Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Fanny Huang and Version 1 by Cristina Maccallini.
The dysregulated activation of nNOS in neurons is critical in the development of different conditions affecting the SNC. An excessive production of NO by nNOS is responsible for a number of proteins’ posttranslational modifications (PTMs) which can lead to aberrant biochemical pathways, impairing the SNC functions. HResere we havearchers briefly revised the main implications of the dysregulated nNOS activation in the progression of the most prevalent CNS diseases, suggesting that compounds able to modulate the nNOS activity could be promising therapeutics to tackle different neuronal pathologic conditions.
- Central Nervous System
- neuronal Nitric Oxide Synthase
1. The Overstimulation of nNOS in CNS Disease Development
nNOS shares a similar architecture to eNOS and iNOS, being a homodimer in which each monomer contains an N-terminal oxygenase domain hosting the catalytic site, and a reductase domain containing the cofactors binding sites (Figure 1). The two domains are connected by a Ca2+-calmodulin (CaM) binding sequence and, specifically for nNOS, the oxygenase domain is also connected to the postsynaptic density protein, discs-large, ZO-1 (PDZ) domain, which is characterized by a β-hairpin sequence. Through the PDZ domain, nNOS can interact with other proteins, triggering different cascades of protein–protein interactions [1]. This also determines the specific subcellular localization and functions of nNOS. Indeed, nNOS can be found both in the neurons’ cytosol as well as bound to the postsynaptic membrane through the interaction with the postsynaptic density protein-95 (PSD-95)-NMDAR complex, or with other different proteins, such as Capon, syntrophin, or postsynaptic density-93 (PSD-93) [2][3][4]. These proteins regulate the activation of nNOS and its physiological functions.
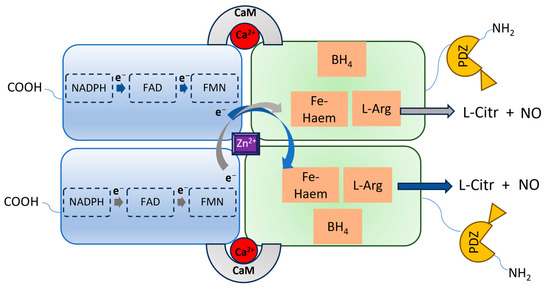
Figure 1. Representation of the nNOS homodimeric architecture. Each monomer has a carboxy-terminal reductase domain and an amino-terminal oxygenase domain, containing the L-Arg binding site and linked to the PDZ domain. In the reductase domain, electrons via the NADPH-FAD-FMN transport chain reach the heme iron of the opposite monomer oxygenase domain, facilitated by CaM, enabling the oxidation of L-arginine to L-citrulline, accompanied by the release of NO.
In physiological conditions, the stimulated N-methyl-D-aspartate receptors (NMDARs) mediate the intracellular influx of Ca2+, which can bind the CaM sequence of nNOS, initiating its activity. The produced NO can behave as an anterograde or retrograde neurotransmitter, regulating memory, learning, and synaptic plasticity [5][6][7]. Indeed, nNOS-derived NO induces the full expression of c-Fos, Egr-1, Arc, and brain-derived neurotrophic factor (BDNF), which are key proteins associated with neuroplasticity. This was observed both in cortical cultures after bicuculline-evoked synaptic activity, and in in vivo mice models of experience-dependent plasticity in the whisker barrel cortex [8][9]. In particular, the signaling pathways involved in this effect include GMP, PKG, extracellular-signal-regulated kinase (ERK), and calcium–calmodulin (CaM)-dependent protein kinase II (CaMKII) [8].
However, in the early phases of pathological conditions such as stroke, AD, and PD, as well as in other neuropsychiatric conditions such as epilepsy and autism, an overactivation of NMDARs can be observed, followed by an excessive Ca2+ influx, with a loss of its homeostasis (Figure 2). These events lead to the prolonged overstimulation of nNOS, due to excessive intracellular Ca2+ levels [10][11]. The overproduced NO contributes to the development of such diseases by mediating proteins’ PTM, such as nitrotyrosination (Tyr-NO2), i.e., the reaction of tyrosine residues with ONOO−, and cysteine nitrosylation (SNO) (Figure 1). In the specific diseases’ context, these PTMs are mostly pathological, leading to proteins’ loss- or gain-of-function.
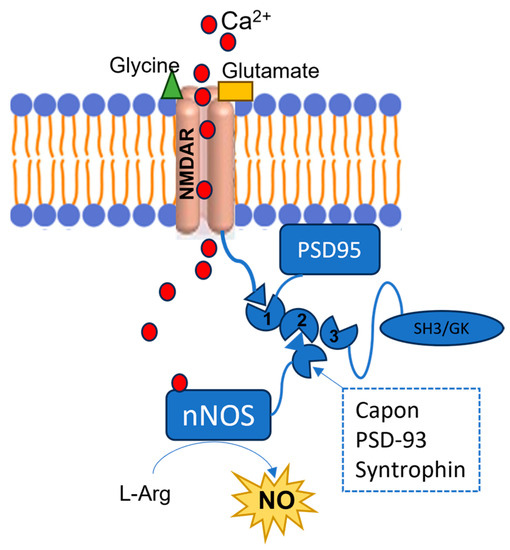
Figure 2. Representation of the NMDARs-PSD-95-nNOS complex. The red circles represent the Ca2+ ions, the green triangle represents a glycine molecule, and the yellow rectangle represents a glutamate molecule. NMDARs are bound to the PDZ1 domain of the PSD95, and when they are stimulated by the interaction with glycine and glutamate, they allow Ca2+ intracellular influx. This cation binds the nNOS CaM, and the enzyme interacts with the PSD95’s PDZ2 domain by its PDZ β-hairpin motif or with other proteins such as Capon, synthropin, or PSD-93. As a consequence, the enzyme’s activity is stimulated, although an excessive nNOS activation is observed in different SNC diseases, due to the NMDARs’ overstimulation.
2. NO-Mediated PTM and Alzheimer Disease
AD is a progressive type of dementia characterized by the aggregation into β-sheets of the amyloid β peptide (Aβ), which is derived from the cleavage of the amyloid precursor protein (APP). Further biological markers of this disease are the intracellular neurofibrillary tangles caused by the excessive phosphorylation of the tau protein. The only FDA-approved AD medications are the acetylcholinesterase inhibitors (AChEIs) donepezil, galantamine, and rivastigmine, and the NMDA antagonist memantine (Figure 3) [12]. Despite the many clinical trials, no new drug has received FDA approval in the last 20 years, and, among the reasons of the clinical trial failures, the inadequate comprehension of the pathophysiology of the AD is a widely accepted explanation [13][14].
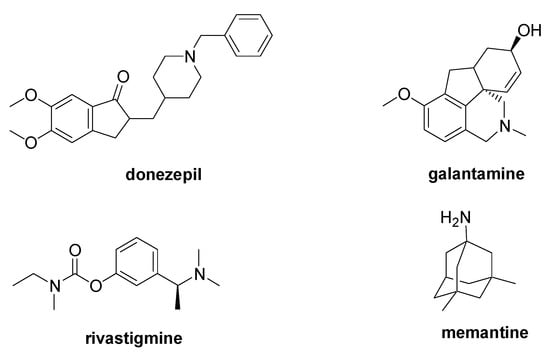
Figure 3. Chemical structure of the FDA-approved drugs for AD therapy.
In general, cerebral regions of AD patients, specifically the hippocampus and the cerebral cortex, display higher Tyr-NO2 levels, and there is a positive correlation between nNOS expression and neurofibrillary tangles in neurons, as well as amyloid plaque accumulations and nitrergic neurons [15][16][17]. Moreover, the activity of gamma-secretase is affected by nitrotyrosination [18]. Together with β-site APP cleaving enzyme type 1 (BACE1), gamma-secretase is responsible for the production of Aβ1–40 or Aβ1–42 peptides. In AD patients, it has been demonstrated that the nitrotyrosination of this enzyme leads to the imbalanced production of Aβ1–42, which is more prone to aggregate and toxic species [19]. Furthermore, Aβ1–42 nitrotyrosination was also demonstrated to increase aggregates’ stability and toxicity [20]. In AD, the nitrotyrosination of important neuronal metabolic enzymes is also observed, such as lactate dehydrogenase and triosephosphate isomerase (TPI), with a reduction in their activity and important metabolic changes [21]. It was reported that nitro-TPI contributes to intracellular neurofibrillary tangle formation [21].
Besides nitrotyrosination, the extensive S-nitrosylation of proteins is also crucial for synaptic function and neuronal survival and it is associated with AD development [22][23]. Indeed, SNO protein modifications may induce further protein misfolding and neuronal and synaptic damage, leading to mitochondrial stress. In safe cells, these modifications are reversible thanks to the presence of antioxidants such as glutathione and de- and trans-nitrosylating enzymes [24][25]. However, this balance is compromised in the developing AD due to the excessive NO released from the overstimulated nNOS, which leads to massive SNO-proteins. For example, the S-nitrosylation at Cys 83/157 of Cyclic-dependent kinase 5 (CDK5), which is responsible for the cleavage of p35 to p25, upregulates the kinase activity, leading to dendritic spine loss and neuronal apoptosis [26][27]. It was observed that S-nitrosylation of the insulin-degrading enzyme (IDE) inhibits its activity to degrade Aβ [28], while S-nitrosylation of vesicular acetylcholine transporter (VAChT) and vesicular glutamate transporter 1 (VGLUT1) worsens the ACh turnover [29].
In addition, aberrant transnitrosylation reactions, i.e., transfer of the NO group from one protein to another, are highly implicated in AD synaptic loss due to activation of the alternative biochemical network to the physiologic functions of the involved enzymes [30].
2. NO-Mediated PTM and Parkinson Disease
PD is a degenerative condition of the brain associated with motor symptoms (rigidity, tremor, bradykinesia, and postural instability) and non-motor disorders (apathy, depression, cognitive dysfunction, and sleep disorders). The main cause of PD is the loss of dopaminergic neurons in the substantia nigra [31] due to the aggregation of the α-synuclein, a protein that regulates the trafficking and release of neurotransmitter vesicles [32]. Currently, there is no therapy to modify the course of PD, with its treatment only being palliative to alleviate the motor and non-motor symptoms that occur during the disease’s development. The administration of the levodopa (Figure 4) is considered the principal therapeutic approach to restore dopamine levels [33], and it can be combined with carbidopa or benserazide (Figure 4), two decarboxylase inhibitors useful to increase levodopa bioavailability [34]. Moreover, the simultaneous treatment with monoamine oxidase B inhibitors such as rasagiline, safinamide, and selegiline is recommended to increase dopamine levels (Figure 4) [34]. Entacapone and tolcapone, two catechol-O-methyltransferase inhibitors, are also used to promote the gastrointestinal absorption of levodopa [34].
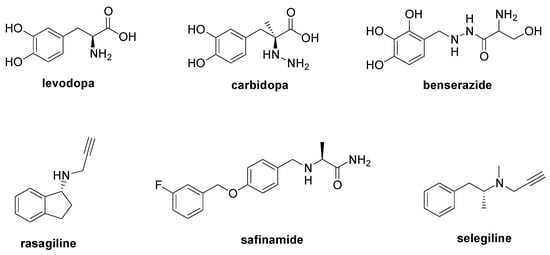
Figure 4. Chemical structures of approved drugs used in the management of PD.
Although the loss of dopaminergic neurons is a well-established mechanism involved in PD, the reasons leading to the initiation of this process are still unknown. Different studies have reported the accumulation of 3-nitrotyrosinated proteins and a neuronal upregulation of nNOS in cells isolated from PD patients [35][36][37].
It was demonstrated that α-synuclein nitrotyrosination induces its aggregation and inhibits its interaction with dopamine vesicles [38]. Moreover, the nitration of tyrosine hydrolase (TH) seems to be implicated in PD onset. This enzyme is involved in catecholamine synthesis from tyrosines, and its activity is impaired through nitration, lessening dopamine availability [39]. On the contrary, it was reported that TH nitrosylation at Cys 279 enhances its enzymatic activity both in vitro and in vivo, confirming the important role of NO in the subtle regulation of proteins involved in PD progression [40]. It was reported that the overproduced NO is responsible for the S-nitrosylation of different, other proteins, such as the disulfide isomerase and microtubule-associated protein 1b, as well as of CDK5, impairing axo-dendritic function and neurite length [41]. Moreover, the S-nitrosylation of parkin, a protein involved in the degradation of specific substrates, reduces its activity, and consequently neurotoxic proteins can accumulate, leading to ER stress [41]. These proteins’ PTMs alter network connectivity, which is associated with cognitive decline and neuronal death.
3. NO-Mediated PTM and Neurological Disorders
NO plays an important role in neurodevelopment, and its altered signaling appears implicated in the progression of a variety of neurodevelopmental and neuropsychiatric diseases. NO can either facilitate or suppress synaptic plasticity, depending on the brain area, concentration, and cellular environment [42][43]; therefore, both overactivation and downregulation of nNOS can be implied in the development of such diseases.
Recently, interesting studies have put in light connections between the specific genetic mutation of the Shank3 gene occurring in autism, a condition associated with deficits in communication and social skills, and excessive NO synthesis, which is responsible for aberrant protein nitrosation and S-nitrosylation [44][45][46][47]. Elevated levels of different SNO proteins functionally involved in the synaptic vesicle cycle, neurotransmission, and glutamatergic pathway, such as protein phosphatase catalytic subunit α-Ppp3ca, syntaxin-1a, vesicle-associated membrane protein 3, and others, were found in Shank3 KO mouse models [46][47]. Collectively, these observations provide insights into the specific pathological role of dysregulated NO production in autism spectrum disorders.
Epilepsy is the most common neurological disease and it was reported that nNOS-derived NO is neurotoxic in the epileptic brain, due to the formation of peroxynitrite after its reaction with the superoxide radical, triggering PTZ kindling epilepsy-induced neural damage [48]. Indirect evidence suggests that the inhibition of nNOS in pilocarpine-induced temporal lobe epilepsy mice can protect against hippocampal neuronal injuries by increasing neuropeptide Y expression, which has been implicated in energy homeostasis and neuroprotection [49]. Moreover, overexpression of NO and lipid peroxidation was reported in the brain of pentylenetetrazol (PTZ)-induced epilepsy rats, and antioxidant treatment normalized their levels [50]. Specific NO-mediated PTMs are implicated in epilepsy; for example, NO is responsible for type 1 ryanodine receptor (RyR1) S-nitrosylation, inducing Ca2+ release from the endoplasmic reticulum through the Ca2 + release channel, and worsening the disease progression [51].
In an animal model of epilepsy, the kainate receptor, specifically glutamate ionotropic receptor kainate type subunit 2 (GluK2), undergoes S-nitrosylation, and SNO-GluK2 further potentiates calcium influx [52].
References
- Merino-Gracia, J.; Costas-Insua, C.; Canales, M.Á.; Rodríguez-Crespo, I. Insights into the c-terminal peptide binding specificity of the pdz domain of neuronal nitric-oxide synthase: Characterization of the interaction with the tight junction protein claudin-3. J. Biol. Chem. 2016, 291, 11581–11595.
- Sattler, R.; Xiong, Z.; Lu, W.Y.; Hafner, M.; MacDonald, J.F.; Tymianski, M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 1999, 284, 1845–1848.
- Brenman, J.E.; Chao, D.S.; Gee, S.H.; McGee, A.W.; Craven, S.E.; Santillano, D.R.; Wu, Z.; Huang, F.; Xia, H.; Peters, M.F.; et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 1996, 84, 757–767.
- Rothe, F.; Canzler, U.; Wolf, G. Subcellular localization of the neuronal isoform of nitric oxide synthase in the rat brain: A critical evaluation. Neuroscience 1998, 83, 259–269.
- Park, J.H.; Straub, V.A.; O’Shea, M. Anterograde signaling by nitric oxide: Characterization and in vitro reconstitution of an identified nitrergic synapse. J. Neurosci. 1998, 18, 5463–5476.
- Hardingham, N.; Dachtler, J.; Fox, K. The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front. Cell. Neurosci. 2013, 7, 190.
- Paul, V.; Ekambaram, P. Involvement of nitric oxide in learning & memory processes. Indian J. Med. Res. 2011, 133, 471–478.
- Gallo, E.F.; Iadecola, C. Neuronal nitric oxide contributes to neuroplasticity-associated protein expression through cGMP, protein kinase G, and extracellular signal-regulated kinase. J. Neurosci. 2011, 31, 6947–6955.
- Moosavi, M.; Abbasi, L.; Zarifkar, A.; Rastegar, K. The role of nitric oxide in spatial memory stages, hippocampal ERK and CaMKII phosphorylation. Pharmacol. Biochem. Behav. 2014, 122, 164–172.
- Bezprozvanny, I.; Mattson, M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008, 31, 454–463.
- Gallego-Sandín, S.; Alonso, M.T.; García-Sancho, J. Calcium homoeostasis modulator 1 (CALHM1) reduces the calcium content of the endoplasmic reticulum (ER) and triggers ER stress. Biochem. J. 2011, 437, 469–475.
- Atri, A. Current and future treatments in Alzheimer’s disease. Semin. Neurol. 2019, 39, 227–240.
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397.
- Ramesh, M.; Gopinath, P.; Govindaraju, T. Role of Post-translational Modifications in Alzheimer’s Disease. Chembiochem 2020, 21, 1052–1079.
- Hensley, K.; Maidt, M.L.; Yu, Z.; Sang, H.; Markesbery, W.R.; Floyd, R.A. Electrochemical Analysis of Protein Nitrotyrosine and Dityrosine in the Alzheimer Brain Indicates Region-Specific Accumulation. J. Neurosci. 1998, 18, 8126–8132.
- Quinn, J.; Davis, F.; Woodward, W.R.; Eckenstein, F. Beta-amyloid plaques induce neuritic dystrophy of nitric oxide-producing neurons in a transgenic mouse model of Alzheimer’s disease. Exp. Neurol. 2001, 168, 203–212.
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: A versatile oxidative stress biomarker for major neurodegenerative diseases. Int. J. Neurosci. 2020, 130, 1047–1062.
- Guix, F.X.; Wahle, T.; Vennekens, K.; Snellinx, A.; Chávez-Gutiérrez, L.; Ill-Raga, G.; Ramos-Fernandez, E.; Guardia-Laguarta, C.; Lleó, A.; Arimon, M.; et al. Modification of γ-secretase by nitrosative stress links neuronal ageing to sporadic Alzheimer’s disease. EMBO Mol. Med. 2012, 4, 660–673.
- Jarrett, J.T.; Berger, E.P.; Lansbury, P.T. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry 1993, 32, 4693–4697.
- Guivernau, B.; Bonet, J.; Valls-Comamala, V.; Bosch-Morató, M.; Godoy, J.A.; Inestrosa, N.C.; Perálvarez-Marín, A.; Fernández-Busquets, X.; Andreu, D.; Oliva, B.; et al. Amyloid-β peptide nitrotyrosination stabilizes oligomers and enhances NMDAR-mediated toxicity. J. Neurosci. 2016, 36, 11693–11703.
- Guix, F.X.; Ill-Raga, G.; Bravo, R.; Nakaya, T.; de Fabritiis, G.; Coma, M.; Pietro Miscione, G.; Villà-Freixa, J.; Suzuki, T.; Fernàndez-Busquets, X.; et al. Amyloid-dependent triosephosphate isomerase nitrotyrosination induces glycation and tau fibrillation. Brain 2009, 132, 1335–1345.
- Castegna, A.; Thongboonkerd, V.; Klein, J.B.; Lynn, B.; Markesbery, W.R.; Butterfield, D.A. Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J. Neurochem. 2003, 85, 1394–1401.
- Nakamura, T.; Prikhodko, O.A.; Pirie, E.; Nagar, S.; Akhtar, M.W.; Oh, C.-K.; McKercher, S.R.; Ambasudhan, R.; Okamoto, S.-i.; Lipton, S.A. Aberrant protein Snitrosylation contributes to the pathophysiology of neurodegenerative diseases. Neurobiol. Dis. 2015, 84, 99–108.
- Anand, P.; Stamler, J.S. Enzymatic mechanisms regulating protein S-nitrosylation: Implications in health and disease. J. Mol. Med. 2012, 90, 233–244.
- Di Giacomo, G.; Rizza, S.; Montagna, C.; Filomeni, G. Established principles and emerging concepts on the interplay between mitochondrial physiology and S-(De) nitrosylation: Implications in cancer and neurodegeneration. Int. J. Cell Biol. 2012, 2012, 361872.
- Qu, J.; Nakamura, T.; Cao, G.; Holland, E.A.; McKercher, S.R.; Lipton, S.A. S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by β-amyloid peptide. Proc. Natl. Acad. Sci. USA 2011, 108, 14330–14335.
- Qu, J.; Nakamura, T.; Holland, E.A.; McKercher, S.R.; Lipton, S.A. S-nitrosylation of Cdk5. Prion 2012, 6, 364–370.
- Cordes, C.M.; Bennett, R.G.; Siford, G.L.; Hamel, F.G. Redox regulation of insulin degradation by insulin-degrading enzyme. PLoS ONE 2011, 6, e18138.
- Wang, Y.; Zhou, Z.; Tan, H.; Zhu, S.; Sun, Y.; Li, M.X.; Wang, J.F. Nitrosylation of vesicular transporters in brain of amyloid precursor protein/presenilin 1 double transgenic mice. J. Alzheimer’s Dis. 2017, 55, 1683–1692.
- Lipton, S.A. Hidden networks of aberrant protein transnitrosylation contribute to synapse loss in Alzheimer’s disease. Free Radic. Biol. Med. 2022, 193, 171–176.
- Robinson, P. Understanding the molecular basis of Parkinson’s disease, identification of biomarkers and routes to therapy. Expert. Rev. Proteom. 2010, 2010, 565–578.
- Murphy, D.D.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 2000, 20, 3214–3220.
- LeWitt, P.A. Levodopa Therapy for Parkinson’s Disease: Pharmacokinetics and Pharmacodynamics. Mov. Disord. 2015, 30, 64–72.
- Dhanawat, M.; Mehta, D.K.; Gupta, S.; Das, R. Understanding the Pathogenesis Involved in Parkinson’s Disease and Potential Therapeutic Treatment Strategies. Cent. Nerv. Syst. Agents Med. Chem. 2020, 20, 88–102.
- Gatto, E.M.; Riob’o, N.A.; Carreras, M.C.; Chernavsky, A.; Rubio, A.; Satz, M.L.; Poderoso, J.J. Overexpression of neutrophil neuronal nitric oxide synthase in Parkinson’s disease. Nitric Oxide 2000, 4, 534–539.
- Eve, D.J.; Nisbet, A.P.; Kingsbury, A.E.; Hewson, E.L.; Daniel, S.E.; Lees, A.J.; Marsden, C.D.; Foster, O.J. Basal ganglia neuronal nitric oxide synthase mRNA expression in Parkinson’s disease. Brain Res. Mol. Brain Res. 1998, 63, 62–71.
- Joniec, I.; Ciesielska, A.; Kurkowska-Jastrzebska, I.; Przybylkowski, A.; Czlonkowska, A.; Czlonkowski, A. Age- and sex-differences in the nitric oxide synthase expression and dopamine concentration in the murine model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 2009, 1261, 7–19.
- Burai, R.; Ait Bouziad, N.; Chiki, A.; Lashuel, H.A. Elucidating the role of site-specific nitration of α-synuclein in the pathogenesis of Parkinson’s disease via protein semisynthesis and mutagenesis. J. Am. Chem. Soc. 2015, 137, 5041–5052.
- Blanchard-Fillion, B.; Souza, J.M.; Friel, T.; Jiang, G.C.T.; Vrana, K.; Sharov, V.; Barrón, L.; Schöneich, C.; Quijano, C.; Alvarez, B.; et al. Nitration and Inactivation of Tyrosine Hydroxylase by Peroxynitrite. J. Biol. Chem. 2001, 276, 46017–46023.
- Wang, Y.; Sung, C.C.; Chung, K.K. Novel enhancement mechanism of tyrosine hydroxylase enzymatic activity by nitric oxide through S-nitrosylation. Sci. Rep. 2017, 7, 44154.
- Stykel, M.G.; Ryan, S.D. Nitrosative stress in Parkinson’s disease. NPJ Park. Dis. 2022, 8, 104.
- Zhu, L.J.; Li, F.; Zhu, D.Y. nNOS and Neurological, Neuropsychiatric Disorders: A 20-Year Story. Neurosci. Bull. 2023, 39, 1439–1453.
- Zoupa, E.; Pitsikas, N. The Nitric Oxide (NO) Donor Sodium Nitroprusside (SNP) and Its Potential for the Schizophrenia Therapy: Lights and Shadows. Molecules 2021, 26, 3196.
- Kartawy, M.; Khaliulin, I.; Amal, H. Systems biology reveals S-nitrosylation dependent regulation of mitochondrial functions in mice with Shank3 mutation associated with autism spectrum disorder. Brain Sci. 2021, 11, 677.
- Monteiro, P.; Feng, G. SHANK proteins: Roles at the synapse and in autism spectrum disorder. Nat. Rev. Neurosci. 2017, 18, 147–157.
- Amal, H.; Barak, B.; Bhat, V.; Gong, G.; Joughin, B.A.; Wang, X.; Wishnok, J.S.; Feng, G.; Tannenbaum, S.R. Shank3 mutation in a mouse model of autism leads to changes in the S-nitroso-proteome and affects key proteins involved in vesicle release and synaptic function. Mol. Psychiatr. 2018, 25, 1835–1848.
- Tripathi, M.K.; Ojha, S.K.; Kartawy, M.; Hamoudi, W.; Choudhary, A.; Stern, S.; Aran, A.; Amal, H. The NO Answer for Autism Spectrum Disorder. Adv. Sci. 2023, 10, 2205783.
- Zhu, X.; Dong, J.; Han, B.; Huang, R.; Zhang, A.; Xia, Z.; Chang, H.; Chao, J.; Yao, H. Neuronal nitric oxide synthase contributes to PTZ kindling epilepsy-induced hippocampal endoplasmic reticulum stress and oxidative damage. Front. Cell. Neurosci. 2017, 11, 377.
- Yao, Y.; Hu, Y.; Yang, J.; Zhang, C.; He, Y.; Qi, H.; Zeng, Y.; Zhang, A.; Liu, X.; Zhu, X. Inhibition of neuronal nitric oxide synthase protects against hippocampal neuronal injuries by increasing neuropeptide Y expression in temporal lobe epilepsy mice. Free Radic. Biol. Med. 2022, 188, 45–61.
- Bashkatova, V.; Narkevich, V.; Vitskova, G.; Vanin, A. The influence of anticonvulsant and antioxidant drugs on nitric oxide level and lipid peroxidation in the rat brain during penthylenetetrazole-induced epileptiform model seizures. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 487–492.
- Mikami, Y.; Kanemaru, K.; Okubo, Y.; Nakaune, T.; Suzuki, J.; Shibata, K.; Sugiyama, H.; Koyama, R.; Murayama, T.; Ito, A.; et al. Nitric Oxide-induced Activation of the Type 1 Ryanodine Receptor Is Critical for Epileptic Seizure-induced Neuronal Cell Death. eBioMedicine 2016, 11, 253–261.
- Wang, L.; Liu, Y.; Lu, R.; Dong, G.; Chen, X.; Yun, W.; Zhou, X. The role of S-nitrosylation of kainate-type of ionotropic glutamate receptor 2 in epilepsy induced by kainic acid. J. Neurochem. 2017, 144, 255–270.
More
