Dilated cardiomyopathy (DCM) is a cardiac condition with structural and functional impairment, where either the left ventricle or both ventricular chambers are enlarged, coinciding with reduced systolic pump function (reduced ejection fraction, rEF). The prevalence of DCM is more than 1:250 individuals, and mortality largely due to heart failure in two-third of cases, and sudden cardiac death in one-third of patients. Damage to the myocardium, whether from a genetic or environmental cause such as viruses, triggers inflammation and recruits immune cells to the heart to repair the myocardium. Examination of myocardial biopsy tissue often reveals an inflammatory cell infiltrate, T lymphocyte (T cell) infiltration, or other activated immune cells. Despite medical therapy, adverse outcomes for DCM remain. The evidence base and existing literature suggest that upregulation of CX3CR1, migration of immune cells, together with cytomegalovirus (CMV) seropositivity is associated with worse outcomes in patients with dilated cardiomyopathy.
- dilated cardiomyopathy
- CX3CR1
- fractalkine
- T lymphocytes
- cytomegalovirus
1. Introduction
2. Phenotypic Clustering of Dilated Cardiomyopathy Patients
-
PG1 included 331 patients with mild systolic dysfunction
-
(Mean ejection fraction: 43% ± 9%),
-
PG2 included 83 patients with auto-immune disease background
-
PG3 included 165 patients with cardiac arrhythmias (mainly atrial fibrillation and ventricular tachycardias), including patients with genetic causes (Familial cardiomyopathy)
-
PG4 included 216 patients with severe systolic dysfunction (Mean ejection fraction: 23% ± 8%)
3. Aetiologies of Dilated Cardiomyopathy
3.1. Familial Cardiomyopathy
3.2. Autoimmune Myocarditis
Autoimmune myocarditis is a condition in which cardiac muscle is damaged by self-reactive immune cells. This condition is typically associated with numerous genetic and environmental risk factors. Various systemic autoimmune diseases, such as sarcoidosis or SLE (lupus), can compromise cardiac function by a variety of mechanisms [7][11]. The prognosis for patients with either of these conditions is typically dismal. However, it has been demonstrated that early administration of glucocorticosteroids and immunosuppressive agents improves patients’ conditions [8][12]. Restoring the equilibrium between autoimmunity and immune tolerance, which is regulated by Th17 and regulatory T cells (Tregs), is one of the primary therapeutic approaches for autoimmune disease [9][13]. Treg cells are capable of inhibiting autoreactive T cells, and multiple studies have repeatedly identified a deficiency in the quantity or function of Treg cells in autoimmune disorders [9][13]. Therefore, Treg administration has been demonstrated to be a promising treatment for a variety of autoimmune disorders.3.3. Post-Viral Myocarditis
Myocarditis is frequently preceded by infection, with viruses being the most prevalent cause of this condition [10][11][15,16]. There are typically three phases of viral myocarditis. During the first phase, which lasts several days, the virus obtains entry and actively replicates. Therefore, the innate immune response will be triggered against these exogenous substances. During this phase, direct viral burden contributes significantly to myocardial damage. The subsequent phase revealed that an excessive immune response is the leading cause of cardiac damage. During this phase, T cell infiltration has been reported alongside an increase in fibrosis and calcification of the myocardium. In the final phase, patients may experience either remission or progression to dilated cardiomyopathy (DCM), depending on the heart’s capacity to recover from previous insults of direct viral injury and immune persistence [10][11][15,16].3.4. Immune Checkpoint Inhibitor-Related Myocarditis
Immune checkpoint inhibitors (ICIs) are a novel type of cancer treatment that is being applied to a growing number of cancer types for example malignant melanoma, Hodgkin’s lymphoma and non small cell lung cancer to name a few. The immune modulators CTLA-4, PD-1, and PD-L1 are the targets of ICIs [12][19]. ICIs may, however, stimulate T cell activity against host tissues, leading to immune-related adverse events (irAEs). Myocarditis is a rare adverse reaction associated with ICIs, with incidence rates ranging from 0.1% to 2% [13][20]. With ICI myocarditis, there is a reduction in absolute lymphocyte count and an increase in neutrophils, both of which are associated with subsequent significant adverse cardiac events [13][20]. Cardiovascular magnetic resonance (CMR) utilising tissue characterisation techniques such as late gadolinium enhancement (LGE) and the presence of myocardial oedema, is the gold standard non-invasive imaging test for diagnosis and risk prediction in myocarditis of other aetiologies. CTLA-4 is a crucial regulator of T cell inhibition via multiple mechanisms, including negative signalling of B7-CD28, inhibition of IL-2 mRNA expression, and interaction with the TCR-CD3 pathway to inhibit T cell activation [14][15][22,23]. After IFN-g exposure, PD-1 and PD-L1 were highly expressed to prevent T-cell-mediated signalling on host cells. The FDA has currently approved seven ICI medications for cancer therapy. Although ICI-associated myocarditis is uncommon, its mortality rate can reach 25 to 50 percent [15][23]. In addition, this adverse event was reported to occur within 1 to 2 months of the initial ICI dose. The underlying pathogenesis of ICI-associated myocarditis remains unknown.4. Role of Fractalkine Signalling in Cardiovascular Disease
4.1. Fractalkine (CX
3
CL1) and Its Receptor CX
3
CR1 in Atherogenesis
Fractalkine consists of 373 amino acids and is the only member of the CX3C chemokine subfamily with both membrane-bound and soluble variants [16][17][18][19][25,26,27,28]. The former is an adhesion molecule with four sections: an extracellular N-terminal domain, a mucin-like stalk, a transmembrane alpha helix, and a brief cytoplasmic tail. Fractalkine can be cleaved at the juncture of its stalk and transmembrane helix my metalloproteinases ADAM 10 or ADAM 17 to produce its soluble form, which functions as a chemoattractant (see Figure 2) for monocytes, NK cells, and T cells.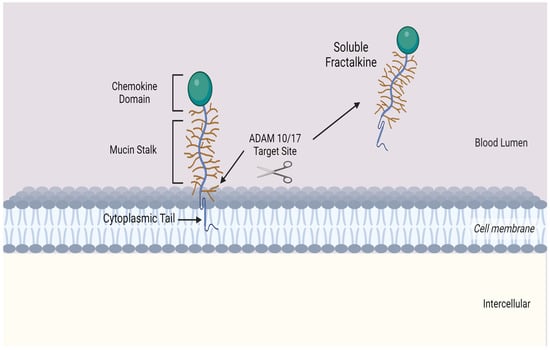
4.2. CX
3
CR1 and Myocardial Infarction
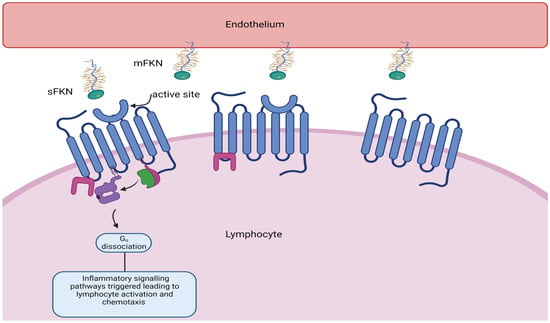
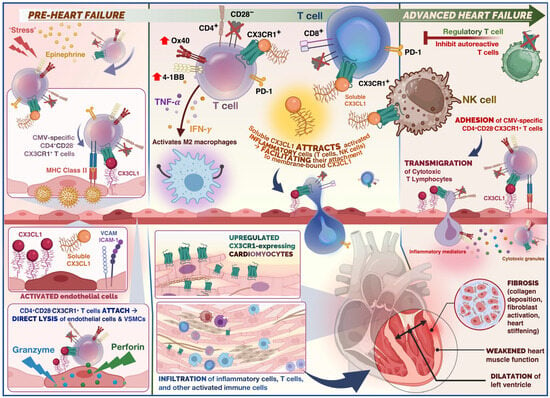
4.3. Fractalkine Signalling in Heart Failure
5. Link between CX3CR1 and Cytomegalovirus
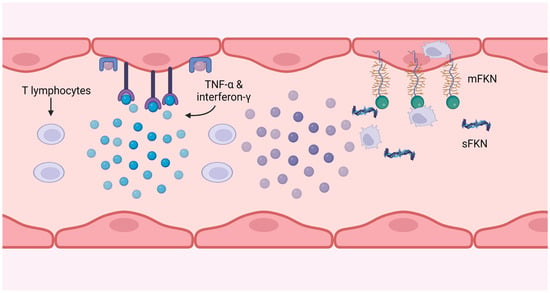
Cytomegalovirus (CMV) is a pervasive herpes virus with a seroprevalence of greater than 60% in individuals aged 50 and older in the majority of studies [23][54] and 85% in those aged 80 and older [24][46]. CMV causes an asymptomatic or moderate initial response in immunocompetent hosts, but it is never eliminated from the body, resulting in lifelong latent infection with the possibility of reactivation [23][54]. Seropositivity for CMV induces significant changes in the T cell composition of the host. CMV-specific CD8+ and, to a lesser extent, CD4+ memory T cells, particularly TEMRA cells, account for a disproportionately high proportion of total T cells; this phenomenon is known as memory inflation [25][26][27][55,56,57]. There is evidence that memory expansion in the CD8 compartment is greater in older patients, indicating that the CMV-specific memory population continues to expand with age, but this is less evident and has been less thoroughly studied in CD4 T cells [28][48]. Memory inflation may be an adaptive response to suppress reactivations [29][58], and whether this unbalanced expansion impedes the immune system’s function remains debatable, as some contend that the CD8 compartment is sufficiently plastic to permit expansion of one phenotype without disruption of others [30][59]. In the presence of viral antigen, CMV-specific CD4+ T cells have been shown to cause endothelial injury, with more damage occurring in donors with higher frequencies of CMV-specific CD4+ T cells [31][32][33][60,61,62]. This injury is caused by the release of IFN-g and TNF-α by T cells at sufficient levels to induce endothelial cell induction of fractalkine, thereby attracting natural killer (NK) cells and monocyte-macrophages, as depicted in Figure 4.