipid environment [30][39]. While five reports of SMA copolymer nanodiscs (native nanodiscs) were identified at the protein membrane extraction step, the vitrification step featured the same five reports of native nanodiscs in addition to 50 reports of membrane scaffold protein (MSP)-based nanodiscs, four of saposin–lipid-protein complexes (Salipro, Stockholm, Sweden) and three of circularized nanodiscs.Circularized nanodiscs (3 out of the 62 reports of nanodiscs) have been recently designed based on engineered scaffold proteins based on Apo1 scaffold protein where the N and C-terminus are covalently linked [31][32][44,45]. As a result of covalent circularization, larger (up to 80 nm diameter) and more homogeneous and stable nanodiscs were produced compared to the standard ones [32][45]. Two MP structures identified by this study were obtained at high resolution using circularizable scaffold protein NW9 (~8.5 nm of diameter) and one using NW11 (~11 nm of diameter). In terms of lipid composition, a report that used NW9 and a report that used NW11 employed soybean polar lipid extract. The NW9 remaining report utilized E. coli total extract lipid. Finally, 4 out of 61 reports of nanodiscs have been identified using a system related to MSP-nanodiscs, known as Salipro, wherein another protein, Saposin A, replaces MSPs. Regarding the lipid composition of MP structures that used Salipro at the vitrification step, two reports employed soybean polar lipid extract (one of them mixed with 25% cholesterol), while the other two reports used brain polar lipids.In addition, a single report was found regarding the use of peptidiscs among the MP structures resolved at below 3.0 Å resolution. Peptidiscs constitute a detergent-free surfactant system very recently developed (2018) that uses multiple copies of a short sequence of a unique helical peptide (NSPr) redesigned to have optimal hydrophobic and hydrophilic properties. These helical peptides wrap around the hydrophobic transmembrane domains of the MPs and shield them from the watery solution [33][52].
34. Sample Vitrification
The vitrification step consists of applying approximately 3 μL of protein solution onto a grid, followed by blotting with filter paper to eliminate excess solution and create a very thin layer of protein suspension on the grid. The grid is then rapidly frozen by plunging it into liquid ethane cooled by liquid nitrogen [6]. Plunge freezing could be performed manually or using some commercial devices, such as VitrobotTM (Thermo Fischer ScientificTM, Waltham, MA, USA), EM GP2 (Leica Microsystems GmnH, Wetzlar, Germany) and CryoplungeTM 3 (Gatan, Inc., Pleasanton, CA, USA). However, optimal ice thickness is difficult to reproduce from one grid to another due to the uneven surface properties of the blotting papers [34][60]. Moreover, the dwell time of the samples on the grids before freezing at the level of seconds is sufficient to favor particle adsorption at the air–water interface [35][61]. In order to solve these limitations, several efforts have been made recently to develop alternative blotting-free methods that guarantee a more reliable and reproducible grid preparation and that diminish the deleterious effects of the air–water interface. For these blotting-free methods, several automated devices have been developed employing inkjet printing (Spotiton) [36][62], gas pressure spray (microfluidic devices) [37][38][63,64], ultrasonic spray [39][40][41][65,66,67], electrostatic spray [42][68], and sample scribing [43][44][69,70] (for a detailed description of these methods, see [11]). Here, scholarswe did not identify the use of any of these new blotting-free methods for the obtention of MP cryo-EM structures at high resolution, likely due to the limited commercial availability of most of these new devices. However, very recently, the commercialized form of Spotiton, known as Chamaleon (SPT Labtech Ltd., Covina, CA, USA) [45][71], has been acquired by several cryo-EM facilities worldwide. This system employs an inkjet mechanism to spray picoliter-sized droplets of the sample solution are sprayed onto self-wicking nanowire grids. The entire sample volume can be accurately dispensed through the piezoelectric inkjet head by applying voltage pulses to control ice thickness [36][46][47][62,72,73]. This method potentially provides more reproducible grid preparations than blotting-dependent methods and reduces the dwell time on the grids before freezing for seconds to hundreds of milliseconds [48][74]. This minimizes the number of particles adsorbed at the air–water interface and increases the number of particles adsorbed in the vitreous ice. Consequently, particle distribution is enhanced in the grids, and the preferred particle orientation problem is alleviated [46][48][72,74]. The Chamaleon system has been tested in several cryo-EM facilities.45. Type of the Grids
Grids serve as the sample carriers for TEM and cryo-EM. They have a standard diameter of 3 nm and typically consist of two main components: a mesh base and a foil [11]. The mesh base is made of a metal that is capable of offering mechanical stability, electron beam conduction and heat dissipation. The foil coats the grid by being placed on top of this mesh and contains micrometer-sized holes that support the vitreous ice with the embedded protein particles and allow electron passage during microscope imaging [49][76]. The foil can contain a regular repeating array of circular holes (Quantifoil® or C-flatTM grids, Großlöbichau, Germany) or an irregular geometry of holes (Lacey grids) [6][11][6,11]. These varying designs of mesh base and foils meet specific experimental needs and preferences in TEM and cryo-EM applications. The three major types of the grids used for cryo-EM data collection of MP solved by using cryo-EM SPA at near-atomic resolution (below 3.0 Å resolution) in the last two years (2021–2022) are in order of frequency of use:- (1)
-
Regular holey-carbon coated copper mesh grids (Quantifoil®): 112 of 310 reports (36%);
- (2)
-
Regular holey-carbon coated gold mesh grids (Quantifoil®): 82 of 310 reports (26.5%);
- (3)
-
Regular holey-gold coated gold mesh grids (UltrAuFoil®): 75 of 310 reports (24%).
56. Conclusions
An exciting feature of cryo-EM SPA is its capacity to capture images representing various conformational states of the same macromolecule because of the rapid freezing of protein in solution [54][93]. Consequently, cryo-EM SPA can provide a window into the dynamics properties of proteins, revealing different conformational states and enabling inferences about how a structure transitions from one conformation to another. In this context, a notable trend in the cryo-EM SPA field in recent years is the development of several different pieces of software to extract protein dynamics and continuous conformational flexibility data from the cryo-EM image datasets, such as 3DVA [55][94], MSPACE [56][95], e2gmm [57][96], Scipion-EM-ProDy workflow [58][97] and others. Additionally, recently released software advancements have enhanced the flexible fitting of atomic structural models into cryo-EM maps through molecular dynamic (MD) simulations, such as Namdinator [59][98] or by approaches that combine normal mode analysis and MD simulations, such as NMMD [60][99] and MDeNM-EMfit [61][100]. These latter approaches are particularly noteworthy as they are able to perform a comprehensive exploration of the free energy landscapes of large conformational changes. Consequently, it becomes possible to resolve various states of a given structure in atomic detail. Therefore, the pursuit of high-resolution MP structures serves not only to provide intricate structural details but also to yield profound insights into their functional dynamics and underlying mechanisms. Despite the remarkable advances in cryo-EM SPA in recent years, it remains an evolving technique. The continuous development of novel sample preparation approaches has the potential to usher in groundbreaking progress in the structural knowledge at the near-atomic level of an increasing number of MPs. Moreover, it can offer novel, accessible experimental conditions for obtaining high-resolution structures of challenging samples and large membrane protein complexes.
