Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Diego Guidolin.
Dopamine (DA) is a catecholamine, that is, an ethylamine with an attached catechol group (a phenyl group with two hydroxyl groups in meta- and para positions). DA-producing neurons were first identified and mapped in animals by Dahlström and Fuxe in 1964 [1,2], indicating the existence of neuronal circuits using DA as a neurotransmitter.
- receptor–receptor interactions
- receptor complexes
- GPCR
- dopaminergic pathways
- Parkinson’s disease
1. Introduction
Dopamine (DA) receptors belong to class A GPCRs [91][1], well known for being able to signal as monomers [92][2]. In addition, however, the overall available evidence (obtained through multiple approaches with consistent results) strongly supports the presence of class A GPCR complexes in native systems [72][3]. In this respect, studies concerning the kinetics of complex formation and its dependence on the involved interaction energy [93][4] are of substantial interest. The observed half-lives of dimers indicate that they are often transient (lasting few hours) and may undergo recombination (“kiss-and-run” encounters [80][5]). These processes may lead to a dynamic equilibrium between monomers and receptor complexes for class A GPCRs, as suggested by studies on the corticotropin-releasing factor receptor type 1 in the endoplasmic reticulum [94][6], indicating that the ratio of monomers/receptor complexes was maintained at an almost constant level in the plasma membrane, even in spite of agonist activation of the receptors. Receptor complexes including DA receptors (see also [95][7]) are shown in Table 1.
Table 1.
Receptor complexes involving dopamine receptors.
| Receptor Complex | Cell Location | Reference |
|---|---|---|
| A2A-D2 | Neurons Astrocytes |
[68][8] [96][9] |
| A2A-D3 | Neurons | [97][10] |
| A2A-D4 | Neurons | [98][11] |
| NMDA-D2 | Neurons | [99][12] |
| NTS1-D2 | Neurons | [100,[13101]][14] |
| CB1-D2 | Neurons | [102][15] |
| D2-5HT1 | Astrocytes | [103][16] |
| D2-5HT2A | Neurons | [101,104][14][17] |
| D2-OTR | Neurons Astrocytes |
[105][18] [106][19] |
| D2-GHS1A | Neurons | [107][20] |
| D2-D4 | Neurons | [108][21] |
| α2A-D4 | Neurons | [108][21] |
| β2-D4 | Neurons | [109][22] |
| D4-MOR | Neurons | [110][23] |
| CCK2-D2 (putative) | Neurons | [66,111][24][25] |
| α1-D2 (putative) | Astrocytes | [112][26] |
| A2A-D2-sigma1 | Neurons | [113][27] |
| A2A-D2-mGluR5 | Neurons | [84][28] |
| A2A-D2-CB1 | Neurons | [82][29] |
| D1-D2 | Neurons | [114][30] |
| D1-D3 | Neurons | [115][31] |
| A1-D1 | Neurons | [116,117][32][33] |
| NMDA-D1 | Neurons | [118][34] |
| GABAA-D5 | Neurons | [119][35] |
| α1-D1 (putative) | Astrocytes | [112][26] |
NMDA—N-methyl-D-aspartate glutamate receptor; 5HT1, 5HT2A—type 1 and type 2A serotonin receptors; GHS1A—type 1a ghrelin receptor; OTR—oxytocine receptor; CB1—type 1 cannabinoid receptor; NTS1—type 1 neurotensin receptor; α1, β2—type α1 and type β2 adrenergic receptor; sigma1—sigma1 receptor; mGluR5—type 5 metabotropic glutamate receptor; MOR—μ-opioid receptor; type A1 and type A2A—adenosine receptor.
2. Receptor Complexes Involving Dopamine Receptors
Receptor Complexes Involving D
2
-like Dopamine Receptors
A first aspect emerging from the available data is that D2 appears to be a hub receptor which interacts with many other GPCRs.
Probably, the most studied interaction is between dopamine D2 and adenosine A2A receptors, leading to the formation of A2A-D2 heterodimers (see [95,120][7][36] for reviews). By using pull-down and mass spectrometry techniques, it has been demonstrated [121,122][37][38] that the heteromerization between A2A and D2 receptors significantly depends on charged residues located at the intracellular part of the transmembrane helix 5 (TM5) of the D2 receptor. The role of TM helix interactions within the A2A-D2 heteroreceptor complex interface has also been explored by using synthetic TM α-helix peptides of the D2 receptor [123][39], and the results allowed for the identification of a TM4/5 interface between the two monomers. The A2A-D2 heterodimer is also representative of many aspects concerning the signaling outcome from a receptor complex. Experimental evidence has shown that the receptor complex formation modifies the signaling from the single protomers. In particular, early in vitro experiments on membrane preparations showed a reduction in the affinity of the high-affinity D2-agonist-binding site after incubation with the A2A agonist CGS21680 [124[40][41],125], demonstrating that antagonistic interactions occur in the A2A-D2 heterodimer. By using receptor autoradiography, this finding was subsequently confirmed by studies on brain tissue from rats and humans [126][42]. They showed a strong reduction in D2 receptor affinity for dopamine in the nucleus accumbens core and shell after the A2A receptor agonist treatment. By using functional, biochemical, and biophysical techniques (such as co-immunoprecipitation and proximity ligation assay), antagonistic interactions between A2A and D2 receptors were also recently demonstrated in astrocytes [96,127][9][43]. In this context, observations indicating that agonist activation of the A2A protomer in the A2A-D2 heteroreceptor complex inhibits D2 Gi/o-mediated signaling but increases the D2 β-arrestin2-mediated signaling are of interest. This marks a difference compared with the action of D2 receptor antagonists, which block all the D2 signaling pathways. Thus, through the allosteric receptor–receptor interaction, an A2A agonist becomes a biased inhibitory modulator of the Gi/o-mediated D2 signaling [128][44]. The possible formation, as a consequence of the formation of a receptor complex, of new allosteric sites allowing the binding of some ligand is a further modulatory mechanism that the A2A-D2 heteromer illustrates. Homocysteine can, indeed, bind to the heterodimer without interfering with the RRI between A2A and D2 and acts as an allosteric antagonist of the D2 receptor [129][45]. Thus, the inhibitory effect of A2A agonists is amplified by homocysteine. These modulatory actions were demonstrated in striatal neurons [129][45], as well as in astrocytes [130][46], where homocysteine reduces the D2-mediated inhibition of glutamate release. An intriguing process involving A2A and D2 receptors was highlighted by studies on cell lines [19][47] that demonstrated intercellular transfer of these GPCRs by exosomes, resulting in the incorporation of functional receptors into acceptor cells. As shown by photo-bleaching fluorescence resonance energy transfer, the transferred receptors may also undergo A2A-D2 receptor heteromerization in the target cell. Thus, the release of extracellular micro vesicles (the so-called “roamer type” of volume transmission [19][47]) may represent a significant mechanism for the modulation of neuron-neuron and astrocyte–neuron intercellular signaling.
Evidence has been provided indicating that the adenosine A2A receptor can establish antagonistic RRIs with the other D2-like receptors as well, namely, D3 [97][10] and D4 [98][11], leading to a reduction in the affinity of their binding site for DA. Antagonistic RRIs also characterize other receptor complexes involving the D2 receptor, as, for instance, the heterodimers it can form with the glutamate NMDA [99][12] and mGluR5 [84][28] receptors, the neurotensin NTS1 [100][13] receptor, and the cannabinoid CB1 [102,131][15][48] receptor. Higher-order heteroreceptor complexes, involving both A2A and D2, have also been identified. Examples include the heterotrimers formed by A2A and D2 receptors with the metabotropic glutamate receptor 5 (A2A-D2-mGluR5 [84][28]), the sigma1 receptor (A2A-D2-sigma1 [113][27]), and the cannabinoid CB1 receptor (A2A-D2-CB1 [82][29]). In these receptor complexes, the pattern of allosteric interactions on the D2 protomer also inhibits the recognition and signaling of the DA receptor.
Synergistic RRIs involving the D2 receptor, however, were also identified. A first example is provided by the receptor complex between the D2 receptor and the serotonin 5-HT2A receptor [104][17], where the activation of the 5-HT2A protomer by 5-HT2A agonists produced an enhancement of D2 signaling. In astrocytes, receptor complexes between the dopamine D2 receptor and the serotonin 5-HT1A receptor have been observed [103][16]. However, the functional consequences of the signaling pathways mediated by D2-5-HT1 heteromers in these cells are still not known in detail [132][49]. A further example is represented by the D2-OTR heterodimer, involving D2 and the oxytocin receptor. In neurons [105][18], oxytocin, via the allosteric RRI established in the heterocomplex, markedly increased D2 receptor recognition (increased affinity of the high-affinity state) and increased the coupling of Gi/o to the receptor. The D2-OTR heterodimer was recently identified in astrocytes as well [106][19], and the activation of OTR was shown to have a facilitatory effect on the response of D2 receptors, causing them to be activated by subthreshold D2 agonist concentrations and leading to an inhibition of glutamate release by the cells.
Synergistic RRIs are also in operation in the heterodimer involving the dopamine D4 receptor and μ-opioid receptor (MOR) [110][23], since D4 activation causes a substantial increase in the affinity of the MOR agonist binding sites. Evidence was also obtained that the D4 and β2-adrenergic receptor may form a D4-β2 receptor complex that integrates Gs- and Gi-mediated regulation of adenylyl cyclase [109][22]. In this context, of particular interest are also studies (see [108][21]) focused on the dopamine D4 receptor polymorphic variants D4.4 (four repeats in exon 3) and D4.7 (seven repeats in exon 3), both able to heterodimerize with the norepinephrine α2A receptor. However, only heteromerization with D4.7, but not with D4.4, increases the potency of norepinephrine in terms of activating the α2A receptor, indicating the possible polymorphic variants of a D2-like receptor as a factor conferring significantly different pharmacological properties onto the receptor complexes it may form.
Receptor Complexes Involving D
1
-like Dopamine Receptors
The potentiation of immediate early gene expression and of arachidonic acid release have been described as functional interactions between activated dopamine D1 and D2 receptors (see [45][50]). However, it was also demonstrated that stably co-expressed D1 and D2 receptors may form heteromeric units [114][30]. It is of substantial interest that the two receptors, when coactivated in the same cell, produce a phospholipase C-mediated calcium signal that is not seen when the receptors are activated alone. The pharmacological analysis of this receptor complex indicated a specific coupling to the Gq/11 pathway to produce such a response. Activation of Gq/11, however, could not be elicited through activation of either receptor when activated alone. Thus, the recruitment of G proteins other than those expected for the monomers has been observed after D1-D2 dimerization, a further mechanism of signal transduction modulation associated with receptor complex formation.
Antagonistic interactions between D1 and the adenosine A1 receptor, associated with the formation of A1-D1 heterodimers [116[32][33],117], were also characterized. A1 agonists, indeed, were found to reduce the number of D1 agonist binding sites in the high-affinity state, and with receptor autoradiography, A1 agonists were found to antagonistically modulate D1 binding sites, causing a reduction in their affinity (see [133][51] for details).
Receptor complexes between dopamine D1 and D3 have been demonstrated using several techniques, giving evidence for synergistic intramembrane D1-D3 interactions at the level of D1 recognition, since D3 activation was able to increase the affinity of the D1 agonist binding sites [115][31]. Synergistic RRIs also exist in the D1-NMDA heterodimer [118][34], by which NMDA receptor activation can recruit D1 receptors to the plasma membrane, thereby leading to an increase in D1 signaling and cAMP accumulation.
Recent interesting findings on prefrontal cortex astrocytes indicated a significant functional interaction between α1-adrenergic and DA receptors, driving downstream Ca2+ signaling [112][26]. Also, in light of the abovementioned data showing that DA receptors may form receptor complexes with adrenergic receptors [109[22][52],134], and of neuroanatomical data showing that D1 and α1-adrenergic receptors colocalize on prefrontal cortex dendrites and may undergo co-trafficking [119][35], the hypothesis has been put forward that in cortical astrocytes as well, heterodimers involving DA receptors and adrenergic receptors could be present [112][26]. A direct experimental demonstration, however, is still lacking.
GABAA and dopamine D5 heteromerization, demonstrated by Liu and collaborators [135][53], was the first identification of a receptor complex involving a GPCR and an ion-channel receptor. The results indicated that co-activation of the monomers was required for the formation of the complex, which allowed for a bidirectional crosstalk, leading to a reduction in GABAA signaling and a reduced coupling between D5 and Gs proteins.
3. Possible Differences in Receptor Complex Dynamics in Neurons and Astrocytes
As briefly illustrated before, a number of receptor complexes (such as, for instance, the A2A-D2 heterodimer) are expressed both in neurons and astrocytes. In this respect, it is reasonable to assume that the conformation of a receptor complex in the two cases may exhibit some difference because of differences in the membrane microenvironment. Differences in the energy landscape, indeed, modulate the pattern of allosteric interaction between monomers and may lead to changes in the signaling features of the complex that they can form [80][5].
Differences in membrane potential between the two cell types, for instance, have been documented [136][54]. Unlike neurons, astrocytes do not generate action potentials, but they are electrically dynamic cells. Indeed, in contrast to most non-excitable cells that have relatively depolarized membrane potentials, astrocytes have a hyperpolarized membrane (at a level that typically rests significantly below that of neurons) and a low membrane resistance. For the present discussion, membrane composition is another factor deserving consideration. This aspect was the focus of an extensive lipidome analysis by Fitzner and collaborators [137][55], showing that each cell type was characterized by a unique lipid composition: neurons, for instance, exhibited quite high levels of cholesterol, while astrocytes were enriched in phosphatidylinositol.
All these features of the membrane microenvironment, therefore, have the potential to modulate the pharmacological properties of a given receptor complex. To illustrate this concept, the results of a simulation based on molecular modeling methods and focused on the A2A-D2 heterodimer in two different membrane environments (neuron-like and astrocyte-like) are shown in Figure 21.
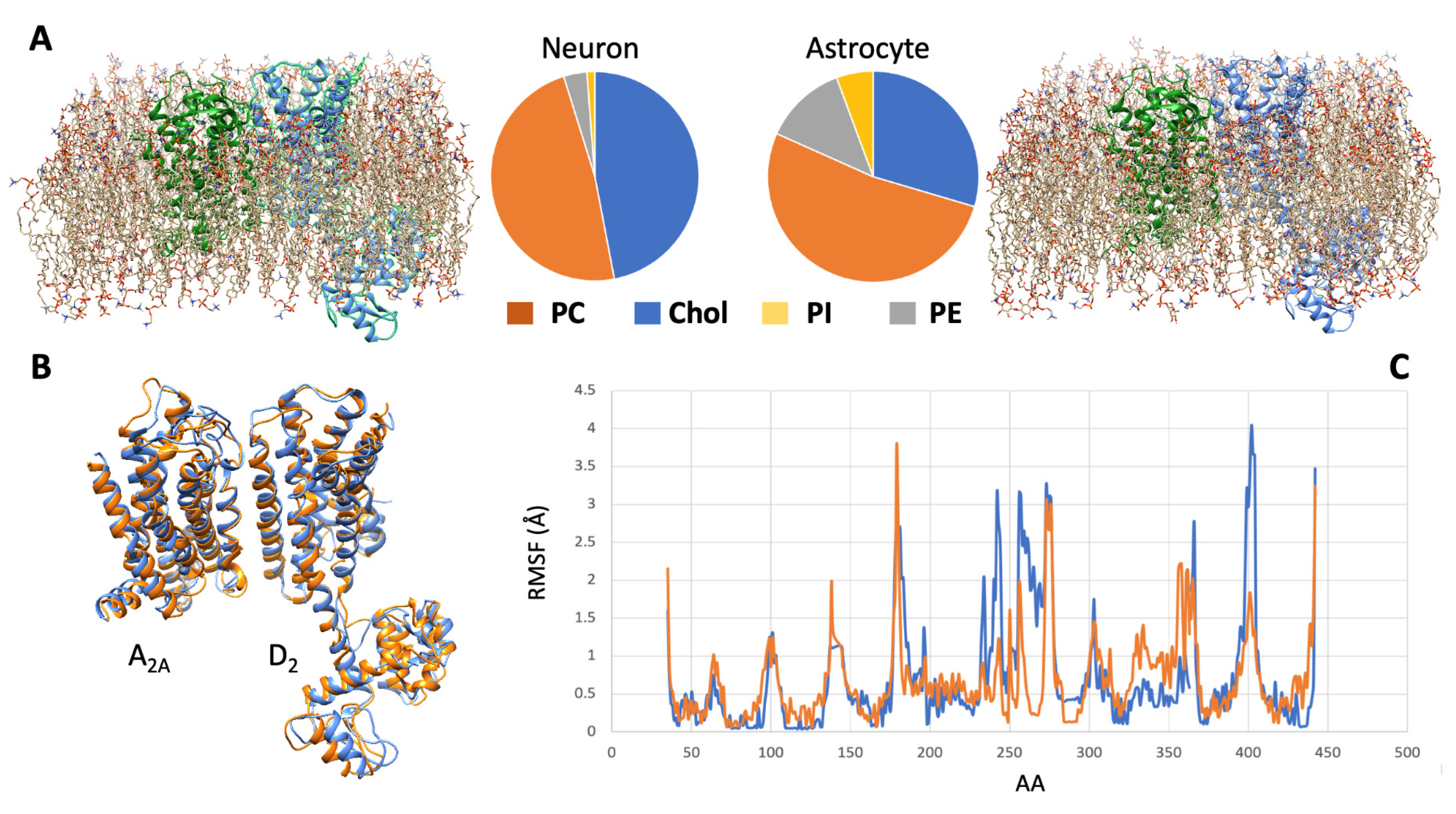
Figure 21. Molecular dynamics simulation of the A2A-D2 receptor complex in different cell membranes. (A) By using the CHARMM-GUI membrane builder web server (http://www.charmm-gui.org/?doc=input [138][56], accessed on 5 July 2023), four phospholipids, namely, phosphatidylcholine (PC), cholesterol (Chol), phosphatidylinositol (PI), and phosphatidylethanolamine (PE), were used to model two different membrane bilayers around the molecular model [120,123][36][39] of the heterodimer. The first (left panel) approximated the neuronal membrane composition, the second one (right panel) the astrocytic one (see [137][55]). A molecular dynamics procedure, based on the CABSflex method [139][57] and available as a web server (https://biocomp.chem.uw.edu.pl/CABSflex2, accessed on 6 July 2023), was then used to evaluate the conformations that the receptor complex may acquire in the two environments. (B) Configurations of minimal energy of the A2A-D2 heterodimer in neuronal (orange) and astrocytic (blue) membrane. (C) Root mean square fluctuations (RMSF) diagrams, per amino acid position, of the D2 monomer chain when in neuronal (orange) and astrocytic (blue) membrane. The estimated differences in configuration and dynamical behavior of the heterodimer suggest that different membrane environments could represent a factor modulating the pharmacological properties of the receptor complex.
References
- Foord, S.M.; Jupe, S.; Holbrook, J. Bioinformatics and type II G-protein-coupled receptors. Biochem. Soc. Trans. 2002, 30, 473–479.
- Whorton, M.R.; Bokoch, M.P.; Rasmussen, S.G.F.; Huang, B.; Zare, R.N.; Kobilka, B.; Sunahara, R.K. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. USA 2007, 104, 7682–7687.
- Franco, R.; Martinez-Pinilla, E.; Lanciego, J.L.; Navarro, G. Basic pharmacological and structural evidence for class A G-protein-coupled receptor heteromerization. Front. Pharmacol. 2016, 7, 76.
- Gurevich, V.V.; Gurevich, E.V. How and why do GPCRs dimerize? Trends Pharmacol. Sci. 2008, 29, 234–240.
- Guidolin, D.; Ciruela, F.; Genedani, S.; Guescini, M.; Tortorella, C.; Albertin, G.; Fuxe, K.; Agnati, L.F. Bioinformatics and mathematical modeling in the study of receptor-receptor interactions and receptor oligomerization. Focus on adenosine receptors. Biochim. Biophys. Acta 2011, 1808, 1267–1283.
- Teichmann, A.; Gibert, A.; Lampe, A.; Grzesik, P.; Rutz, C.; Furkert, J.; Schmoranzer, J.; Krause, G.; Wiesner, B.; Schülein, R. The specific monomer/dimer equilibrium of the corticotropin-releasing factor receptor type 1 is established in the endoplasmic reticulum. J Biol. Chem. 2014, 289, 24250–24262.
- Fuxe, K.; Marcellino, D.; Guidolin, D.; Woods, A.; Agnati, L.F. Dopamine Receptor Oligomerization. In The Dopamine Receptors; Neve, K.A., Ed.; Humana Press: Totowa, NJ, USA; Springer: Berlin/Heidelberg, Germany, 2010; pp. 255–280.
- Trifilieff, P.; Rives, M.L.; Urizar, E.; Piskorowski, R.A.; Vishwasrao, H.D.; Castrillon, J.; Schmauss, C.; Stätmann, M.; Gullberg, M.; Javitch, J.A. Detection of antigen interactions ex vivo by proximity ligation assay: Endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 2011, 51, 111–118.
- Pelassa, S.; Guidolin, D.; Venturini, A.; Averna, M.; Frumento, G.; Campanini, L.; Bernardi, R.; Cortelli, P.; Buonaura, G.C.; Maura, G.; et al. A2A-D2 heteromers on striatal astrocytes: Biochemical and biophysical evidence. Int. J. Mol. Sci. 2019, 20, 2457.
- Torvinen, M.; Marcellino, D.; Canals, M.; Agnati, L.F.; Lluis, C.; Franco, R.; Fuxe, K. Adenosine a2a receptor and dopamine d3 receptor interactions: Evidence of functional a2a/d3 heteromeric complexes. Mol. Pharmacol. 2005, 67, 400–407.
- Fuxe, K.; Borroto-Escuela, D.O. Receptor-Receptor Interactions in the Central Nervous System; Humana Press: New York, NY, USA, 2018; Volume 140, p. 346.
- Liu, X.Y.; Chu, X.P.; Mao, L.M.; Wang, M.; Lan, H.X.; Li, M.H.; Zhang, G.C.; Parelkar, N.K.; Fibuch, E.E.; Haines, M.; et al. Modulation of D2R–NR2B interactions in response to cocaine. Neuron 2006, 52, 897–909.
- Koschatzky, S.; Tschammer, N.; Gmeiner, P. Cross-receptor interactions between dopamine D2L and neurotensin NTS1 receptors modulate binding affinities of dopaminergics. ACS Chem. Neurosci. 2011, 2, 308–316.
- Plach, M.; Schäfer, T.; Borroto-Escuela, D.O.; Weickert, D.; Gmeiner, P.; Fuxe, K.; Friedland, K. Differential allosteric modulation within dopamine D2R-neurotensin NTS1R and D2R-serotonin 5-HT2AR receptor complexes gives bias to intracellular calcium signaling. Sci. Rep. 2019, 9, 16312.
- Przybyla, J.A.; Watts, V.J. Ligand-induced regulation and localization of cannabinoid CB1 and dopamine D2L receptor heterodimers. J. Pharmacol. Exp. Ther. 2010, 332, 710–719.
- Kolasa, M.; Solich, J.; Faron-Górecka, A.; Żurawek, D.; Pabian, P.; Łukasiewicz, S.; Kuśmider, M.; Szafran-Pilch, K.; Szlachta, M.; Dziedzicka-Wasylewska, M. Paroxetine and Low-dose Risperidone Induce Serotonin 5-HT1A and Dopamine D2 Receptor Heteromerization in the Mouse Prefrontal Cortex. Neuroscience 2018, 377, 184–196.
- Borroto-Escuela, D.O.; Romero-Fernandez, W.; Tarakanov, A.O.; Marcellino, D.; Ciruela, F.; Agnati, L.F.; Fuxe, K. Dopamine D2 and 5-hydroxytryptamine 5-HT2A receptors assemble into functionally interacting heteromers. Biochem. Biophys. Res. Commun. 2010, 401, 605–610.
- Romero-Fernandez, W.; Borroto-Escuela, D.O.; Agnati, L.F.; Fuxe, K. Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor–receptor interactions. Mol. Psychiatry 2013, 18, 849–850.
- Amato, S.; Averna, M.; Guidolin, D.; Ceccoli, C.; Gatta, E.; Candiani, S.; Pedrazzi, M.; Capraro, M.; Maura, G.; Agnati, L.F.; et al. Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes. Int. J. Mol. Sci. 2023, 24, 4677.
- Kern, A.; Albarran-Zeckler, R.; Walsh, H.E.; Smith, R.G. Apo-grelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron 2012, 73, 317–332.
- Ferré, S.; Becher, A.M.; Bonaventura, J.; Quiroz, C.; Sanchez-Soto, M.; Casadò-Anguera, V.; Cai, N.-S.; Moreno, E.; Boateng, C.A.; Keck, T.M.; et al. Functional and pharmacological role of the dopamine D4 receptor and its polymorphic variants. Front. Endocrinol. 2022, 13, 1014678.
- Rebois, R.V.; Maki, K.; Meeks, J.A.; Fishman, P.H.; Hébert, T.E.; Northup, J.K. D2-like dopamine and β-adrenergic receptors form a signaling complex that integrates Gs- and Gi-mediated regulation of adenylyl cyclase. Cell. Signal. 2012, 24, 2051–2060.
- Gago, B.; Fuxe, K.; Agnati, L.; Penafiel, A.; De La Calle, A.; Rivera, A. Dopamine D(4) receptor activation decreases the expression of mu-opioid receptors in the rat striatum. J. Comp. Neurol. 2007, 502, 358–366.
- Agnati, L.F.; Fuxe, K.; Giardino, L.; Calzà, L.; Zoli, M.; Battistini, N.; Benfenati, F.; Vanderhaeghen, J.J.; Guidolin, D.; Ruggeri, M. Evidence for cholecystokinin-dopamine receptor interactions in the central nervous system of the adult and old rat. Studies on their functional meaning. Ann. N. Y. Acad. Sci. 1985, 448, 315–333.
- Petkova-Kirova, P.; Giovannini, M.G.; Kalfin, R.; Rakovska, A. Modulation of acetylcholine release by cholecystokinin in striatum: Receptor specificity; role of dopaminergic neuronal activity. Brain Res. Bull. 2012, 89, 177–184.
- Pittolo, S.; Yokoyama, S.; Willoughby, D.D.; Taylor, C.R.; Reitman, M.E.; Tse, V.; Wu, Z.; Etchenique, R.; Li, Y.; Poskanzer, K.E. Dopamine activates astrocytes in prefrontal cortex via α1-adrenergic receptors. Cell Rep. 2022, 40, 111426.
- Pinton, L.; Borroto-Escuela, D.O.; Narváez, M.; Oflijan, J.; Agnati, L.F.; Fuxe, K. Evidence for the existence of dopamine D2R and Sigma 1 allosteric receptor–receptor interaction in the rat brain: Role in brain plasticity and cocaine action. Springerplus 2015, 4, P37.
- Beggiato, S.; Tomasini, M.C.; Borelli, A.C.; Borroto-Escuela, D.O.; Fuxe, K.; Antonelli, T.; Tanganelli, S.; Ferraro, L. Functional role of striatal A2A, D2, and mGlu5 receptor interactions in regulating striatopallidal GABA neuronal transmission. J. Neurochem. 2016, 138, 254–264.
- Pinna, A.; Bonaventura, J.; Farré, D.; Sànchez, M.; Simola, N.; Mallol, J.; Lluís, C.; Costa, G.; Baqi, Y.; Müller, C.E.; et al. L-DOPA disrupts adenosine A(2A)-cannabinoid CB(1)-dopamine D(2) receptor heteromer cross-talk in the striatum of hemiparkinsonian rats: Biochemical and behavioral studies. Exp. Neurol. 2014, 253, 180–191.
- Rashid, A.J.; So, C.H.; Kong, M.M.C.; Furtak, T.; El-Ghundi, M.; Cheng, R.; O’Dowd, B.F.; George, S.R. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. USA 2007, 104, 654–659.
- Marcellino, D.; Ferré, S.; Casado, V.; Cortés, A.; Le Foll, B.; Mazzola, C.; Drago, F.; Saur, O.; Stark, H.; Soriano, A.; et al. Identification of dopamine D1-D3 receptor heteromers: Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J. Biol. Chem. 2008, 283, 26016–26025.
- Ginés, S.; Hillion, J.; Torvinen, M.; Le Crom, S.; Casadó, V.; Canela, E.I.; Rondin, S.; Lew, J.Y.; Watson, S.; Zoli, M.; et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc. Natl. Acad. Sci. USA 2000, 97, 8606–8611.
- Franco, R.; Lluis, C.; Canela, E.I.; Mallol, J.; Agnati, L.; Casadó, V.; Ciruela, F.; Ferré, S.; Fuxe, K. Receptor-receptor interactions involving adenosine A1 or dopamine D1 receptors and accessory proteins. J. Neural Transm. 2007, 114, 93–104.
- Lee, F.J.; Xue, S.; Pei, L.; Vukusic, B.; Chéry, N.; Wang, Y.; Wang, Y.T.; Niznik, H.B.; Yu, X.-M.; Liu, F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell 2002, 111, 219–230.
- Mitrano, D.A.; Pare, J.F.; Smith, Y.; Weinshenker, D. D1-dopamine and a1-adrenergic receptors co-localize in dendrites of the rat prefrontal cortex. Neuroscience 2014, 258, 90–100.
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L.F. Adenosine A2A-Dopamine D2 Receptor-Receptor Interaction in Neurons and Astrocytes: Evidence and Perspectives. Prog. Mol. Biol. Transl. Sci. 2020, 169, 247–277.
- Ciruela, F.; Burgueno, J.; Casado, V.; Canals, M.; Marcellino, D.; Goldberg, S.R.; Bader, M.; Fuxe, K.; Agnati, L.F.; Lluis, C.; et al. Combining mass spectrometry and pull-down techniques for the study of receptor heteromerization. Direct epitope-epitope electro- static interactions between adenosine A2A and dopamine D2 receptors. Anal. Chem. 2004, 76, 5354–5363.
- Woods, A.S.; Ciruela, F.; Fuxe, K.; Agnati, L.F.; Lluis, C.; Franco, R.; Ferré, S. Role of electrostatic interaction in receptor–receptor heteromerization. J. Mol. Neurosci. 2005, 26, 125–132.
- Borroto-Escuela, D.O.; Rodriguez, D.; Romero-Fernandez, W.; Kapla, J.; Jaiteh, M.; Ranganathan, A.; Lazarova, T.; Fuxe, K.; Carlsson, J. Mapping the interface of a GPCR dimer: A structural model of the A2A adenosine and D2 dopamine receptor heteromer. Front. Pharmacol. 2018, 9, 829.
- Ferré, S.; von Euler, G.; Johansson, B.; Fredholm, B.B.; Fuxe, K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc. Natl. Acad. Sci. USA 1991, 88, 7238–7241.
- Fuxe, K.; Ferré, S.; Zoli, M.; Agnati, L.F. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res. Rev. 1998, 26, 258–273.
- Diaz-Cabiale, Z.; Hurd, Y.; Guidolin, D.; Finnman, U.B.; Zoli, M.; Agnati, L.F.; Vanderhaeghen, J.J.; Fuxe, K.; Ferré, S. Adenosine A2A agonist CGS 21680 decreases the affinity of dopamine D2 receptors for dopamine in human striatum. Neuroreport 2001, 12, 1831–1834.
- Cervetto, C.; Venturini, A.; Passalacqua, M.; Guidolin, D.; Genedani, S.; Fuxe, K.; Borroto- Escuela, D.O.; Cortelli, P.; Woods, A.; Maura, G.; et al. A2A-D2 receptor- receptor interaction modulates gliotransmitter release from striatal astrocyte processes. J. Neurochem. 2016, 140, 268–279.
- Borroto-Escuela, D.; Romero-Fernandez, W.; Tarakanov, A.; Ciruela, F.; Agnati, L.; Fuxe, K. On the existence of a possible A2A-D2-beta- arrestin2 complex: A2A agonist modulation of D2 agonist-induced beta-arrestin2 recruitment. J. Mol. Biol. 2011, 406, 687–699.
- Agnati, L.F.; Ferré, S.; Genedani, S.; Leo, G.; Guidolin, D.; Filaferro, M.; Carriba, P.; Casado, V.; Lluis, C.; Franco, R.; et al. Allosteric modulation of dopamine D2 receptors by homocysteine. J. Proteome Res. 2006, 5, 3077–3083.
- Cervetto, C.; Venturini, A.; Guidolin, D.; Maura, G.; Passalacqua, M.; Tacchetti, C.; Cortelli, P.; Genedani, S.; Candiani, S.; Ramoino, P.; et al. Homocysteine and A2A-D2 receptor-receptor interaction at striatal astrocyte processes. J. Mol. Neurosci. 2018, 65, 456–466.
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; Maura, G.; Agnati, L.F. Receptor-receptor interactions and microvesicle exchange as mechanisms modulating signaling between neurons and astrocytes. Neuropharmacology 2023, 231, 109509.
- Borgkvist, A.; Marcellino, D.; Fuxe, K.; Greengard, P.; Fisone, G. Regulation of DARPP-32 phosphorylation by Delta(9)-tetrahydrocannabinol. Neuropharmacology 2008, 54, 31–35.
- Łukasiewicz, S.; Błasiak, E.; Szafran-Pilch, K.; Dziedzicka-Wasylewska, M. Dopamine D2 and serotonin 5-HT1A receptor interaction in the context of the effects of antipsychotics—In Vitro studies. J Neurochem. 2016, 137, 549–560.
- Rashid, A.; O’Dowd, B.F.; Verma, V.; George, S.R. Neuromal Gq/11-coupled dopamine receptors: An uncharted role for dopamine. Trends Pharmacol. Sci. 2007, 28, 551–555.
- Fuxe, K.; Marcellino, D.; Rivera, A.; Diaz-cabiale, Z.; Filip, M.; Gago, B.; Roberts, D.C.S.; Lange, U.; Genedani, S.; Ferraro, L.; et al. Receptor-receptor interactions within receptor mosaics. Impact on neuropharmacology. Brain Res. Rev. 2008, 58, 415–452.
- Ferré, S.; Sarasola, L.I.; Quiroz, C.; Ciruela, F. Presynaptic adenosine receptor heteromers as key modulators of glutamatergic and dopaminergic neurotransmission in the striatum. Neuropharmacology 2023, 223, 109329.
- Liu, F.; Wan, Q.; Pristupa, Z.B.; Yu, X.M.; Wang, Y.T.; Niznik, H.B. Direct protein-protein coupling enables cross-talk between dopamine D5 and gamma-aminobutyric acid A receptors. Nature 2000, 403, 274–280.
- McNeill, J.; Rudyk, C.; Hildebrand, M.E.; Salmaso, N. Ion channels and electrophysiological properties of astrocytes: Implications for emergent stimulation technologies. Front. Cell. Neurosci. 2021, 15, 644126.
- Fitzner, D.; Bader, J.M.; Penkert, H.; Bergner, C.G.; Su, M.; Weil, M.-T.; Surma, M.A.; Mann, M.; Klose, C.; Simons, M. Cell-type- and brain-region-resolved mouse brain lipidome. Cell Rep. 2020, 32, 108132.
- Lee, J.; Patel, D.S.; Ståhle, J.; Park, S.-J.; Kern, N.R.; Kim, S.; Lee, J.; Cheng, X.; Valvano, M.A.; Holst, O.; et al. CHARMM-GUI Membrane Builder for Complex Biological Membrane Simulations with glycolipids and lipoglycans. Chem. Comput. 2019, 15, 775–786.
- Kuriata, A.; Gierut, A.M.; Oleniecki, T.; Ciemny, M.P.; Kolinski, A.; Kurcinski, M.; Kmiecik, S. CABS-Flex 2.0: A Web Server for fast simulations of flexibility of protein structures. Nucleic Acids Res. 2018, 46, W338–W343.
More
