You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by DAIVA ŽADEIKĖ.
Acoustic technology is characterized as environmentally friendly and is considered an alternative method due to its sustainability and economic efficiency. This technology provides advantages such as the intensification of processes, increasing the efficiency of processes and eliminating inefficient ones, improving product quality, maintaining the product’s texture, organoleptic properties, and nutritional value, and ensuring the microbiological safety of the product.
- ultrasound
- safety monitoring
- bioprocess regulation
1. Introduction
In recent years, EU and global efforts have been aimed at solving the problems of the efficient use of resources, ecology, and the development of food production technologies, to increase the sustainability and efficiency of processing. Various traditional processing and preservation methods, such as extrusion, filtration, extraction, drying, frying, cooking, fermentation, etc., are still widely used to process raw food materials. The underlying principle of most traditional food-processing methods depends on the use of high-temperature regimes to inhibit foodborne pathogens, thus ensuring the safety of food products [1]. Therefore, non-thermal technologies for food processing, such as ultrasound, irradiation, pulsed electric fields, cold plasma, and high hydrostatic pressure, have been widely evaluated [2]. These technologies enable prolonging the shelf life of the food, maintaining its nutritional, texture, and sensory characteristics, as well as increasing the bioavailability of food nutrients [3].
Acoustic technology is one of the sustainable alternatives to thermal processing, which is recognized as economically efficient and environmentally friendly and can be applied in the food (cereals, milk, meat, fruits/vegetables, drinks, etc.) production industry and also in the development of bio-based and biodegradable materials for safe food packaging [4,5][4][5]. This technology provides advantages, such as the intensification of technological processes, increased extraction efficiency, the modification of food components, maintaining its texture, organoleptic properties, and nutritional value, and ensuring the microbiological safety of the product [6,7,8,9,10,11][6][7][8][9][10][11]. Depending on the intensity, ultrasound (US) can be used for the activation or deactivation of enzymes, mixing, homogenization, emulsification, preservation, stabilization, ripening, and solid–liquid extraction to improve the extraction yields of active ingredients from different matrices. Moreover, the advances in the development of innovative non-thermal technologies can meet consumer demand for high-quality, safe, nutritious, and minimally processed foods [12].
In the food industry, most applications of high-power low-frequency US (>1 W/cm2; <100 kHz) are based on systems in which a liquid or a gaseous medium (such as air) is used for the propagation of the ultrasonic waves [13]. This type of US, known as power US, induces mechanical, physical, chemical, and biochemical changes through acoustic cavitation, caused by the formation, growth, and collapse of bubbles, releasing a large amount of energy. The energy is used in food-processing operations, such as drying, extraction, emulsification, and inactivation of pathogenic bacteria and their enzymes in the food matrix or on its surface [14,15][14][15].
2. Ultrasound Generation Systems
Acoustic technology is based on mechanical waves, and the frequency, amplitude, and wavelength are intrinsic characteristics of acoustic waves (Figure 1).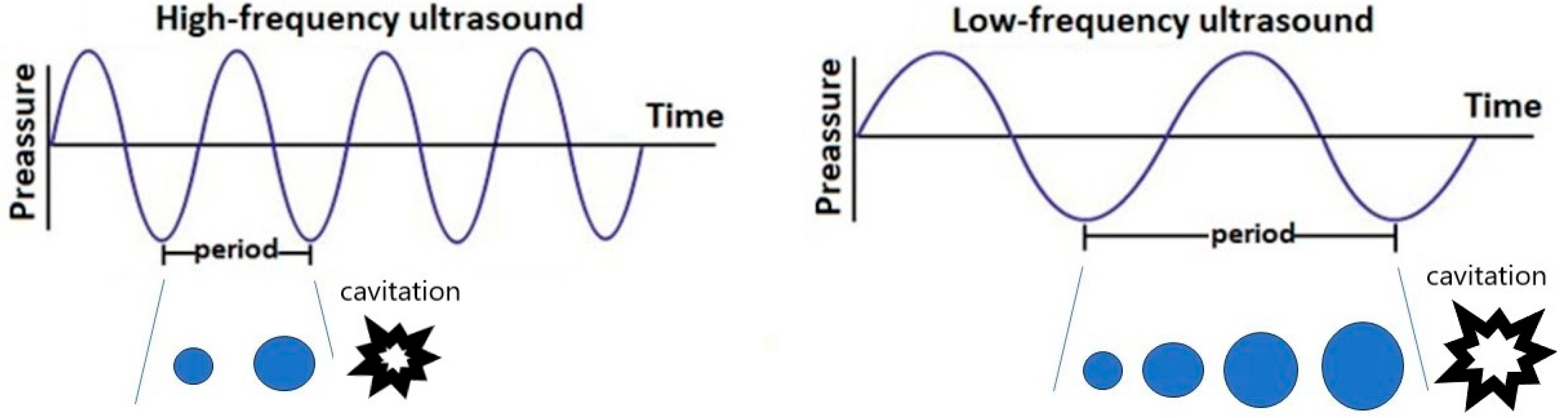
Figure 1.
Acoustic waves of high- and low-frequency ultrasound. Blue dots—cavitation bubble.
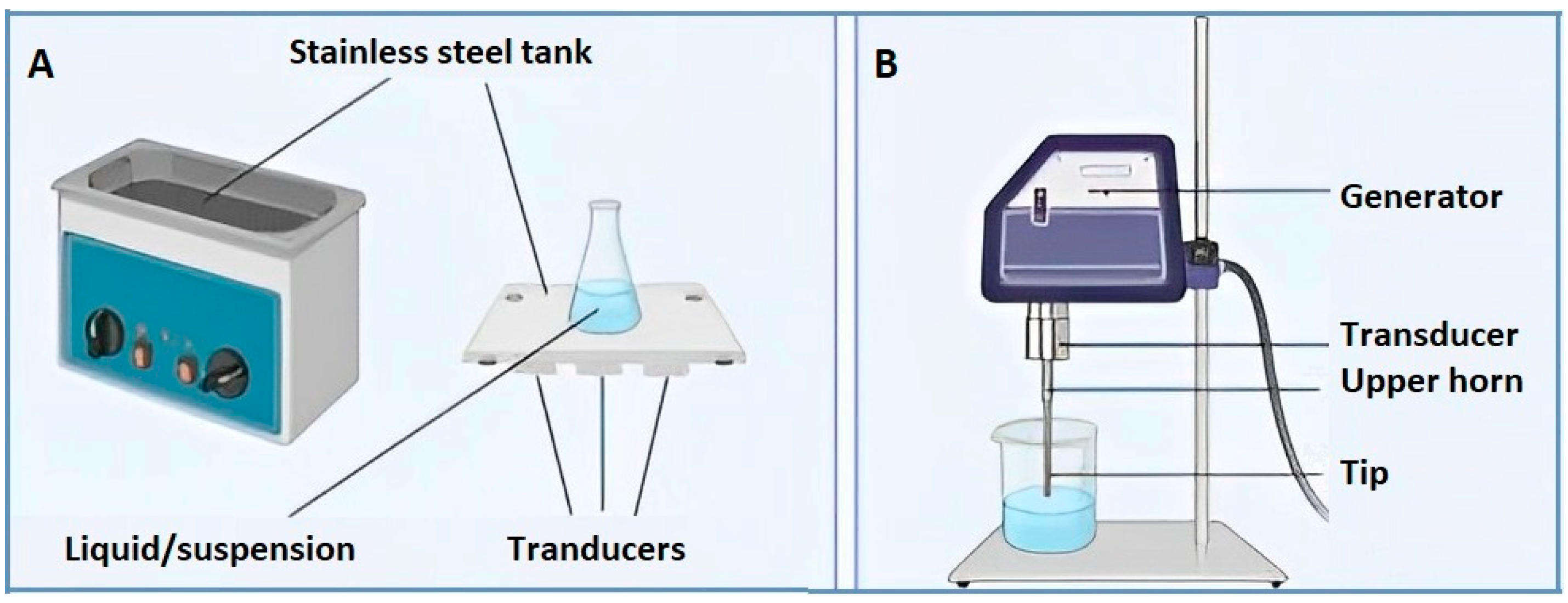
Figure 2.
Acoustic systems: ultrasonic bath (
A
) and ultrasonic probe (
B
).
3. Application of Acoustic Technology in Food Processing for Quality and Safety Control
3.1. Fruits and Vegetables
The extension of the shelf life of fresh or minimally processed fruits and vegetables is a key problem to be solved during their post-harvest storage [35][30]. Fully removing microorganisms on the surface of products remains a challenge in the food industry [35][30]. In the review of Chen et al. [36][31], US processing emerged as a novel tool for food preservation, providing antimicrobial effects due to the cavitation process. As water is used in this technology, it could be a promising method, which could be implemented in the washing step to obtain safe fresh or fresh-cut fruits and vegetables [36][31]. Hereby, US-assisted fruit drying reduces wastewater toxicity and energy consumption and improves productivity. US was reported to be effective for application in minimally processed products, causing only minimal losses in natural aromas and colors, maintaining an acceptable quality of fresh and cut fruits and vegetables and inhibiting or limiting the formation of microbes [37][32]. US (frequencies usually used during ultrasonic-assisted drying are between 20 and 40 kHz to avoid the continuous loss of sound wave energy) can be used as a pre-treatment prior to drying fruits and vegetables, since it increases the drying kinetics [38][33]. Ultrasound-assisted treatment is a pesticide-removal technology, which is safer for the environment and more effective at pesticide removal for a number of fruits and vegetables, including grapes, cabbage, carrots, tomatoes, and cucumbers [39,40][34][35]. For this purpose, in the study of Cengiz et al. [41][36], two kinds of US treatments, an ultrasonic bath at 40 kHz and an ultrasonic probe at 24 kHz, in combination with a low-intensity electrical current (1400 mA + 40 kHz, 800 mA + 24 kHz, and 1400 mA + 24 kHz) were tested for the determination of US’s effectiveness in the reduction of some important pesticide residues in tomato samples. These combinations led to a reduction of the captan, thiamethoxam, and metalaxyl residues by 94.24%, 69.80%, and 95.06%, respectively [41][36]. Lozowicka and co-authors demonstrated that, for strawberries, a 5 min cleaning step with US (40 kHz; 5 min) efficiently reduced sixteen pesticide residues by 91.2% [42][37]. Similarly, for lettuce surfaces, US with frequencies of 20–60 kHz can be used to remove insecticide contents by up to 89–95% after 8 min of US treatment without any changes in the nutritional properties [43][38]. It was found that the residue levels of organophosphorus pesticides (trichlorfon, dimethoate, dichlorvos, fenitrothion, and chlorpyrifos) on raw cucumber were significantly reduced by up to around 85% after US treatment (US bath, 40 kHz) for 20 min [44][39]. Concerning transient cavitation, under high-intensity ultrasound, bubbles attain the required size promptly and rupture, which would cause a high pressure (up to 100 MPa) and temperature (up to 5000 K) in a short period [36,44][31][39]. This causes the pyrolysis of pesticides and cell disruption, which promote reactions between the reactive species and the pesticide molecules. Acoustic technology has gained much attention due to its inhibitory effect on browning enzymes thanks to the capability of breaking the cell membranes. In particular, it has been discovered that US in combination with temperature and high pressure is more effective against polyphenol oxidase (PO) [45][40]. The PO in the original juice of various fruits was less inactivated than the US-treated purified form [46][41]. Ultrasonication at an intensity >200 W induced the aggregation and dissociation of PO particles and significantly decreased the α-helix structure. Similarly, pineapple juice had its content of PO reduced after 10 min of US treatment, as well as a viscosity decline of 75% [47][42]. PO decreased also on fresh cut potatoes after 5 min of US treatment without a change in color, while a 10 min treatment damaged the cells of potatoes [48][43]. The anti-browning effectivity, measured as PO activity increase, improved when ultrasound (40 kHz) was combined with ascorbic acid (1%) in fresh-cut apple in storage at 10 °C during 12 days [49][44]. In summary, US may be valid for the fresh-cut fruit/vegetable industry since the majority of components and sensory attributes are not affected. Furthermore, ultrasound can be considered as an alternative technique to heat treatments.3.2. Cereals and Cereal Products
Contamination of certain foods with toxins, produced by some organisms, poses challenges to ensuring food safety and quality. Cereal-based food products comprise vital nutrients that have the potential to be naturally contaminated by various fungi, as well as their secondary toxic metabolites, considered as mycotoxins, which can infect the crops before or after harvest and can be typically found in foods, such as cereals, dried fruits, nuts, and spices [50][45]. Cereal crops give filamentous fungi, such as Fusarium and Aspergillus spp., an important opportunity to grow using the edible parts of the plants, such as starch granules [51][46]. The result of the fungal invasion is grains shriveling and becoming more porous. Among various approaches with the aim of eliminating or at least reducing the presence of mycotoxins in food, ultrasonication is one of the novel techniques being discussed, partially due to the avoidance of direct heat during the processing [53][47]. As was shown, US treatment under different conditions allowed a reduction in the amounts of selected mycotoxins from about 40% (aflatoxins B1, B2, G1, and G2) [54][48] to 96% (aflatoxin B1, deoxynivalenol (DON), zearalenone (ZEA), ochratoxin A (OTA)) [55][49]. The degradation of mycotoxins in aqueous solutions and maize was significantly affected by the US intensity (2.2–11 W/cm2) and sonication time range from 10 to 50 min, showing DON being more stable than AFB1, ZEA, and OTA [54][48]. According to Liu et al. [55][49], during the initial stage of US treatment (1.1–1.65 W/cm2; 8 min), power US can promote the dissolution of AFB1 and ZEA, which were subsequently dissolved in water and were partly oxidized by free radicals under the effect of power US. A possible mechanism of mycotoxin degradation was proposed by Liu et al. in another study [56][50], when 550 W power US (20 kHz; 6.6 W/cm2) with a 13 mm probe was applied for processing aqueous AFB1, which was degraded by 85.1% after 80 min of US exposure, significantly reducing its bioactivity and toxicity because of mycotoxin degradation by the free radicals generated during the acoustic treatment. The dissolution of mycotoxins in water also facilitates their degradation. In this case, the presence of water plays a critical role, as the covalent bonds of the water molecules break upon US treatment, thus yielding highly reactive radicals, hydroxyl and hydrogen, together with the subsequent formation of hydrogen peroxide outside cavitation bubbles, which then attack the organic molecules of toxins and initiate their decomposition. Due to the adverse health effects caused by mycotoxins, the EU has been working for almost two decades on the harmonization of mycotoxin standards for foods based on established international regulations and methods of analysis and sampling. Because of the high cost of state-of-the-art invasive, relatively slow, multi-step, and expensive wet chemistry methods and the reluctance of industry to perform representative sampling for food safety purposes, the EU felt obliged to enforce rather strict measures for sampling (EC 401/2006). Taking into account the above-mentioned aspects, there is a growing interest in the application of new, faster methods for inline or online monitoring of the presence of mycotoxins in food. Rapid methods, such as near-infrared (NIR) spectroscopy, appear to provide a new approach to monitoring the quality of agricultural products [57,58][51][52]. Yearly, the potential of using IR spectroscopy for the detection of mycotoxins, including deoxynivalenols (DON), ochratoxin, fumonisins, aflatoxins, and fungal contamination in cereals and cereal products, has also been demonstrated [59,60][53][54]. However, the technique is not particularly suitable for routine batch analysis because of the limited applications, while the slow scan speed and low sensitivity appear to be the main disadvantages of using IR instruments. Acoustic waves generated by an acoustic spectrometer, working in a range of 4.95–35.70 kHz and measuring the amplitude of the acoustic signal penetrating through the tested sample (thickness of grain layer: 30 mm; diameter: 50 mm) have been proposed to be applied to determine the preliminary level of mycotoxin contamination based on changes in the microstructure of unaffected (wholesome) and affected (contaminated/scabby) grains and aflatoxin-inoculated corn kernels [52,61,62][55][56][57]. The speed and non-invasive character of an acoustic method make it suitable to be used to carry out inline high-throughput analysis, by testing product/matrix porosity, which is influenced by the structure (grain size, shape) and moisture content of contaminated grain matrix, preferably at the point of harvest or in the cereal-processing chain before milling and further processing [61][56]. Moreover, the efficiency of US application in the reduction of mycotoxins in contaminated wheat-based products can be explored by using a multi-step prevention system: acoustic screening of grains with the elimination of contaminated grain from the production chain and, in the second step, a detoxification approach (e.g., ultrasonication and fermentation) [63,64][58][59]. Trakselyte-Rupsiene and co-authors used fermentation with lactic acid bacteria (LAB) in combination with an acoustic screening method (acoustic spectrophotometer; frequency: 10–60 kHz; duration: 10 s; thickness of the grain layer: 50 mm; diameter: 80 mm) for the prevention of Fusarium spp. mycotoxins in wheat grain, as well as in fermented products [63][58]. The study suggested that the acoustic technique used could identify DON, as well as DON-3-β-d-glucoside (D3G) contamination in raw wheat and is a promising tool in the wheat-grain-processing chain, and also, fermentation, using appropriate LABs, can reduce DON and D3G content in the fermented product by up to 75% and 84%, respectively. All authors indicated that the US power density and intensity, the solid–liquid ratio, and the US treatment modes significantly affect the degradation rates of mycotoxins. Even more, in an interesting study by S.O. Kerhervea et al. [65][60], the development of the US technique was reported, intended for quality control by measuring the mechanical properties of noodle doughs. These noncontact measurements were applied online during dough processing at a pilot plant, giving the advantage of avoiding contamination arising from direct contact with the monitoring equipment. Furthermore, the authors also declared that this ultrasonic technique could be promising if monitoring changes in the properties of noodles caused by proteases that can be associated with biological contamination, mycotoxins, sometimes present in wheat [65][60].3.3. Beverages
Currently, juice is one of the most-popular beverages in the food industry. However, juice usually suffers from the loss of important nutrients, freshness, and quality during the thermal processing necessary for food safety and quality preservation. Thermal treatment (>70–90 °C) denatures proteins, inactivating microorganisms and enzymes, which causes undesirable changes in the juice and shorten its shelf life. Incomplete inactivation of enzymes results in browning and cloudiness [36][31] and changes in the biochemical, physicochemical, organoleptic properties, and nutritional components (vitamins, phenolic, etc.) of juices [66][61]. For example, the hot water treatment of tomato juice at 90 °C for 90 s resulted in low lycopene retention (67%) and changes in color [67][62]. Unwanted color reduction (50%) and a decrease in ascorbic acid content (15–40%) occurred in strawberry juice after thermal processing (90 °C, 5 min) compared to a pulsed electric field (100 Hz, 500 µs) [68][63]. To maintain the desirable sensory characteristics of food, such as taste, flavor, texture, and overall acceptability, some acoustic-assisted processes have been developed. The study of Lagnika et al. [69][64], investigating the effects of US processing on the physicochemical and nutritional quality of pineapple juice, showed US treatment (500 W; 20 kHz; probe diameter of 10 mm; 15 min; <65 °C) had a significantly lower browning degree. US was effective at retaining the total phenolic content and delaying microbial growth in pineapple juice as compared to the thermally treated or untreated juice sample during 60 days of storage at room temperature. The study demonstrated that US combined with mild heat pasteurization treatment (65 °C; 15 min) could be able to effectively inactivate the microorganisms and pectin methylesterase in pineapple juice, whilst preserving a relatively high amount of phenols [69][64]. According to Shen et al., US treatment (26 kHz, 9 min) of green grape juice preserved its sensory attributes, providing the significant inactivation of microorganisms (<1 log CFU/mL) and the enrichment of bioactive compounds (up to 482.47–543.62 μg/mL) [70][65]. Similarly, temperature-controlled US treatment (55 °C; 10 min; intensity 75%) can promote better appearance and odor in apple juice, indicating the improvement of the sensory characteristics through microbiological stabilization [70][65]. Evidence of the advantage of US as a nonthermal approach in stabilizing the food matrix was demonstrated on cherry tomato [71][66]. According to the authors, applying of dual-frequency US (20/40 kHz) for 10 min to cherry tomato resulted in preserved quality parameters (inactivation of microorganisms around 2.12–3.10 log CFU/g) and even higher retention (31.64–33.09 mg GAE/100 g) of the total phenolic compounds [71][66]. HPUS has recently been approved as a highly promising technology that can be adopted for several purposes in the winemaking process for the treatment of crushed grapes. The effect of US used at different amplitudes (30–90%) for different periods of time (2–10 min) showed that an increase in the amplitude and sonication time did not affect the main polyphenol contents of red wines [72][67]. The application of a pilot-scale power ultrasound system (30 kHz, 2500 W, 8 W/cm2) to crushed grapes facilitated the extractability of compounds from grapes to the must-wine, increasing the total phenol (18% and 23%) and tannin concentrations (43% and 30%) in the wine from less-ripened grapes and in the wine from partially rotten grapes, respectively [73][68]. The application of HPUS to obtain value-added red wines, using short maceration times, led to producing wine of a higher color intensity and a higher total polyphenol and anthocyanin content from grapes macerated for 4 h [74][69]. In this case, the application of US treatment (30 kHz, 2500 W, 8 W/cm2) led to enhancing the extraction of volatile compounds in the must, especially the free terpene and norisoprenoid content (from 3.37 to 4.92 μg/L). However, US caused the degradation of some phenolic compounds and vitamins, changes in color, the loss of anthocyanin, and other adverse effects on the food characteristics [74][69].3.4. Milk and Dairy Products
The most-common effects of acoustic cavitation on dairy products were recently reviewed by Carrillo-Lopez et al. [75][70] and Chavez-Martínez et al. [76][71]. The main impacts of US treatment were observed on the physicochemical, functional, and microbiological properties of milk and dairy products. In the study by Carrillo-Lopez et al., the application of 10 min of sonication (50% and 100%; 24 kHz; 400 W in continuous mode) of fresh raw milk led to increasing the yield of Panela cheese by 24.29%. Moreover, increasing the US time resulted in an evident yellow tone of the cheese [75][70]. The differences between low- and high-intensity US, as well as the advantages and disadvantages of each one in terms of the processing, quality, and preservation of milk and dairy products were reported in detail by Chávez-Martínez and co-authors [76][71]. As low-power US (LPUS) (<3 W/cm2) does not have a destructive effect on raw material components in the food industry, its applications are focused on as quality control tools for the monitoring of the microbial growth, enzymatic reactions, fermentation, and gelling processes of milk [77,78][72][73]. Low-frequency US (22 kHz; 60–120 W/L) treatment, used to reconstitute powdered milk, yields a higher nutritional final product with a higher content of bioactive compounds, such as exopolysaccharides, vitamin C content, and antioxidants, when applied prior to fermentation, which improves the growth of fermenting bacteria [79][74]. HPUS modifies the biological, physical, and chemical properties of materials through destructive tests, and it has been used during the production of various dairy products [80][75]. The rheological properties of dairy products developed by sonication during the fermentation (sonotrode of 20 kHz; 20 W; 10% protein; 43.5 °C; pH 5.8–5.1) of Greek yogurt could facilitate the subsequent stirring within the production process of this product type [81][76]. LPUS applied to monitor cheese maturation by measuring the US velocity with a couple of narrow-band US transducers (1 MHz) showed that the US velocity increased with the ripening time from 1630 to 1740 m/s, depending on the cheese texture [82][77]. The possibility of using US as a pre-treatment method to improve the nutritional attributes of cheese was demonstrated by Munira and co-authors [83][78]. The authors, evaluating the potential of US, comparing it to different milk processing techniques, such as microwaves (MWs) and high-pressure (HP), for the antioxidant and angiotensin-I converting enzyme (ACE)-inhibitory activity of cheddar cheese during ripening, indicated that the antioxidant activity and ACE-inhibitory potential of cheeses made from pre-treated milk significantly increased in the following order: US-II (41 J/g; 20 kHz; milk flow rate of 35 mL/min) > HP (400 MPa; 15 min; <40 °C) > US-I (23 J/g; 20 kHz; milk flow rate of 15 mL/min) > MW (86.5 J/g; 3 min; <40 °C) > untreated control. Otherwise, acoustic treatments may not only consequently improve, but can also decrease the quality characteristics of foods. Thermo-sonication as a method for milk pre-treatment before fermentation offers the possibility to obtain gels with rheological properties superior to those obtained from conventionally heated milk. However, HPUS applied during the fermentation process has not shown the most-desired results for this product. According to Nöbel et al. [84][79], HPUS (45 kHz; 17 kW/m2) applied during the fermentation of skim milk resulted in the formation of lumps and increased graininess, which are textural defects in yogurt. Lara-Castellanos et al. [85][80] showed a cheese prepared with 30% ultrasound-modified (20%, w/v; sonicator power of 50%, with pulses of 10 s for 30 min; temperature was controlled at 35 ± 5 °C) casein delayed the appearance of molds, but gave lower overall acceptability due to the changes in the microstructural, functional, and textural properties of the ultrasonicated casein. The application of low-intensity acoustic energy could have less impact on the quality characteristics of foods. Thereby, the use of acoustic energy can have mixed effects on the taste, aroma, appearance, freshness, and texture of food products. This effect is related to the US processing conditions and the properties of the processed foods. Since no studies evaluating the health effects of food produced by acoustic technology have been found, it would be appropriate to assess the toxicity of ultrasonicated food ingredients on consumer health.3.5. Meat and Meat Products
Recent studies have reported the prospective application of high-intensity US on fresh meat [86][81], mostly in tenderizing, brining, cooking and fermentation, thawing, freezing, and bacterial inhibition. US treatment increases meat tenderness and shortens the period of aging, without any effect on other quality parameters [87][82]. This is attributed to the rupture of the myofibrillar structure of the protein, collagen macromolecules’ fragmentation, and protein migration, among others, which accelerate proteolysis. In the study of Caraveo et al. [88][83], the redness of ultrasound-treated meat (40 kHz; 11 W/cm2; 90 min) was lower after treatment than that of control meat, but no difference was observed after Day 8 of storage. US can significantly decrease coliform, mesophilic, and psychrophilic bacteria in the meat during storage; however, the original microbial loads increased constantly during refrigeration. It has also been reported that the quality parameters of food products, such as the color, nutritional substances, and texture, are closely correlated with the heat transfer rate during the freezing process [89][84]. Ultrasound treatment is able to generate smaller ice crystals by accelerating the heat transfer, thus retaining the original quality properties of the frozen food products [90][85]. Visy et al. evaluated the combined effect of US-induced acoustic cavitation (20 kHz frequency; 5.09 W/cm2 power density; intensity of 100 W) and microbubbles during the brining of pork loin (Longissimus dorsi) [91][86]. The US brining enhanced the NaCl diffusion into the meat compared to meat brined under static conditions and the formation of microscopic pores on the surface of meat myofibers [91][86]. Xu et al., evaluating the changes of US-assisted (power of 350 W; frequency of 40 kHz; 10 °C) thawing on lamb meat quality and differential metabolite profiles during refrigerated storage, found that ultrasound-assisted thawing improved the water-holding capacity and increased the color of the lamb during refrigerated storage [92][87]. Furthermore, ultrasound-assisted thawing also reduced the sulfhydryl content in the lamb and inhibited protein oxidation. Moreover, potential metabolites associated with amino acids, carbohydrates and their conjugates, and peptides could be identified after US-assisted thawing [92][87]. HPUS, by producing high-speed jets, increases the temperature of the thawing water, subsequently generating asymmetric bubble collapse, improving heat transfer, thereby shortening the thawing process [93][88]. Slightly different results were reported by Bao et al. [94][89], showing increased tenderness and overall acceptability of dry-cured yak meat, but negatively affecting the meat color, smell, and taste after the US treatment (20 kHz; 200–400 W). Therefore, the optimization of the sonication time for different applications is inevitable. Meat products are usually non-homogeneous and extremely attenuating materials, which make it difficult for US waves to transmit through the material, due to the inability to penetrate the inner parts of the product and the absorption of US by the outer layers. Localized heating and overheating are common phenomena in ultrasonication [93][88]. The standardization of the HIUS-assisted freezing process and product variables is a major challenge to scale this technology for industrialization. Sonication can also be used as a suitable tool to produce marinated food products with a lesser amount of salt (sodium chloride) in comparison to presently available commercial marinades. Gómez-Salazar et al. [95][90] studied the effect of acid marination assisted by power ultrasound (40 kHz; 110 W) on the quality of rabbit meat. It was observed that the ultrasound-assisted marinating increased the NaCl uptake in rabbit meat in comparison with marinating without additional US treatment. Furthermore, the acoustic treatment also reduced the time required for salting and the coloring of raw meat, allowing producing a product with a uniform salt content [95][90]. Contreras-Lopez et al. [96][91] observed that the application of high-intensity acoustic energy increased the overall salt concentration in pork loins and retained the color and quality. Furthermore, in the study by Sanches et al. [97][92], US treatment led to a higher NaCl content in a shorter time during beef wet brining, reduced the denaturation temperature of myofibrillar proteins, and did not affect the lipid oxidation of the beef when compared to samples in static brine.References
- Gizaw, Z. Public health risks related to food safety issues in the food market: A systematic literature review. Environ. Health Prev. Med. 2019, 24, 68.
- Zhang, Z.H.; Wang, L.H.; Zeng, X.A.; Han, Z.; Brennan, C. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2018, 54, 1–13.
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Recent Advances toward the application of nonthermal technologies in food processing: An insight on the bioaccessibility of health-related constituents in plant-based products. Foods 2021, 10, 1538.
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, applications, and comparison of thermal (pasteurization, sterilization, and aseptic packaging) against non-thermal (ultrasounds, UV radiation, ozonation, high hydrostatic pressure) technologies in food processing. Appl. Sci. 2022, 12, 2202.
- Kirsh, I.; Frolova, Y.; Bannikova, O.; Beznaeva, O.; Tveritnikova, I.; Myalenko, D.; Romanova, V.; Zagrebina, D. Research of the influence of the ultrasonic treatment on the melts of the polymeric compositions for the creation of packaging materials with antimicrobial properties and biodegrability. Polymers 2020, 12, 275.
- Lafarga, T.; Alvarez, C.; Bobo, G.; Aguilo-Aguayo, I. Characterization of functional properties of proteins from Ganxet beans (Phaseolus vulgaris L. var. Ganxet) isolated using an ultrasound-assisted methodology. LWT Food Sci. Technol. 2018, 98, 106–112.
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325.
- Zhang, M.; Xia, X.; Liu, Q.; Chen, Q.; Kong, B. Changes in microstructure, quality and water distribution of porcine longissimus muscles subjected to ultrasound-assisted immersion freezing during frozen storage. Meat Sci. 2019, 151, 24–32.
- Wang, N.; Shi, N.; Fei, H.; Liu, Y.; Zhang, Y.; Li, Z.; Ruan, C.; Zhang, D. Physicochemical, structural, and digestive properties of pea starch obtained via ultrasonic-assisted alkali extraction. Ultrason. Sonochem. 2022, 89, 106136.
- Hashemi, M.M.; Mousavi, K.A.; Javanmardi, F.; Hadidi, M.; Hadian, Z.; Jafarzadeh, S.; Huseyn, E.; SantAna, A.S. A review of recent advances in the decontamination of mycotoxin and inactivation of fungi by ultrasound. Ultrason. Sonochem. 2021, 79, 105755.
- Lauteri, C.; Ferri, G.; Piccinini, A.; Pennisi, L.; Vergara, A. Ultrasound technology as inactivation method for foodborne pathogens: A review. Foods 2023, 12, 1212.
- Song, X.; Pendenza, P.; Díaz Navarro, M.; Valderrama García, E.; Di Monaco, R.; Giacalone, D. European Consumers’ perceptions and attitudes towards non-thermally processed fruit and vegetable products. Foods 2020, 9, E1732.
- Astráin-Redín, L.; Alejandre, M.; Raso, J.; Cebrián, G.; Álvarez, I. Direct contact ultrasound in food processing: Impact on food quality. Front. Nutr. 2021, 8, 633070.
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 2020, 60, 104726.
- Alzamora, S.M.; Guerrero, S.N.; Schenk, M.; Raffellini, S.; López-Malo, A. Inactivation of microorganisms. In Ultrasound Technologies for Food and Bioprocessing; Springer: New York, NY, USA, 2011; pp. 321–3431.
- Dong, Z.; Udepurkar, A.P.; Kuhn, S. Synergistic effects of the alternating application of low and high frequency ultrasound for particle synthesis in microreactors. Ultrason. Sonochem. 2020, 60, 104800.
- Hersh, A.M.; Bhimreddy, M.; Weber-Levine, C.; Jiang, K.; Alomari, S.; Theodore, N.; Manbachi, A.; Tyler, B.M. Applications of focused ultrasound for the treatment of glioblastoma: A New Frontier. Cancers 2022, 14, 4920.
- Bhatta, A.K.; Keyal, U.; Liu, Y. Application of high frequency ultrasound in dermatology. Discov. Med. 2018, 26, 237–242.
- Tellez-Morales, J.A.; Hernandez-Santo, B.; Rodríguez-Miranda, J. Effect of ultrasound on the techno-functional properties of food components/ingredients: A review. Ultrason. Sonochem. 2020, 61, 104787.
- Mao, Q.; Coutris, N.; Rack, H.; Fadel, G.; Gibert, J. Investigating ultrasound-induced acoustic softening in aluminium and its alloys. Ultrasonics 2020, 102, 106005.
- Estivi, L.; Brandolini, A.; Condezo-Hoyos, L.; Hidalgo, A. Impact of low-frequency ultrasound technology on physical, chemical and technological properties of cereals and pseudocereals. Ultrason. Sonochem. 2022, 86, 106044.
- Strieder, M.M.; Neves, M.I.L.; Silva, E.K.; Meireles, M.A.A. Low-frequency and high-power ultrasound-assisted production of natural blue colorant from the milk and unripe Genipa americana L. Ultrason. Sonochem. 2020, 66, 105068.
- Harpreet, K.; Singh, G.B. Effect of high-intensity ultrasound treatment on nutritional, rheological and structural properties of starches obtained from different cereals. Int. J. Biol. Macromol. 2019, 126, 367–375.
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications: A review. Ultrason. Sonochem. 2017, 34, 540–560.
- Strieder, M.M.; Silva, E.K.; Meireles, M.A.A. Advances and innovations associated with the use of acoustic energy in food processing: An updated review. Innov. Food Sci. Emerg. Technol. 2021, 74, 102863.
- Pérez-Andrés, J.M.; Charoux, C.M.G.; Cullen, P.J.; Tiwari, B.K. Chemical modifications of lipids and proteins by nonthermal food processing technologies. J. Agric. Food Chem. 2018, 66, 5041–5054.
- Milićević, N.; Kojić, P.; Sakač, M.; Mišan, A.; Kojić, J.; Perussello, C.; Banjac, V.; Pojić, M.; Tiwari, B. Kinetic modelling of ultrasound-assisted extraction of phenolics from cereal brans. Ultrason. Sonochem. 2021, 79, 105761.
- Astráin-Redín, L.; Skipnes, D.; Cebrián, G.; Álvarez-Lanzarote, I.; Rode, T.M. Effect of the application of ultrasound to homogenize milk and the subsequent pasteurization by pulsed electric field, high hydrostatic pressure, and microwaves. Foods 2023, 12, 1457.
- Maghsoudlou, Y.; Alami, M.; Mashkour, M.; Shahraki, M.H. Optimization of ultrasound-assisted stabilization and formulation of almond milk. J. Food Process. Preserv. 2016, 40, 828–839.
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms 2020, 8, 952.
- Chen, F.; Zhang, M.; Yang, C.H. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020, 63, 104953.
- Fan, K.; Wu, J.; Chen, L. Ultrasound and its combined application in the improvement of microbial and physicochemical quality of fruits and vegetables: A review. Ultrason. Sonochem. 2021, 80, 105838.
- Rodríguez, Ó.; Santacatalina, J.V.; Simal, S.; Garcia-Perez, J.V.; Femenia, A.; Rosselló, C. Influence of power ultrasound application on drying kinetics of apple and its antioxidant and microstructural properties. J. Food Eng. 2014, 129, 21–29.
- Alaei, B.; Chayjan, R.A.; Zolfigol, M.A. Improving tomato juice concentration process through a novel ultrasound-thermal concentrator under vacuum condition: A bioactive compound investigation and optimization. Inn. Food Sci. Emerg. Technol. 2022, 77, 102983.
- Azam, S.M.R.; Ma, H.; Xu, B.; Devi, S.; Siddique, M.A.B.; Stanley, S.L.; Bhandari, B.; Zhu, J. Efficacy of ultrasound treatment in the and removal of pesticide residues from fresh vegetables: A review. Trends Food Sci. Technol. 2020, 97, 417–432.
- Cengiz, M.F.; Başlar, M.; Basançelebi, O.; Kılıçlı, M. Reduction of pesticide residues from tomatoes by low intensity electrical current and ultrasound applications. Food Chem. 2018, 267, 60–66.
- Lozowicka, B.; Jankowska, M.; Hrynko, I.; Kaczynski, P. Removal of 16 pesticide residues from strawberries by washing with tap and ozone water, ultrasonic cleaning and boiling. Environ. Monit. Assess. 2016, 188, 51.
- Azam, S.M.R.; Ma, H.; Xu, B.; Devi, S.; Stanley, S.L.; Siddique, M.A.B.; Mujumdar, A.S.; Zhu, J. Multi-frequency multimode ultrasound treatment for removing pesticides from lettuce (Lactuca Sativa L.) and effects on product quality. LWT 2021, 143, 111147.
- Zhang, A.-A.; Sutar, P.P.; Bian, Q.; Fang, X.-M.; Ni, J.-B.; Xiao, H.-W. Pesticide residue elimination for fruits and vegetables: The mechanisms, applications, and future trends of thermal and non-thermal technologies. J. Future Food. 2022, 2, 223–240.
- Zawawi, N.A.F.; Hazmi, N.A.M.; How, M.S.; Kantono, K.; Silva, F.V.M.; Sulaiman, A. Thermal, high pressure, and ultrasound inactivation of various fruit cultivars’ polyphenol oxidase: Kinetic inactivation models and estimation of treatment energy requirement. Appl. Sci. 2022, 12, 1864.
- Liu, S.; Liu, Y.; Huang, X.; Yang, W.; Hu, W.; Pan, S. Effect of ultrasonic processing on the changes in activity, aggregation and the secondary and tertiary structure of polyphenol oxidase in oriental sweet melon (Cucumis melo Var. makuwa Makino). J. Sci. Food Agric. 2017, 97, 1326–1334.
- Costa, M.G.M.; Fonteles, T.V.; de Jesus, A.L.T.; Almeida, F.D.L.; de Miranda, M.R.A.; Fernandes, F.A.N.; Rodrigues, S. High intensity ultrasound processing of pineapple juice. Food Bioprocess Technol. 2013, 6, 997–1006.
- Amaral, R.D.A.; Benedetti, B.C.; Pujola, M.; Achaerandio, I.; Bachelli, M.L.B. Effect of ultrasound on quality of fresh-cut potatoes during refrigerated storage. Food Eng. Rev. 2015, 7, 176–184.
- Jang, J.H.; Moon, K.D. Inhibition of polyphenoloxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011, 124, 444–449.
- Adebo, O.A.; Molelekoa, T.; Makhuvele, R.; Adebiyi, J.A.; Oyedeji, A.B.; Gbashi, S.; Adefisoye, M.A.; Ogundele, O.M.; Njobeh, P.B. A review on novel non-thermal food processing techniques for mycotoxin reduction. Int. J. Food Sci. Technol. 2021, 56, 13–27.
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Food 2022, 2, 91–102.
- Mortazavi, S.M.; Sani, A.M.; Mosheni, S. Destruction of AFT by ultrasound treatment. J. Appl. Environ. Biol. Sci. 2015, 4, 198–202.
- Liu, Y.; Li, M.; Liu, Y.; Bian, K. Structures of reaction products and degradation pathways of aflatoxin B1 by ultrasound treatment. Toxins 2019, 11, 526.
- Liu, Y.; Li, M.; Liu, Y.; Bai, F.; Bian, K. Effects of pulsed ultrasound at 20 kHz on the sonochemical degradation of mycotoxins. World Mycotoxin J. 2019, 14, 357–366.
- Liu, Y.; Liu, Y.; Zhao, W.; Li, M.; Liu, N.; Bian, K. Reduction of aflatoxin B1 and zearalenone contents in corn using power ultrasound and its effects on corn quality. Toxins 2022, 14, 834.
- Caporaso, N.; Whitworth, M.B.; Fisk, I.D. Near-infrared spectroscopy and hyperspectral imaging for non-destructive quality assessment of cereal grains. Appl. Spectrosc. Rev. 2018, 53, 667–687.
- Cortes, V.; Blasco, J.; Aleixos, N.; Cubero, S.; Talens, P. Monitoring strategies for quality control of agricultural products using visible and near-infrared spectroscopy: A review. Trends Food Sci. Technol. 2019, 5, 138–148.
- De Girolamo, A.; Cervellieri, S.; Visconti, A.; Pascale, M. Rapid analysis of deoxynivalenol in durum wheat by FT-NIR spectroscopy. Toxins 2014, 6, 3129–3143.
- Jia, B.; Wang, W.; Ni, X.Z.; Chu, X.; Yoon, S.C.; Lawrence, K.C. Detection of mycotoxins and toxigenic fungi in cereal grains using vibrational spectroscopic techniques: A review. World Mycotoxin J. 2020, 13, 163–177.
- Juodeikiene, G.; Basinskiene, L.; Vidmantiene, D.; Bartkiene, E.; Bakutis, B.; Baliukoniene, V. Acoustic sensing of deoxynivalenol in co-occurrence with zearalenone and T-2/HT-2 toxin in winter wheat cultivar Sirvinta from Lithuania. World Mycotoxin J. 2011, 4, 395–404.
- Juodeikiene, G.; Vidmantiene, G.; Basinskiene, L.; Cernauskas, D.; Klupsaite, D.; Bartkiene, E.; Petrauskas, A.; de Koe, W.J. Recent advances in the rapid acoustic screening of deoxynivalenol in wheat grains. World Mycotoxin J. 2014, 7, 517–525.
- Juodeikiene, G.; Cernauskas, D.; Trakselyte-Rupsiene, K.; Bartkiene, E.; Zadeike, D.; Banyte, G.; Santini, A. Acoustic-based screening method for the detection of total aflatoxin in corn and biological detoxification in bioethanol production. Front. Microbiol. 2020, 11, 543.
- Trakselyte-Rupsiene, K.; Juodeikiene, G.; Janić Hajnal, E.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Cernauskas, D.; Lele, V.; Zadeike, D.; Bartkiene, E. Challenges of fermentation in combination with acoustic screening for deoxynivalenol and deoxynivalenol conjugates reduction in contaminated wheat-based products. Food Control 2022, 134, 108699.
- Ademola, O.; Turna, N.S.; Liverpool-Tasie, L.S.O.; Obadina, A.; Wu, F. Mycotoxin reduction through lactic acid fermentation: Evidence from commercial ogi processors in southwest Nigeria. Food Control 2021, 121, 107620.
- Kerhervé, S.O.; Guillermic, R.M.; Strybulevych, A.; Hatcher, D.W.; Scanlon, M.G.; Page, J.H. Online non-contact quality control of noodle dough using ultrasound. Food Control. 2019, 104, 349–357.
- Fernández, G.A.; Butz, P.; Bognar, A.; Tauscher, B. Antioxidative capacity, nutrient content and sensory quality of orange juice and an orange-lemon-carrot juice product after high pressure treatment and storage in different packaging. Eur. Food Res. Technol. 2011, 213, 290–296.
- Jabbari, S.-S.; Jafari, S.M.; Dehnad, D.; Shahidi, S.-A. Changes in lycopene content and quality of tomato juice during thermal processing by a nanofluid heating medium. J. Food Eng. 2018, 230, 1–7.
- Charles-Rodríguez, A.; Nevárez-Moorillón, G.; Zhang, Q.; Ortega-Rivas, E. Comparison of thermal processing and pulsed electric fields treatment in pasteurization of apple juice. Food Bioprod. Process. 2007, 85, 93–97.
- Lagnika, C.; Adjovi, Y.C.S.; Lagnika, L.; Gogohounga, F.O.; Do-Sacramento, O.; Koulony, R.K.; Sanni, A. Effect of combining ultrasound and mild heat treatment on physicochemical, nutritional quality and microbiological properties of pineapple juice. Food Nutr. Sci. 2017, 8, 227–241.
- Shen, Y.; Zhu, D.; Xi, P.; Cai, T.; Cao, X.; Liu, H.; Li, J. Effects of temperature controlled ultrasound treatment on sensory properties, physical characteristics and antioxidant activity of cloudy apple juice. LWT 2021, 142, 111030.
- Mustapha, A.T.; Zhou, C.; Amanor-Atiemoh, R.; Ali, T.A.A.; Wahia, H.; Ma, H.; Sun, Y. Efficacy of dual-frequency ultrasound and sanitizers washing treatments on quality retention of cherry tomato. Innov. Food Sci. Emerg. Technol. 2020, 62, 102348.
- Natolino, A.; Celotti, E. Ultrasound treatment of red wine: Effect on polyphenols, mathematical modeling, and scale-up considerations. LWT 2022, 154, 112843.
- Pérez-Porras, P.; Gómez-Plaza, E.; Osete-Álcaraz, A.; Martínez-Pérez, P.; Jurado, R.; Bautista-Ortín, A.B. The effect of ultrasound on Syrah wine composition as affected by the ripening or sanitary status of the grapes. Eur. Food Res. Technol. 2023, 249, 641–651.
- Fernández, L.L.; Pérez-Porras, P.; Díaz-Maroto, M.C.; Gómez-Plaza, E.; Pérez-Coello, M.S.; Bautista-Ortín, A.B. The technology of high-power ultrasound and its effect on the color and aroma of rosé wines. J. Sci. Food Agric. 2023, 103, 6616–6624.
- Carrillo-Lopez, L.M.; Juarez-Morales, M.G.; Garcia-Galicia, I.A.; Alarcon-Rojo, A.D.; Huerta-Jimenez, M. The effect of high-intensity ultrasound on the physicochemical and microbiological properties of Mexican Panela cheese. Foods 2020, 9, 313.
- Chávez-Martínez, A.; Reyes-Villagrana, R.A.; Rentería-Monterrubio, A.L.; Sánchez-Vega, R.; Tirado-Gallegos, J.M.; Bolivar-Jacobo, N.A. Low and high-intensity ultrasound in dairy products: Applications and effects on physicochemical and microbiological quality. Foods 2020, 9, 1688.
- Zlatev, Z.; Pehlivanova, T.; Dimitrova, A.; Baycheva, S.; Taneva, I.; Keremidchieva, K. Development of an ultrasonic device for quality evaluation of yogurt. Eng. Rev. 2018, 38, 279–287.
- Benedito, J.; Simal, S.; Clemente, G.; Mulet, A. Manchego cheese texture evaluation by ultrasonics and surface probes. Int. Dairy J. 2006, 16, 431–438.
- Potoroko, I.; Kalinina, I.; Botvinnikova, V.; Krasulya, O.; Fatkullin, R.; Bagale, U.; Sonawane, S. Ultrasound effects based on simulation of milk processing properties. Ultrason. Sonochem. 2018, 48, 463–472.
- Guimarães, J.T.; Balthazar, C.F.; Scudino, H.; Pimentel, T.C.; Esmerino, E.A.; AshokKumar, M.; Freitas, M.Q.; Cruz, A.G. High-intensity ultrasound: A novel technology for the development of probiotic and prebiotic dairy products. Ultrason. Sonochem. 2019, 57, 12–21.
- Körzendörfer, A.; Schäfer, J.; Hinrichs, J.; Nöbel, S. Power ultrasound as a tool to improve the processability of protein-enriched fermented milk gels for Greek yogurt manufacture. J. Dairy Sci. 2019, 102, 7826–7837.
- Benedito, J.; Cárcel, J.A.; Clemente, G.; Mulet, A. Cheese maturity assessment using ultrasonics. J. Dairy Sci. 2000, 83, 248–254.
- Munira, M.; Nadeema, M.; Qureshid, T.M.; Gamlathb, C.J.; Martine, G.J.O.; Hemarf, Y.; Ashokkumar, M. Effect of sonication, microwaves and high-pressure processing on ACE inhibitory activity and antioxidant potential of Cheddar cheese during ripening. Ultrason. Sonochem. 2020, 67, 105140.
- Nöbel, S.; Ross, N.-L.; Protte, K.; Körzendörfer, A.; Hitzmann, B.; Hinrichs, J. Microgel particle formation in yogurt as influenced by sonication during fermentation. J. Food Eng. 2016, 180, 29–38.
- Lara-Castellanos, M.; Azuara, E.; Jimenez-Fernandez, V.; Luna-Solano, G.; Jimenez, M. Effect of casein replacement by modified casein on physicochemical, textural, sensorial properties and microbiological stability of fresh cheese. Int. Dairy J. 2021, 112, 104864.
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jiménez, M.; Garcia-Galicia, I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019, 55, 369–382.
- Peña-González, E.M.; Alarcón-Rojo, A.D.; Rentería, A.; García, I.; Santellano, E.; Quintero, A.; Luna, L. Quality and sensory profile of ultrasound-treated beef. Ital. J. Food Sci. 2017, 29, 463–475.
- Caraveo, O.; Alarcon-Rojo, A.D.; Renteria, A.; Santellano, E.; Paniwnyk, L. Physicochemical and microbiological characteristics of beef treated with high intensity US and stored at 4 °C. J. Sci. Food Agric. 2015, 95, 2487–2493.
- Qiu, L.; Zhang, M.; Chitrakar, B.; Bhandari, B. Application of power ultrasound in freezing and thawing processes: Effect on process efficiency and product quality. Ultrason. Sonochem. 2020, 68, 105230.
- Mahato, S.; Zhu, Z.; Sun, D.-W. Glass transitions as affected by food compositions and by conventional and novel freezing technologies: A review. Trends Food Sci. Technol. 2019, 94, 1–11.
- Visy, A.; Jónás, G.; Szakos, D.; Horváth-Mezőfi, Z.; Hidas, K.I.; Barkó, A.; Friedrich, L. Evaluation of ultrasound and microbubbles effect on pork meat during brining process. Ultrason. Sonochem. 2021, 75, 105589.
- Xu, C.; Zang, M.; Qiao, X.; Wang, S.; Zhao, B.; Shi, Y.; Bai, J.; Wu, J. Effects of ultrasound-assisted thawing on lamb meat quality and oxidative stability during refrigerated storage using non-targeted metabolomics. Ultrason. Sonochem. 2022, 90, 10621.
- Firouz, M.S.; Sardari, H.; Chamgordani, P.A.; Behjati, M. Power ultrasound in the meat industry (freezing, cooking and fermentation): Mechanisms, advances and challenges. Ultrason. Sonochem. 2022, 86, 106027.
- Bao, G.; Niu, J.; Li, S.; Zhang, L.; Luo, Y. Effects of ultrasound pretreatment on the quality, nutrients and volatile compounds of dry-cured yak meat. Ultrason Sonochem. 2022, 82, 105864.
- Gómez-Salazar, J.A.; Ochoa-Montes, D.A.; Cerón-García, A.; Ozuna, C.; Sosa-Morales, M.E. Effect of acid marination assisted by power ultrasound on the quality of rabbit meat. J. Food Qual. 2018, 2018, 5754930.
- Contreras-Lopez, G.; Carnero-Hernandez, A.; Huerta-Jimenez, M.; Alarcon-Rojo, A.D.; Garcia-Galicia, I.; Carrillo-López, L.M. High-intensity ultrasound applied on cured pork: Sensory and physicochemical characteristics. Food Sci. Nutr. 2020, 8, 786–795.
- Sanches, M.A.R.; Silva, P.M.O.C.; Barretto, T.L.; Darros-Barbosa, R.; da Silva-Barretto, A.C.; Telis-Romero, J. Technological and diffusion properties in the wet salting of beef assisted by ultrasound. LWT 2021, 149, 112036.
More
