Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Long Zhao and Version 2 by Jason Zhu.
Toxic and heavy metals pose significant environmental and health risks, necessitating the development of reliable detection methods. Schiff bases as fluorescent chemosensors have attracted intense attention because of their excellent sensitivity and selectivity towards a range of cations.
- Schiff base
- chemosensor
- functional groups
- sensing strategy
- metal ions
1. Introduction
One of the key strategies employed involves variations in functional groups within the Schiff base structure [1][2][58,59]. By modifying the aromatic rings, substituents, or linker groups, researchers have successfully fine-tuned the properties of the chemosensors to target specific toxic cations selectively. For instance, the introduction of electron-withdrawing groups such as nitro or cyano moieties has been shown to enhance the affinity towards metal cations by increasing the electron density on the Schiff base system [3][4][60,61]. On the other hand, electron-donating groups such as amino or hydroxyl groups have been utilized to improve the sensitivity towards softer toxic cations. Other ways include optimizing the spatial arrangement and rigidity of the receptor portion to facilitate efficient binding and recognition of toxic cations. For instance, introducing bulky substituents or chelating moieties on the receptor platform has enhanced selectivity towards specific metal cations [5][62].
2. Schiff Bases as Chemosensors for Hg2+
Mercury can accumulate in certain fish and seafood species, posing a risk to ecosystems and human health, particularly pregnant women and young children [6][50]. Chronic mercury exposure can lead to serious health problems, including neurological and developmental disorders. Chemosensors for mercury can be used to detect and monitor the levels of mercury in air, water, and soil.
One way of ensuring that chemosensors realize their full potential is by incorporating specific functional groups that can work in synergy to influence the physical and chemical properties of the sensor. Pyrazole derivatives can be used as Schiff base chemosensors because of their versatility. The pyrazole ring consists of a five-membered aromatic ring containing three carbon atoms and two adjacent nitrogen atoms, as shown in Figure 1a [7][65]. The pyrazole itself is non-fluorescent, but when incorporated into other moieties, the compound’s electronic properties will be altered. These changes can be detected through various spectroscopic techniques, such as UV–vis absorption spectroscopy, fluorescence spectroscopy, or electrochemical methods. The sensing mechanism of pyrazole Schiff base chemosensors can involve various interactions, such as coordination bonding, hydrogen bonding, or π–π stacking. These interactions can induce changes in the spectroscopic properties of the compound, allowing for the detection and quantification of the target analyte. An example of this is a report by Yang et al., who prepared a Schiff base probe (probe-1) for detecting Hg2+ in aqueous solutions by linking rhodamine 6G with a pyrazole derivative [8][66]. The methodology was clearly outlined, which enhances reproducibility. This unique combination of moieties produced a functioning probe that is weakly fluorescent due to the C=N isomerization. Adding Hg2+ produced a new π-conjugated structure, leading to a turn-on reaction and a distinct color change from colorless to pink. The turn-on reaction was determined to result from an inhibited PET mechanism, leading to the CHEF process. Sensibility was quite high, with an LOD value estimated at 0.2 nM, which compares well with other comparable probes. For application, the probe was successfully used on paper strips as a calorimetric sensor to track trace amounts of Hg2+ from water. The study, however, relies heavily on spectral changes and neglected to comprehensively evaluate other important parameters, such as stability, solvent effects and response time, which could provide a reference for other researchers pursuing similar structures. Another example where functional groups can induce changes in the electronic properties is a series of pyrene-based Schiff base Hg2+ trackers probes-2a, 2b, and 2c, shown in Figure 1b [9][67]. It sheds light on the performance of pyrene-based compounds as fluorescent chemosensors, and several critical aspects outlined warrant discussion. As expected, the different functional groups produced different performance results. Upon performing a series of analytical techniques, the probe containing thiophene-2-carbohydrazide (2a) performed the best, recording an LOD of 0.27 μM. It exhibited high selectivity and anti-interference properties when tested in other ions. However, while the selectivity towards Hg2+ and the subsequent turn-on response was attributed to the hard and soft acid-base (HSAB) theory and hydrolysis of the imine (C=N) bond, the underlying mechanistic insights into the fluorescence activation upon binding could be explored further to enrich the understanding of the sensor’s behavior. The absence of data on stability and solvent effect also makes it difficult to ascertain the overall performance of this probe.
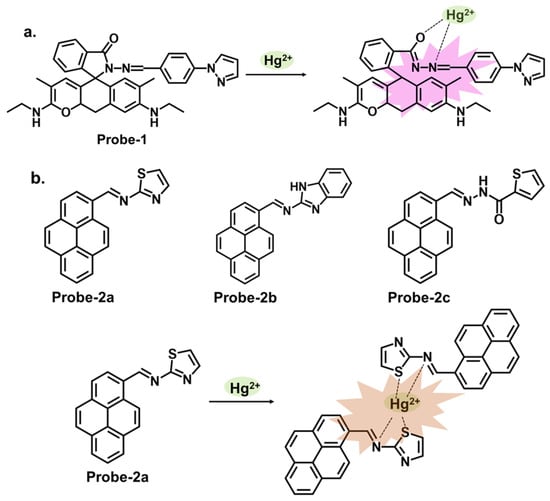
Figure 1. The structures and mechanisms of pyrazole and pyrene-derived Schiff base chemosensors for Hg2+; (a) probe-1, and (b) probes-2a, 2b, and 2c.
Rhodamine is a fluorescent dye that can be used as a chemosensor in applications such as detecting metal ions, pH changes, and biological molecules. Rhodamine-based chemosensors work by undergoing a change in fluorescence intensity or color in the presence of specific analytes. It commonly exists in equilibrium between two forms. The open form is the fluorescent conformation and is dominant in acidic conditions. The closed form is the non-fluorescent spirolactone form, as illustrated in Figure 2. One example is probe-3 studied by Cui et al., which is a Schiff base receptor based on rhodamine-b for tracking Hg2+ [10][68]. The use of rhodamine is commendable since it is well known for its fluorescence enhancement properties. As expected, the turn-on reception resulted from the opening of the spirolactam ring once Hg2+ was added. Various tests showed the probe to be highly selective and sensitive, with an LOD value of 10 nM. Here, however, further investigation on the high selectivity towards Hg2+ compared to other soft acids such as Cd2+ and Ag+ would provide a deeper understanding of the underlying physical phenomena beyond the ring opening. The probe was successfully used for imaging live HeLa cells and zebrafish, further proving to be a valuable contribution to in vivo applications. The study systematically explores the performance of the probes via a series of experiments, including UV–vis, emission studies, and metal ion titration. UV–vis and fluorescence investigations showed the probes linked with Hg2+ in a 1:1 ratio with high selectivity and sensitivity. The LOD values were also very low, estimated at 13.4 nM and 15.6 nM, respectively. The turn-on response was determined to result from the suppression of PET and C=N isomerization once the complex was formed. For application, the probes were successfully used in MCF-7 cells. While there are a lot of interesting findings, the lack of a comprehensive discussion of other rhodamine-based probes with similar mechanisms and their limitations makes it difficult to evaluate the superiority of this probe. Vanjare et al. synthesized probe-5, a rhodamine 6 G-based Schiff base probe for tracking Hg2+ in the nanomolar range [11][70]. The probe’s response towards Hg2+ and other ions is presented with clarified calorimetric and fluorescence changes. The probe’s closed spirocyclic form is non-fluorescent. When Hg2+ is added, it forms a 1:1 complex, resulting in the opening of the spiro ring and a corresponding enhanced fluorescence. Interference from other ions was minimal, and the sensitivity was quite high, with the LOD estimated at 30.37 nM. The probe also efficiently recovered Hg2+ from different water sources at rates above 96%. Cell imaging was also successfully performed in MDA-MB-231 and A375 cells with very low cytotoxicity. While the spectral changes have been comprehensively explored, a study, however, on potential limitations, including photobleaching, side reactions, or other degradation, would be helpful to enhance the scientific rigor and provide more reference points to other researchers seeking to optimize similar structures. Dewangan et al. synthesized unsymmetrical ferrocene-based organometallic Schiff base probes by incorporating ferrocenyl species into rhodamine moieties (probes-6a, 6b, and 6c) [12][71]. A detailed description of the synthesis and purification, as well as the analytical methods employed, is provided, which boosts reproducibility. The probes have poor emission but adding Hg2+ results in enhanced fluorescence and a corresponding increase in quantum yield. Various tests in solution and THP-1 cancer cells showed that the probes can be used to track Hg2+ in solution and at the cellular level. However, detailed comparisons with other similar structures are lacking, which hinders the assessment of its superiority or equivalency in terms of precision and accuracy.
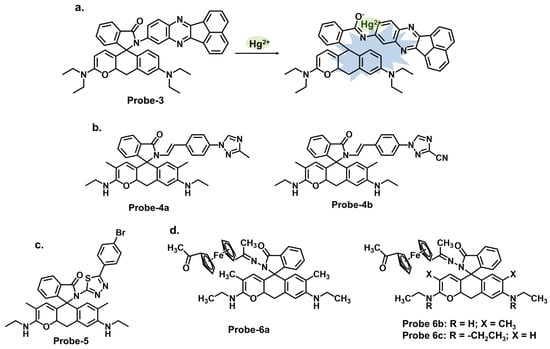
Figure 2. The structures (and mechanisms) of rhodamine-based Schiff base chemosensors for Hg2+; (a) probe-3, (b) probes-4a, 4b, (c) probe-5, and (d) probes-6a, 6b, 6c.
Carbazole is a tricyclic aromatic compound that exhibits fluorescence properties. Carbazole-based chemosensors can be designed and synthesized to detect and respond to specific analytes, such as metal ions, organic molecules, or environmental pollutants. These chemosensors work by changing fluorescence intensity, emission wavelength, or other spectroscopic properties upon interaction with the target analyte. The specific design strategies and applications of carbazole-based chemosensors can vary depending on the desired sensing objective. Leslee et al. reported probe-7, shown in Figure 3a, a Schiff base receptor with carbazole and benzothiazole moieties, for the calorimetric and fluorimetric detection of Hg2+ ions [13][72]. The turn-on sensor is weakly fluorescent because of the ICT process between the carbazole unit and benzothiazole moiety. Fluorescence is enhanced upon complexation with Hg2+ ions, with an estimated LOD of 0.14 μM. The experiments performed clearly illustrate the enhancement mechanism and establish a correlation between the concentration of Hg2+ ions and fluorescence response. The analytical techniques were performed and confirmed the probe to be immune to interference and that the coordination occurred between the Hg2+ ions and the nitrogen and sulfur atoms of the hydrazine and benzothiazole units, respectively. This affinity towards Hg2+ was attributed to the HSAB principle, though there was no deep dive into why other soft acids such as Ag+ and Cd2+ did not have the same effect. Furthermore, the 3D spectral analysis showed that the probe could be viable for tracking Hg2+ ions in cells and cellular organelles. However, the study could benefit from response time analysis to ascertain its potential application in real-time monitoring. Reversibility studies would also provide further insight into how it responds to strong chelating agents like EDTA.
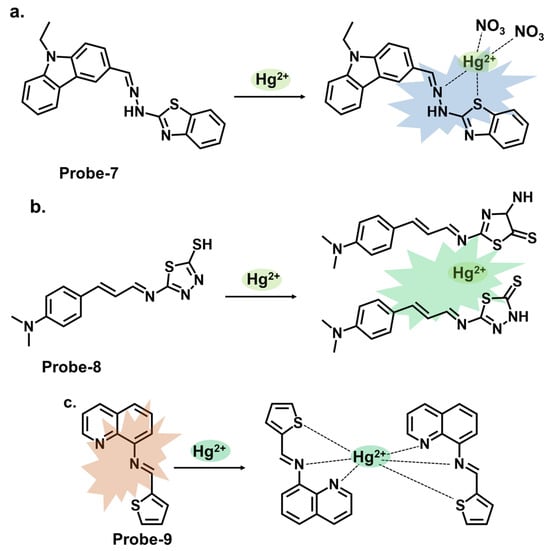
Figure 3. The structures and mechanisms of carbazole-, thiadiazole-, and quinoline-based Schiff base chemosensors for Hg2+; (a) probe-7, (b) probe-8, and (c) probe-9.
3. Schiff Bases as Chemosensors for Cu2+
Copper is widely used in various industries, including electronics, construction, and manufacturing. Monitoring copper levels in industrial processes is important to ensure compliance with regulations and prevent the release of copper into the environment. Chemosensors for copper contribute to scientific research and education by providing tools for studying copper’s behavior, transport, and interactions in various environments. They can aid in understanding copper’s role in biogeochemical cycles, the impact of copper pollution on ecosystems, and the development of remediation strategies.
Carbazole derivatives can be designed to specifically target Cu2+. The principle is the same as those to Hg2+, where changes in color, fluorescence intensity, emission wavelength, or other spectroscopic properties are observed upon interaction with Cu2+. A prime example is 2-amino-3-((Z)-((9-hexyl-9H-carbazol-3-yl) methylene) amino) maleonitrile (probe-10), shown in Figure 4a, reported by Yin et al., which is a carbazole-derived Schiff base probe prepared in a three-step process to detect Cu2+ [14][74]. Probe-10 is weakly fluorescent due to the active C=N isomerism. Upon adding Cu2+, it exhibited a color change from yellow to colorless and a 160-fold fluorescence enhancement with high selectivity and minimal interference. Job’s plot determined a 1:1 binding ratio, while UV–vis titration estimated the LOD at 27.4 nM. The binding was also shown to be reversible by adding EDTA. While the spectral analyses were thoroughly performed and appear to show negligible interference and marked selectivity towards Cu2+, the underlying reasons for this could be explored further to make it easier to predict and tune behavior in complex environments. Unlike carbazoles, monocarbazones consist of one benzene ring instead of two, as shown in Figure 4b. They can be used in synergy with other functional groups to create sensors that target specific analytes. Probe-11 is a monocarbazone Schiff base Cu2+ tracker derived from 2H-benzo[h]chromene-3-carbaldehyde and carbazide by Wang et al. [15][75]. Changes in fluorescence intensity often indicate the only detection signal in most probes so this ratiometric sensor is interesting. Probe-11, which is AIE-active, showed enhanced emission in Cu2+ due to a mechanism involving intermolecular charge transfer coupled with a metal-induced assembly. Its calorimetric behavior was confirmed by the color change from brown to colorless. Job’s plot confirmed the 1:1 binding ratio, while further analysis estimated the LOD to be 10.4 nM, respectively. Additionally, the probe was successfully used to track Cu2+ ions in HeLa cells. Probe-11 proved highly sensitive, reversible, and immune to interference from many other cations. It would be interesting, however, to gain an understanding of its limitations, specifically on photostability, which has been known to affect longevity and performance. Probe-12, shown in Figure 4c, is a rhodamine derivative synthesized by Yang et al. to detect Cu2+ and cysteine [16][76]. The relevant experiments were performed and support the claims made by the authors. A complex is formed upon introducing Cu2+, which opens the lactam ring, resulting in enhanced emission intensity and increased quantum yield. This turn-on reaction was not observed when other divalent and trivalent ions were introduced. Probe-12 was also used to detect Cu2+ in living HepG2 cells and Kunming mice with negligible cytotoxicity. Rhodamine derivatives have been explored extensively in the field of fluorescence chemosensors, and a comprehensive comparison with other similar structures would make it easier to assess probe-12, whether in terms of dynamic range or response time, and make modifications if necessary.
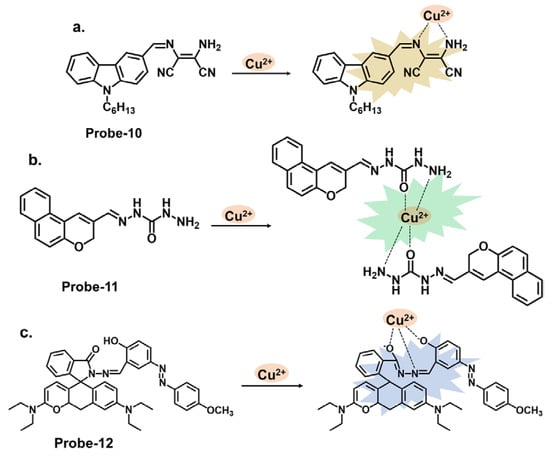
Figure 4. The structures and mechanisms of carbazole-, carbazide-, and rhodamine-based Schiff base chemosensors for Cu2+; (a) probe-10, (b) probe-11, and (c) probe-12.
Benzidine, pyridine, and naphthalene groups, shown in Figure 5a–c, respectively, have been incorporated into various fluorescence probes to track Cu2+ cation. Congo red is a benzidine-based dye that is commonly used as a biological stain, particularly for amyloid fibrils. It has also been explored as a fluorescent chemosensor for detecting various analytes, including metal ions and biological molecules. Ni et al. reported probe-13, a Schiff base Cu2+ tracker synthesized via a one-step process by refluxing 1-hydroxynaphthalene-2-carbaldehyde and Congo red in ethanol [17][77]. The methodology offers a detailed account of the synthesis process and the characterization techniques. In Cu2+, probe-13 showed colorimetric and fluorescence turn-on responses, traced to the inhibition of C=N isomerism and CHEF. This was accompanied by a color change from brown to colorless, while other cations did not cause any emission or color changes. Job’s plot confirmed a 1:1 stoichiometry, while other analyses estimated the LOD to be 0.1 nM. As illustrated in Table 1, this LOD is amongst the lowest in the reviewed probes. Furthermore, probe-13 showed low cytotoxicity and good cell membrane permeability when tested in HepG2 cells. While the key performance indicators were sufficiently explored, assessing the influence of temperature, pH, and solvent polarity on the sensing abilities would contribute to a more comprehensive understanding of its practical utility and durability. Xiao et al. reported probe-14, a naphthalene- and pyridine-bearing Schiff base Cu2+ tracker synthesized from the reaction between 2-naphthaldehyde and 2-hydrazinylpyridine [18][78]. The structure is simple, but several critical aspects, reversibility being one of them, warrant discussion. The probe is weakly fluorescent because PET from the lone pairs on nitrogen to the naphthalene moiety quenches the fluorescence. When Cu2+ forms a complex with probe-14, PET is inhibited, resulting in enhanced emission, which is the basis of the detection process. Job’s plot confirmed a 2:1 binding ratio, with an LOD estimated at 3.90 nM. It was also responsive towards the S2− ion, as the anion could displace the Cu2+ ion from the complex. While the LOD is sufficiently low to reversibly track Cu2+ ions in the nanomolar range, an investigation into the response time would clarify whether it can be used for real-time monitoring. Probe-15 reported by Venkatesan et al. is a Schiff base chemosensor for detecting Cu2+, synthesized by the reaction between thiophene carbaldehyde and diamino malononitrile in methanol [19][79]. The fluorogenic sensor changes color from yellow to colorless in Cu2+, with a 1:1 binding ratio and an LOD of 14.5 nM. Probe-15 exhibited anti-interference properties and high selectivity towards Cu2+ only. The turn-on response was attributed to the CHEF mechanism upon forming the probe-15-Cu2+ complex. It was also observed to be reversible when tested with a powerful chelating agent like EDTA. For practical applications, probe-15 was successfully used to recover Cu2+ in various water samples.
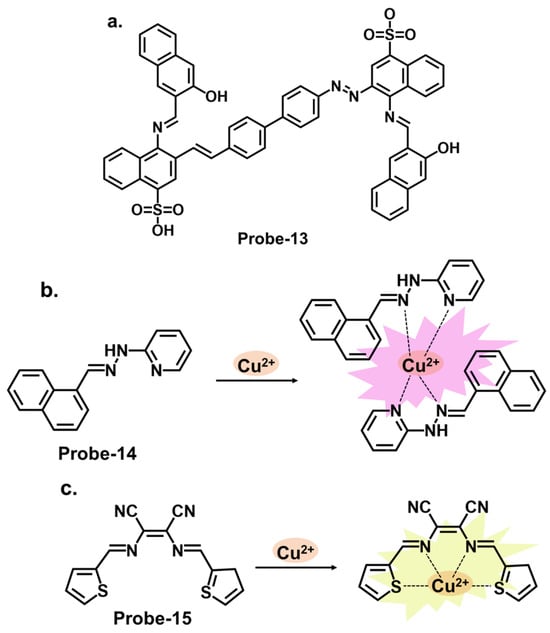
Figure 5. The structures (and mechanisms) of benzidine-, pyridine-, and thiophene-derived Schiff base chemosensors for Cu2+; (a) probe-13, (b) probe-14, and (c) probe-15.
Some successful Schiff base chemosensors for Cu2+ detection are derived from imidazole derivatives. One example is probe-16, shown in Figure 6a, which is synthesized by Slassi et al. from the reaction between N-(3-Aminopropyl) imidazole and 2-Hydroxy-5-(p-tolyldiazenyl) benzaldehyde [20][80]. Probe-16 is weakly fluorescent because of the active C=N isomerism within the structure. In the presence of Cu2+, the probe-16-Cu2+ complex is formed, which restricts C=N isomerism, resulting in CHEF. In this structure, the imidazole moiety is the electron donor, while the hydroxyl and the imine groups form the binding sites. Job’s plot determined the binding ratio to be 2:1, while the detection limit was estimated at 1.8 μM. The range of analytes used to assess selectivity could be expanded to eliminate the chances of other ions being false positives during application. Another example is probe-17, shown in Figure 6b, which is a colorimetric and fluorescent Schiff base sensor for detecting Cu2+, synthesized by refluxing 4-Dimethylamino-benzohydrazide and imidazole-2-formaldehyde [21][81]. NMR spectra and mass spectroscopy confirmed a successful synthesis. This probe had a turn-on reaction towards Cu2+, with a color change from colorless to yellow and an LOD of 15 nM. Job’s plot confirmed a 1:1 binding ratio, while reversibility was tested with S2−, which could displace Cu2+ from the complex. For application, probe-17 could show distinct color changes when used on paper strips. It recovered Cu2+ from various water sources and blood, with recovery rates above 97%. While the sensing mechanism was thoroughly investigated through experimental and theoretical studies, more in-depth discussion on the obtained data, juxtaposed with other similar structures, would provide deeper insights into the underlying physical phenomena that lead to this turn-on response. More chemosensor examples from cyclic compounds with relatively high sensitivity and selectivity for Cu2+ are also reviewed. Pyrenes are polycyclic hydrocarbons consisting of four fused benzene rings, which are typically used as building blocks for the construction of fluorescence chemosensors (Figure 6c). Arjunan et al. reported probe-18, a pyrene motif Schiff base Cu2+ tracker synthesized by refluxing 1-pyrene carboxaldehyde and 3-hydroxy-2-napthoic hydrazide in ethanol [22][82]. Probe-18 is weakly fluorescent because of active PET processes. PET is inhibited upon coordination with Cu2+, resulting in a turn-on response. While the response time was relatively long (5 min), the LOD was quite low (0.26 μM), indicating generally high sensitivity. Furthermore, the probe detected Cu2+ ions in living RAW 264.7 cells with very low cytotoxicity. Although the laboratory-based experiments have been carried out meticulously, the potential real-world applications of the sensor could be investigated further, as the practical utility of the chemosensor is crucial for its broader acceptance and adoption.
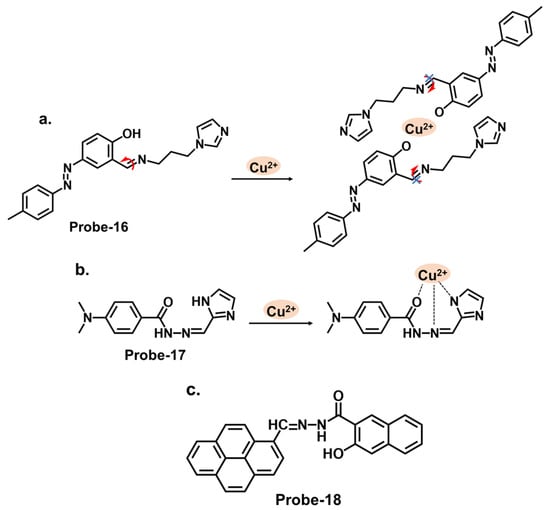
Figure 6. The structures (and mechanisms) of imidazole- and pyrene-based Schiff base chemosensors for Cu2+; (a) probe-16, (b) probe-17, and (c) probe-18.
Diarylethene compounds are noted for their characteristic reversible photochromism, which makes them valuable in optical data storage, molecular switches, and fluorescent sensors. The fluorescence of a diarylethene molecule can be controlled by modulating its molecular conformation through external stimuli, such as light or analyte interactions. Gao et al. reported probe-19, shown in Figure 7a, which is a Schiff base receptor based on a diarylethene with benzo [23][24][25][1,2,5] oxadiazol-4-ylamine, prepared in a two-step reaction to track Cu2+ [26][83]. Probe-19 detects Cu2+ in a turn-on reaction with a 90-fold increase in emission intensity, accompanied by a color change from dark red to bright red. The enhanced emission response was due to the suppression of the C=N isomerization, which led to the CHEF process. Job’s plot confirmed a 2:1 binding ratio, while UV–vis titration estimated the LOD at 1.49 μM. Further analyses showed very little interference and high selectivity. For application, it was used to recover Cu2+ from river water, with recovery rates above 90%. This sensor showed promising results, and further investigations into the stability and photostability under various environmental conditions, such as pH and temperature variations, would provide valuable insights for other potential practical applications. Two more examples are shown in Figure 7. Probe-20, shown in Figure 7b, is a benzimidazole-based Schiff base probe with a bidentate ligand for tracking Cu2+ [27][84]. It is weakly fluorescent, but a turn-on response was observed when Cu2+ was added. This enhanced emission was attributed to the inhibition of C=N isomerization and PET process between the diaminomaleonitrile group and the benzimidazole unit. Job’s plot, mass spectra, and DFT analysis confirmed a 1:2 binding ratio, while UV–vis titration estimated the LOD to be 0.49 μM. Tests in living HepG2 cells showed that the probe possesses high cell penetration to detect Cu2+ in cells sufficiently. While the authors provide a clear and detailed description of the chemosensor’s structural framework, it would be interesting to explore its application in other systems, aside from living cells. Moghadan et al. reported 2-[(9H-fluoren-2-ylmethylene)-amino]-phenol (probe-21), shown in Figure 7c, which is a fluorogenic Schiff base chemosensor designed for tracking Cu2+ [28][85]. Probe-21 is weakly fluorescent because of C=N isomerization and ICT. When Cu2+ is added, both processes are suppressed, resulting in a turn-on response and a color change from pale yellow to colorless. The probe also proved highly selective towards Cu2+, with an LOD of 1.54 nM. While the relevant tests were performed and the results show a functional chemosensor, investigations on temperature stability, pH tolerance, and solvent compatibility are particularly important to determine its environmental robustness and assess its practical applicability.
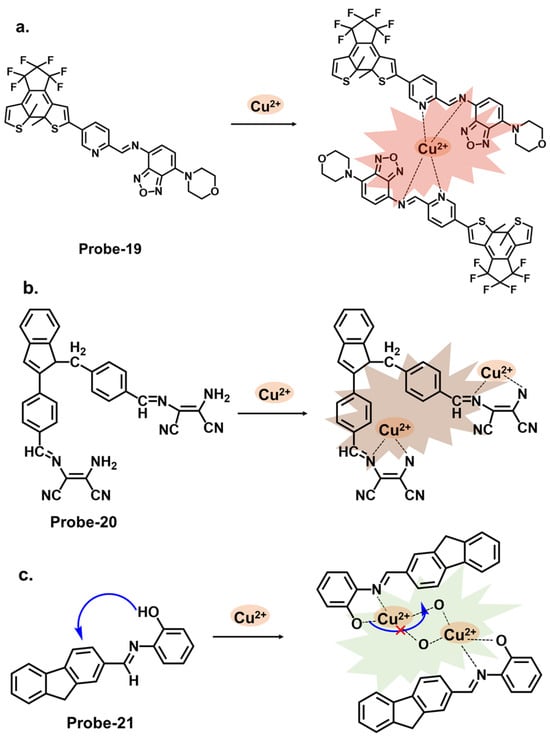
Figure 7. The structures and mechanisms of diarylethene-, benzimidazole-, and fluorene-derived Schiff base chemosensors for Cu2+; (a) probe-19, (b) probe-20, and (c) probe-21.
4. Schiff Bases as Chemosensors for Fe2+ and Fe3+
Excessive Fe2+ in water can promote the growth of certain algae species, leading to algal blooms. These blooms can deplete oxygen levels, cause water turbidity, and create dead zones, affecting other aquatic organisms and ecosystems. In humans, high levels of Fe2+ in drinking water can result in iron overload, known as hemochromatosis. This can cause organ damage, particularly to the liver, heart, and pancreas. It can also lead to gastrointestinal distress, joint pain, and fatigue. Fe3+ is another cation that has been subject to intensive research. In the environment, Fe3+ can contribute to eutrophication in water bodies when it acts as a nutrient and promotes excessive algae and plant growth. This can lead to oxygen depletion, aquatic species imbalance, and deterioration of water quality. In humans, excessive exposure to Fe3+ can disrupt the iron balance in the human body. It can interfere with the absorption, utilization, and storage of iron, leading to iron overload or deficiency, which can have various negative health consequences.
Schiff bases can selectively bind with Fe2+ ions and undergo a color change or fluorescence enhancement, allowing easy detection. The interaction between the Schiff base and Fe2+ can result in a coordination complex, altering the electronic and optical properties of the compound. This change is often observable via visual inspection or spectroscopic techniques. Schiff bases can also act as chemosensors for Fe3+ ions. Fe3+ has a high affinity for oxygen atoms, so Schiff bases with oxygen donor atoms, such as phenols or alcohols, can form stable complexes with Fe3+. The coordination of the metal ion can induce changes in the Schiff base’s electronic structure and spectroscopic properties, leading to a detectable signal such as color change or fluorescence quenching/enhancement.
Figure 8a shows probe-22 by Kouser et al., which is a benzophenone-derived Schiff base Fe2+ tracker prepared via a condensation reaction between 2-hydroxy-1-naphthaldehyde and 3,4-diamino benzophenone in methanolic solution [29][86]. The methodology section offers a detailed account of the synthesis process and characterization techniques utilized. Job’s plot, density Functional theory (DFT) calculations, and 1H NMR studies confirmed a 2:1 binding stoichiometry, while UV–vis titration estimated the detection limit at 0.0363 μM. Adding Fe2+ resulted in a calorimetric response, changing the color from light yellow to brown. In this case, the fluorescence enhancement resulted from the restriction of C=N isomerization processes and PET processes. When tested for application, probe-22 detected Fe2+ in various water samples. While the sensitivity and selectivity of the chemosensor towards Fe2+ are thoroughly discussed, a more detailed exploration into the underlying reasons for the selectivity towards Fe2+ would be beneficial. The Schiff base receptor probe-23, shown in Figure 8b (naphthalic anhydride–(2-pyridine) hydrazone) by Hou et al., was synthesized by the condensation reaction of naphthalimide monaldehyde and 2-hydrazine pyridine to detect Fe3+ [30][87]. The reversible, water-soluble and high Stokes shift (100 nm) probe formed a 1:1 complex with Fe3+, suppressing C=N isomerism and causing a turn-on response. The sensitivity was quite high, with the LOD value estimated at 38.3 nM. Probe-23 showed high anti-interference properties, while the working pH was in the range of 3.2 to 6.4. A detailed comparative study with existing fluorescence chemosensors was performed, which highlighted the unique properties of this sensor. As shown in Table 1, it is one of the few probes that was used in cancerous cells, as it is able to track Fe3+ in liver cancer cell bel-7420 with high cell permeability and low cytotoxicity. To further understand this probe, additional experiments, such as binding studies or computational modeling, could help elucidate the underlying sensor–Fe3+ interactions. Zhang et al. reported probe-24, shown in Figure 8c, which is a Schiff base Fe3+ calorimetric and fluorescent tracker based on a diketopyrrolopyrrole derivative synthesized by reacting the diketopyrrolopyrrole aldehyde with o-aminophenol [31][88]. The authors conducted a thorough sensitivity and selectivity assessment of the chemosensor towards a range of cations, providing an understanding of its capabilities. The probe is weakly fluorescent because of active PET processes. Adding Fe3+ changed the color from amaranth to rose pink. At the same time, the enhanced fluorescence resulted from the inhibition of PET and the onset of CHEF. Further analysis showed a binding constant value of 2.4 × 104 M−1 and a very low LOD of 14.3 nM, with good anti-interference, reversibility, and response time. Furthermore, analysis of various spiked water samples showed recovery rates above 92%.
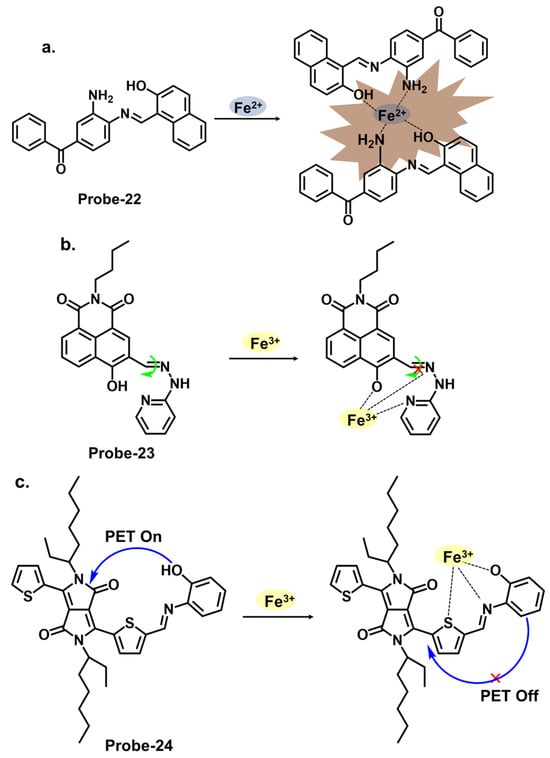
Figure 8. The structures and mechanisms of benzophenone-, pyridine-, and pyrrole-derived Schiff base chemosensors for Fe2+ and Fe3+; (a) probe-22, (b) probe-23, and (c) probe-24.
Figure 9a shows probe-25 by Selvan et al., which is a Schiff base sensor with bis-bidentate N, O sites synthesized from the condensation reaction between naphthalene-1,5-diamine and acetylacetone in ethanol [32][89]. The dual-signaling probe was used to track Fe2+ in a turn-on reaction due to suppression of the PET mechanism. It also detected Cu2+ via a charge transfer mechanism in a turn-off reaction. Emission titration experiments determined the binding ratios between probe-25 and the ions to be 1:2, while the LOD for Fe2+ ions was estimated to be 0.5 μM. The probe showed high selectivity towards Fe2+, with negligible interference from other ions. While the study provides valuable insights into the design and mechanism, further validation with diverse real-world samples would provide a better understanding of its limitations. Probe-26 by Zuo et al., shown in Figure 9b, is a turn-on Schiff base chemosensor prepared from 3,3′-dihydroxybenzidine and 1-naphthaldehyde in ethanol for the colorimetric and fluorescent detection of Fe3+ [33][90]. The authors provide a detailed overview of the synthesis methodology employed, highlighting the characterization techniques and spectroscopic analysis performed. Probe-26 is weakly fluorescent because of active C=N isomerization and PET processes. The two processes are suppressed upon coordination with Fe3+, resulting in enhanced fluorescence and a color change from pale yellow to colorless. Job’s plot confirmed the binding ratio to be 1:2, while fluorescence titration spectra estimated the LOD to be as low as 0.178 μM, reflecting high sensitivity. For application, probe-26 was successfully used in real water and food samples, with recovery rates above 98%. Furthermore, tests on paper strips also showed visible color changes in the presence of Fe3+, attributed to ligand-to-metal charge transfer (LMCT).
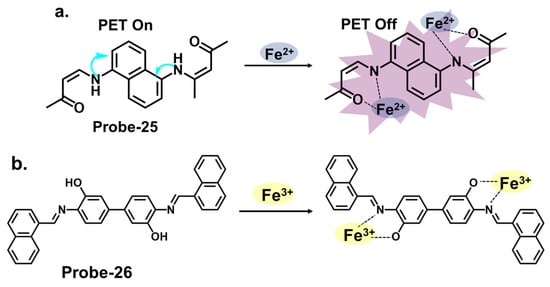
Figure 9. The structures and mechanisms of naphthalene- and dihydroxybenzidine-derived Schiff base chemosensors for Fe2+ and Fe3+; (a) probe-25 and (b) probe-26.
Thiophene derivatives can also be used in tandem with other functional groups to create cation-targeting probes, as shown in Figure 10a. Guo et al. reported probe-27, a dual-functional Schiff base chemosensor based on oligothiophene–phenylamine moieties synthesized to detect Al3+ and Fe3+ ions simultaneously [34][91]. Probe-27 is weakly fluorescent because of twisted intramolecular charge transfer (TICT) processes. Adding Fe3+ resulted in a probe-27-Fe2+ complex and enhanced fluorescence due to suppression of the TICT process. The fluorogenic chemosensor detected Al3+ and Fe3+ with LOD values of 0.177 µM and 0.172 µM, respectively, with a color change from colorless to green. The Job’s plot confirmed 1:1 binding ratios for both ions, and the probe was proven operational in the pH range of 4.0 to 12. Furthermore, real water and food samples tests showed recovery rates above 97%. The findings presented in this study are interesting, and a deeper dive into the mechanistic features, such as rigidity or planarity, would further help explain the selectivity and the subsequent fluorescence activation. Rhodamine derivatives utilize the open and closed form to produce fluorescent changes which form the basis for detection. Figure 10b shows probe-28 by Murugan et al., which is a Schiff base receptor prepared from rhodamine hydrazide and salicylaldehyde to detect Fe3+ and Cu2+ ions [35][92]. The turn-on reception mechanism results from the spirolactum ring cleavage, followed by ICT, resulting in enhanced fluorescence and a color change from colorless to red. The sensitivity was very high, with LOD values of 3.1 nM and 2 nM for Fe3+ and Cu2+ ions, respectively. Emission studies showed that probe-28 possesses strong anti-interference properties, while Job’s plot analysis showed binding ratios of 1:1 for both metals. For application, probe-28 detected Fe3+ in zebrafish embryos while showing negligible cytotoxicity. It would be worthwhile to study the solvent effects or other potential limitations or side reactions associated with the chemosensor’s fluorescence response to gain a better understanding of its applications.
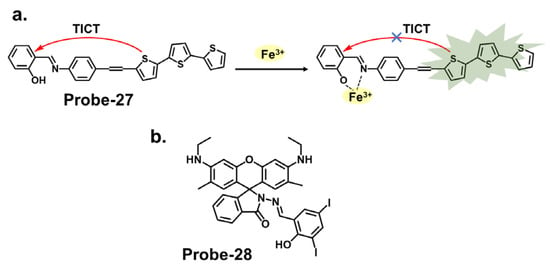
Figure 10. The structures (and mechanisms) of thiophene- and rhodamine-derived Schiff base chemosensors for Fe3+; (a) probe-27 and (b) probe-28.
5. Schiff Bases as Chemosensors for Zn2+
Zinc can enter water bodies through natural processes, such as weathering of rocks and soil erosion, as well as from human activities such as industrial discharges, mining, and agricultural runoff. Elevated zinc levels in water can be toxic to aquatic organisms, including fish, insects, and plants [36][108]. It can disrupt their physiological processes, impair growth and reproduction, and cause mortality. Zinc contamination can also affect the ecosystem balance and biodiversity in aquatic habitats [37][109]. Monitoring zinc levels can help understand its distribution and regulation within living organisms and its role in health and disease [38][110]. Chemosensors for zinc can provide valuable insights into zinc homeostasis and aid in developing therapies for zinc-related disorders.
When pyrrole units are introduced into the Schiff base structure, they enhance the fluorescence response of the sensor. Pyrrole is a conjugated aromatic ring with a high electron density, allowing efficient π-electron delocalization [39][111]. This delocalization leads to enhanced fluorescence emission upon excitation. The functionalization of pyrrole units in the Schiff base chemosensors can be tailored to recognize specific analytes. For example, different substituents or functional groups can be introduced on the pyrrole ring to enable selective detection of metal ions, anions, or organic molecules [40][112]. The interaction between the analyte and the Schiff base sensor leads to changes in the electronic environment and fluorescence properties, providing a means for analyte detection and quantification. Wang et al. reported a condensation reaction between isonicotinohydrazide and ethyl 5-formyl-2,4-dimethyl-pyrrole-3-carboxylate in ethanol to produce a pyrrole-bearing Schiff base hydrazone chemosensor (probe-29) for the calorimetric and fluorescence tracking of Cu2+ and Zn2+, respectively (Figure 11a) [41][93]. CHEF was activated upon adding Zn2+, with a low LOD value of 0.18 μM. Probe-29 also showed high cell penetration, as it was used to detect Zn2+ in U251 glioma cell lines. While the response to Zn2+ is presented with clarified calorimetric and fluorimetric changes, the range of analytes used to assess selectivity could be expanded further. He et al. reported the condensation of 1,2-cyclohexane diamine and 3-(tert-butyl)-5-formyl-4-hydroxybenzoic acid to prepare probe-30 for the selective recognition of Zn2+ and pH changes (Figure 11b) [42][94]. The authors describe the synthesis and characterization of the Schiff base chemosensor in detail, including the reaction conditions and purification techniques employed. Spectroscopic analysis techniques such as UV–vis, fluorescence, and NMR are utilized for structural characterization. The blue luminescence observed was due to the suppression of PET processes. The LOD was determined to be 56 nM, indicating high sensitivity. For applications, probe-30 proved functional in paper strips, zebrafish, and live cells. Benzothiazole has been used in several studies as a backbone for fluorescent chemosensors. The fluorescence response of benzothiazole Schiff base chemosensors relies on the coordination of binding events with target analytes. The imine or azomethine group in the Schiff base provides a binding site for various species through coordination chemistry, hydrogen bonding, or other interactions. When the target analyte binds to the Schiff base, it can induce changes in the benzothiazole moiety’s electronic structure or environment, leading to changes in the fluorescence properties. Wu et al. prepared a substituted Schiff base benzothiazole (probe-31) for the turn-on detection of Zn2+ (Figure 11c) [43][95]. The ratiometric sensor uses benzothiazole as a signaling moiety, while the Schiff base moiety is the receptor site. It is fluorogenic, as the color shifts from orange to green. The 1:1 interaction between the Zn2+ and the phenolic O inhibits the ESIPT process, leading to CHEF. Furthermore, it proved very sensitive, with the LOD estimated at 37.7 nM. While the spectral studies clearly show high selectivity towards Zn2+, reasons for non-selectivity towards other divalent ions such as Co2+ and Cu2+ could be investigated further.
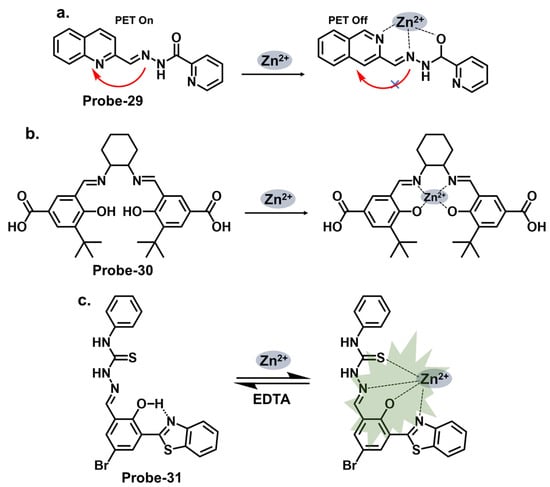
Figure 11. The structures and mechanisms of pyrrole-, hydroxybenzoic-acid-, and benzothiazole-derived Schiff base chemosensors for Zn2+; (a) probe-29, (b) probe-30, and (c) probe-31.
Carbazole macrocycles, shown in Figure 12a, are cyclic structures of multiple carbazole units which offer several advantages as chemosensors. The presence of multiple carbazole units within the macrocycle enhances the rigidity and stability of the structure, which is crucial for efficient fluorescent responses. The extended conjugation provided by the carbazole units allows for a redshift in the absorption and fluorescence spectra, making them suitable for various applications. They offer a promising platform for developing sensing systems with applications in various fields, including environmental monitoring, biological imaging, and chemical analysis. The combination of their inherent fluorescence properties and the versatility of Schiff bases provide ample opportunities for designing efficient and selective chemosensors. Malthus et al. used macrocycles derived from carbazole units to prepare highly selective Schiff base probes (probes-32a and 32b) to detect Zn2+ [44][96]. The condensation reaction between ethylene diamine and carbazole dialdehyde produced two slightly different probes that relied on the suppression of PET and CHEF to produce a turn-on reaction in the presence of Zn2+. Overall, the probes had minimal interference, LODs in the nanomolar range, and strong binding, confirmed by ESI, 1H NMR, and DFT calculations. This introduces new high-yielding and scalable reactions to form a new class of carbazole-based Schiff base macrocycles, whose applications could be explored further. Upadhyay et al. reported the condensation of 1-pyrenemethylamine with the vitamin B6 cofactor pyridoxal to create a three-in-one Schiff base probe (probe-33) for tracking Zn2+, hydrogen phosphate, and cysteine (Figure 12b) [45][97]. Again, the turn-on response originates from the suppression of the PET mechanism between the imine nitrogen and the electron-accepting pyrene fluorophore. The LOD values were sufficiently low (Table 1), determined to be 2.3 μM, 0.21 μM, and 0.16 μM for Zn2+, hydrogen phosphate, and cysteine, respectively. Probe-33 was also functional, as it was successfully used to capture images of treated and untreated HeLa cells. An investigation into the response time across all three analytes will give a clearer assessment into its suitability for real-time monitoring and detection of analytes, which would have significant practical applications in areas such as environmental monitoring, healthcare, and food safety.
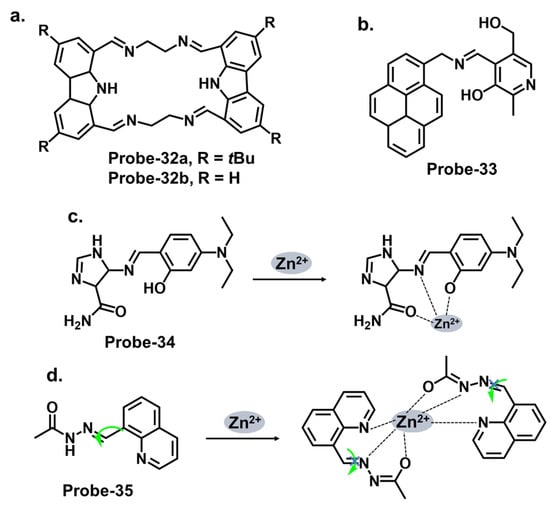
Figure 12. The structures (and mechanisms) of carbazole-, pyrenemethylamine-, imidazole-, and quinoline-derived Schiff base chemosensors for Zn2+; (a) probes-32a, 32b, (b) probe-33, (c) probe-34, and (d) probe-35.
Imidazole-based Schiff base compounds have also been explored as fluorescent chemosensors due to their unique characteristics and potential applications. Imidazole is a five-membered heterocyclic aromatic compound containing two nitrogen atoms (Figure 12c). By incorporating a Schiff base functionality, which includes an imine or azomethine group, imidazole Schiff bases can exhibit interesting fluorescence responses upon interaction with specific analytes. Yun et al. reported an imidazole derivative as a Schiff base probe (probe-34) for the turn-on-off detection of Zn2+, S2−, and Zn2+/3+ ions [46][98]. Probe-34 was prepared from the condensation reaction between (4)-amino-4(5)-(aminocarbonyl)imidazole hydrochloride and 4-diethylamino salicylaldehyde in MeOH. PET between the moieties renders the probe weakly fluorescent. Adding Zn2+ disrupts the PET process, resulting in a turn-on fluorescence via CHEF. Job’s plot confirmed the 1:1 stoichiometry, while LOD values were estimated to be as low as 1.59 μM and 8.03 μM, respectively. Furthermore, the coordination proved reversible, as the addition of EDTA released the analyte. Probe-34 is one of very few sensors that can recognize four analytes. In fact, its reception to four analytes makes the probe vulnerable to false positives, so it would be worthwhile to investigate the underlying physical phenomena to gain understanding of the reported turn-on/-off mechanism.
Quinolines are versatile organic compounds that have been widely employed as fluorescent chemosensors. Their unique photophysical properties, including high quantum yields, large Stokes shifts, and tunable emission wavelengths, make them suitable candidates for sensing applications. By modifying the structure of quinolines, researchers can design chemosensors with specific recognition capabilities for various analytes. One common approach is introducing functional groups or receptors into the quinoline structure to form a Schiff base probe exhibiting selectivity towards a particular analyte. These functional groups can undergo specific interactions such as hydrogen bonding, coordination, or ion-pairing with the target analyte, leading to changes in the fluorescence properties of the quinoline. This fluorescence intensity, color, or lifetime change can be easily monitored and quantified, providing a reliable signal for analyte detection. Wu et al. reported a high-yield condensation reaction to synthesize a Schiff base chemosensor (probe-35) with a large Stokes shift (>200 nm) for the detection of Zn2+ in aqueous media in the micromolar range (Figure 12d) [47][99]. The probe itself is weakly fluorescent because of C=N isomerism. The introduction of Zn2+ inhibits the isomerization, as the probe coordinates with the ion in a 2:1 stoichiometry and an LOD on 89.3 nM. The probe was used to detect Zn2+ in HeLa cells with very low cytotoxicity for application. While spectral studies and computational models were performed to determine selectivity and mechanism, further investigations into the stability and photostability of the chemosensor under various environmental conditions, such as pH and temperature variations, would provide useful information for practical applications.
Salicylaldehyde has been used as an anchoring group in various chemosensor designs [48][113]. Jia et al. reported probe-36, shown in Figure 13a, which is an AIE-active Schiff base receptor based on tetraphenylethylene-functioned salicylaldehyde for tracking Zn2+ [49][100]. The authors provide a clear and detailed description of the chemosensor’s structural framework, with the synthetic route and the characterization techniques adequately explained. The turn-on probe displayed reversible mechanofluorochromism, reflected by the emission color change from yellowish-green to orange-yellow after grinding. The enhanced fluorescence is due to the inhibition of the PET and the ESIPT processes once the Zn2+ ions were added. Probe-36 also showed high sensitivity, reflected by an LOD value of 80.5 nM. Rhodamine Schiff bases are chemosensors derived from coupling rhodamine dyes with various aldehydes or ketones. Incorporating the Schiff base functionality introduces a new chromophore, leading to changes in the photophysical properties of the rhodamine dye. This alteration in the optical properties allows for detecting and quantifying specific analytes or ions in solution. The detection mechanism of rhodamine Schiff base chemosensors is based on molecular recognition. The imine linkage in the Schiff base structure can selectively interact with target analytes through various non-covalent interactions such as hydrogen bonding, coordination, or electrostatic interactions. These interactions lead to changes in the rhodamine dye’s absorption and/or emission spectra, providing a signal response that can be monitored spectroscopically. Liu et al. reported probe-37 (7-methoxychromone-3-carbaldehyde-rhodamine B carbonyl hydrazone), shown in Figure 13b, for the turn-on detection of Zn2+ [50][101]. The probe incorporated the rhodamine and chromone moieties, which facilitated PET processes. The 1:1 coordination with Zn2+ inhibited the PET process, enhancing emission. The sensitivity was high, with the LOD value estimated at 0.34 μM. Its integration into paper strips produced a convenient and low-cost test kit for tracking Zn2+.
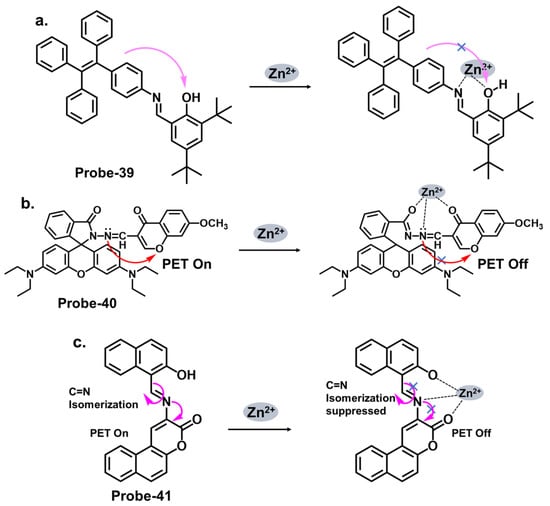
Figure 13. The structures and mechanisms of salicylaldehyde-, rhodamine-, and benzocoumarin-derived Schiff base chemosensors for Zn2+; (a) probe-36, (b) probe-37, and (c) probe-38.
Coumarins exhibit fluorescence due to the presence of conjugated π-electron systems. When excited by light of a specific wavelength, they absorb energy and then emit light at a longer wavelength. The extent of fluorescence can be influenced by the surrounding environment, allowing for the development of fluorescent chemosensors. They are versatile building blocks for developing fluorescent chemosensors. Their inherent fluorescence, combined with appropriate modifications and receptor groups, enables the selective detection and quantification of various analytes, offering significant potential for applications in analytical chemistry and sensor technology. The fluorescence response of coumarin-based Schiff base chemosensors can be attributed to various mechanisms, including ICT, excited-state proton transfer, or heavy atom effect. These mechanisms can result in changes in the fluorescence intensity, emission wavelength, or lifetime of the coumarin moiety. Liu at al. synthesized a multi-analyte probe (probe-38) from 3-Amino-5,6-benzocoumarin and 2-hydroxy-1-naphthaldehyde for the detection of Zn2+, Cu2+, and S2− (Figure 13c) [51][102]. The inherent intramolecular PET and C=N isomerization renders the probe poorly florescent. However, the addition of Zn2+ results in the suppression of these processes, leading to enhanced fluorescence. The binding was 1:1 for this structure, with an LOD value estimated at 3.6 μM. Like coumarins, pyrenes possess a conjugated π-electron system that imparts strong fluorescence properties. While the spectral studies were thoroughly performed to determine selectivity and interference, theoretical calculations could be explored to elucidate the underlying sensor–Zn2+/Cu2+/S2− interactions.
Pyrene derivatives have also been extensively explored as chemosensors for various ions [29][86]. Rani et al. combined pyrene and malonohydrazide groups to develop a receptor (probe-39) for tracking Zn2+, as shown in Figure 14a [52][103]. Because Zn2+ and Cd2+ have similar spectral properties, this sensor was prepared specifically to distinguish between the two. The presence of donor (imine nitrogen) and acceptor groups (pyrene) ensured active PET processes, making the receptor weakly fluorescent. Adding Zn2+ disrupts the PET processes, as it attaches to the NH nitrogen in a 1:1 binding stoichiometry. Probe-39 had low cytotoxicity in HeLa cells and exhibited enhanced fluorescence once Zn2+ was added. It would be interesting to extend the analysis to real-world samples, such as environmental water or other biological fluids, to fully assess the practical applications. Shellaiah et al. reported a one-pot reaction for synthesizing an AIEE-active pyrene-based Schiff base chemosensor (probe-40) for detecting Zn2+ and tyrosine in the solution [53][104]. Characterization data confirmed a successful synthesis. The probe, shown in Figure 14b, coordinated with Zn2+ in a 2:1 stoichiometry, suppressing PET processes and enhancing the fluorescence. The LOD was estimated to be 0.79 nM from fluorescence linear fittings. It also proved to be biocompatible and could track Zn2+ in B16F10 cell lines and zebrafish.
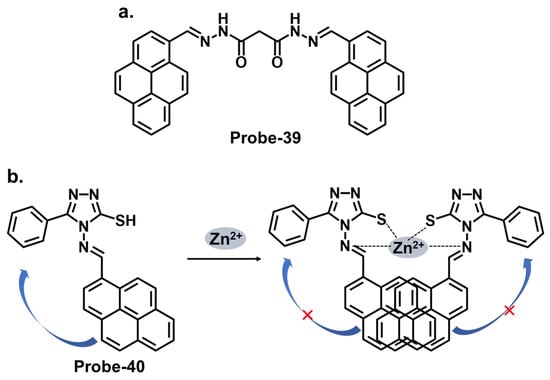
Figure 14. The structures (and mechanisms) of pyrene-derived Schiff base chemosensors for Zn2+; (a) probe-39 and (b) probe-40.
6. Schiff Bases as Chemosensors for Other Ions
Naphthyl hydrazones consist of a naphthyl group bonded to a hydrazine functional group (-NHNH2). Naphthyl hydrazones are utilized as fluorescent chemosensors for the detection of metal ions, anions, and biologically relevant species. The principles behind the use of these compounds in fluorescent chemosensors lie in their ability to undergo specific interactions or reactions with target analytes, leading to changes in their fluorescence properties. These changes can be measured and correlated with the analyte concentration or environmental conditions. Probe-41, shown in Figure 15a, contains a naphthol-hydrazine-based chemosensor skeleton covalently linked to the electron-withdrawing benzothiadiazole group [54][105]. The detailed experimental section enhances the reproducibility of this study and provides a valuable reference for researchers interested in developing similar structures. The probe is strongly fluorescent because of ICT. When Fe3+ is introduced, CHEQ is initiated, with a response time of 55 s and an LOD of 36 nM. Probe-42, shown in Figure 15b, is a naphthol hydrazone Schiff base receptor modified with pyridine to track Al3+ [55][106]. The extensive conjugation and active ICT render it strongly fluorescent. When Al3+ is present, ICT is suppressed, resulting in weaker emission. The LOD was estimated at 0.164 µM, while Job’s plot gave a 1:1 binding ratio. Probe-43, shown in Figure 15c, contains a naphthol hydrazone backbone attached to a thiophene ring [56][107]. The combined structure is an asymmetrical azine derivative, which was used to track Cr3+. The structure undergoes CHEQ in the presence of Cr3+, with no obvious interference from other ions. The affinity towards Cr3+ was explained by the HSAB theory; since Cr3+ is a hard acid, the ligand can be characterized as a hard Lewis base. The sensitivity was high, with the LOD estimated at 41 nM. For application, probe-41 and probe-43 were used to track Fe3+ and Cr3+ in PC3 cells, while probe-42 was used to track Al3+ in HeLa cells.
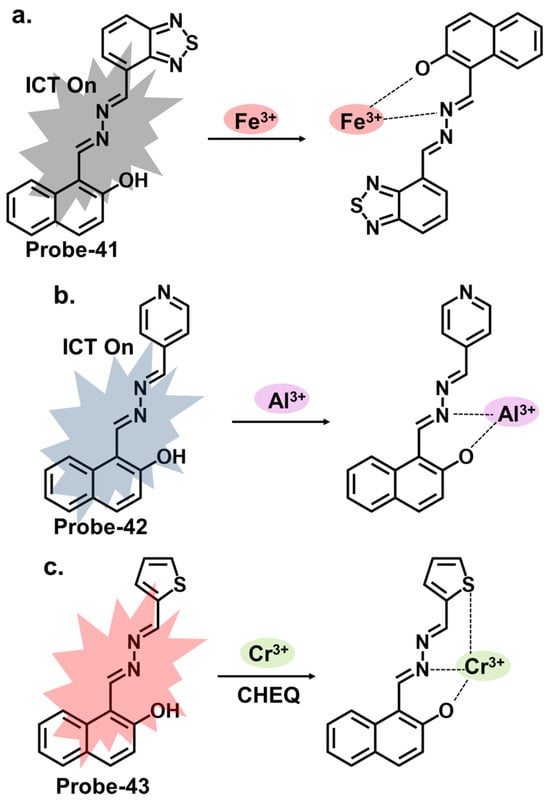
Figure 15. The structures and mechanisms of benzothiadiazole-, pyridine-, and thiophene-based Schiff base chemosensors for Fe3+, Al3+ and Cr3+, respectively; (a) probe-41, (b) probe-42 and (c) probe-43.
