Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Laurent Dufossé.
Microbial pigments play multiple roles in the ecosystem construction, survival, and fitness of all kinds of organisms. Considerably, microbial (bacteria, fungi, yeast, and microalgae) pigments offer a wide array of food, drug, colorants, dyes, and imaging applications.
- Microbial Pigments
- biological properties
- fluorescent pigments
1. Introduction
The survival of life forms on earth is dependent on various pigments, including light-harvesting pigments like chlorophylls, phycoerythrin, and phycobiliproteins [1,2][1][2]; harmful light-filtering pigments like proteorhodopsins [3[3][4],4], melanin’s, pyomelanin, pyocyanin, fluorescent proteins; predator defending pigments like aplysioviolin [5], cephalopods ink [6[6][7],7], Dendrobatidae frog toxins [8], microbial pigments and so on [9]. The quantity, quality, and attractiveness of pigments from various sources such as microbes, algae, invertebrates, and macro-organisms may comprise either beneficial or toxic chemical constituents. Not all colors appealing to our eyes are beneficial to humans. Therefore, investigations on the chemistry of pigment molecules are gaining more interest in the current research. In 1666, Sir Isaac Newton had initiated the beginning of research on colors by developing the first circular diagram of colors, and later various researchers like Harris (1776) and Goethe (1810). Sir Humphry Davy demonstrated the causes of various colors of organic molecules [10]. Later in 1820, Friedrich Accum revealed the many side effects of synthetic colorants in various foods [11]. Sir William Henry Perkin was the first man to develop the first synthetic textile color compound “mauvine” in 1856. With this brief historical background, the visible spectral pigments and invisible nonspectral pigments gain more attention due to numerous applications in ecology, evolution, biomedical, and industrial perspectives. The international color symbolism chart indicates that each color has a specific meaning in different countries and cultures. Despite numerous known applications, evidence shows that visual pigments (color and light) can directly influence the brain [12], psychology [13], taste and flavor of humans [14[14][15][16],15,16], and science communication [17]. The lack of dietary pigments like carotenoids in our daily food intake may lead to various diseases and in rare case death [18]. Visual and food colorants are playing a significant role in decision making in our life to choose different foods and many other things [19], through vision, flavor, olfaction, gustation, and oral somatosensation ways [16].
Humans cannot see nonspectral colors due to a lack of trichromatic or tetrachromatic color vision-related cone types in their eyes. A recent study demonstrated humming birds’ ability to perceive nonspectral colors via the tetrachromacy phenomenon [20]; another example of categorical color perception was observed in Estrildid finches [21]. Numerous studies have been exploring the spectral pigments from microbes and higher organisms for various applications. Nevertheless, nonspectral pigments and their ecological importance in nature and biotechnological applications are not well studied. Thus, studies on nonspectral pigments remain a research gap in the current global science development scenario. Indeed, the planet earth is structured with visible and invisible micro and macromolecules produced by prokaryotes and eukaryotes, regulating various physical, chemical, biological, and geological processes. After going through a vast literature on microbial pigments, it is now understood that microbes and macro-organisms produce varied pigment molecules with a specific purpose in the respective milieus.
The resource of pigments, production rate, transport, price, sustainability, palatability, durability, effectiveness, legislative and regulatory approval, and demand by consumers are the primary requisites for various biotechnological applications in commercial industries. In this context, microbial pigments are attracting great demand to develop food grade, textile grade, and drug grade natural pigments. The reasons for high demand for microbial pigments are their promising unlimited resources, high production of required quantity of pigments, least cost-effective, easy cultivation and can be harvested throughout the year, adaptability to various environments, optimization, stability, genetic engineering, no side effects, eco-friendly, biodegradable, and indispensable applications in multidisciplinary aspects such as ecological, evolutionary, biomedical, agriculture, and industrial studies [9,22,23,24][9][22][23][24]. Many microbes are known to produce a wide variety of pigment molecules with innumerable biological properties and other industrial applications [9,25,26][9][25][26]. Especially, natural pigments of microbial origin have many advantages over synthetic pigments. Although artificial colors are more attractive and have been widely used around the world market (42%) [19,27[19][27][28][29][30],28,29,30], they are found to have many side effects (e.g., teratogenic, cancer, etc.) [29[29][30][31],30,31], and some are not biodegradable (e.g., textile dyes), causing health disorders to aquatic organisms and humans [32,33,34][32][33][34]. Hence, researchers are trying to find alternative physical, chemical, and biological methods to degrade synthetic colors [35,36,37][35][36][37] to avoid the side effects posed to the public and environmental health. Therefore, instead of developing synthetic colors and finding new methods for their degradation, exploring natural pigments from microbes would bring about innumerable advantages for the public and the environment.
 Based on chemical groups, microbial pigments are broadly differentiated into anthraquinones, carotenoids, indoles, phycobiliproteins, prodigiosin, rhodopsins, melanins, and violacein [9,64][9][56]. For understanding the evolutionary aspects, rhodopsins, melanins, and iridescent (structural) pigments are briefly discussed herein. Microbial rhodopsins are light-harvesting photoproteins that bind to retinal and respond to light, which has evolutionary importance. These rhodopsin are found in Archaea, bacteria, fungi, viruses [65][57], and some eukaryotes [66][58]. Based on the known functions, rhodopsins are classified as light sensors (rhodopsins, opsins), energy-conserving transmembrane proton pumps (bacteriorhodopsins, proteorhodopsins, and xanthorhodopsins), and transmembrane chloride pumps (halorhodopsins) [4]. In Haloarchaea, a single cell can possess multiple rhodopsins with varied functions [4]. Melanins are biosynthetically, functionally, and structurally diverse pigments, including five known groups of allomelanin, eumelanin, and neuromelanin pheomelanin, and pyomelanin [67][59]. It is often easy to isolate monochromatic pigment-producing microorganisms from different environments, but isolation of polychromatic pigments producing bacteria such as Pseudomonas aeruginosa (blue and green pigments), Streptomyces sp. (yellow, orange and brown) [25] and iridescent or shimmering bacteria (VIBGYOR) [68][60] (https://www.hoekmine.com; accessed on 10 January 2021; Hoekmine BV, 2020) are rarely isolated. Structural colors are also recorded in fossil feathers, suggesting the importance of evolutionary aspects [69][61].
In general, microbes possess innate pigment traits, but some non-pigmented microbes acquire pigment traits from pigmented microbes (see the Section below: Horizontal Gene Transfer). For this reason, microbial pigments are classified as innate pigments and acquired pigments. Often, pigmented microbes release diffusible and non-diffusible pigments in culture media. However, rarely, some pigments are water-insoluble, for instance, blue pigment indigoidine [70][62], red pigment [71][63], and violacein [72][64]. Some pigments even do not dissolve in solvents; in such incidents, resin extraction can be employed to extract pigments.
Based on chemical groups, microbial pigments are broadly differentiated into anthraquinones, carotenoids, indoles, phycobiliproteins, prodigiosin, rhodopsins, melanins, and violacein [9,64][9][56]. For understanding the evolutionary aspects, rhodopsins, melanins, and iridescent (structural) pigments are briefly discussed herein. Microbial rhodopsins are light-harvesting photoproteins that bind to retinal and respond to light, which has evolutionary importance. These rhodopsin are found in Archaea, bacteria, fungi, viruses [65][57], and some eukaryotes [66][58]. Based on the known functions, rhodopsins are classified as light sensors (rhodopsins, opsins), energy-conserving transmembrane proton pumps (bacteriorhodopsins, proteorhodopsins, and xanthorhodopsins), and transmembrane chloride pumps (halorhodopsins) [4]. In Haloarchaea, a single cell can possess multiple rhodopsins with varied functions [4]. Melanins are biosynthetically, functionally, and structurally diverse pigments, including five known groups of allomelanin, eumelanin, and neuromelanin pheomelanin, and pyomelanin [67][59]. It is often easy to isolate monochromatic pigment-producing microorganisms from different environments, but isolation of polychromatic pigments producing bacteria such as Pseudomonas aeruginosa (blue and green pigments), Streptomyces sp. (yellow, orange and brown) [25] and iridescent or shimmering bacteria (VIBGYOR) [68][60] (https://www.hoekmine.com; accessed on 10 January 2021; Hoekmine BV, 2020) are rarely isolated. Structural colors are also recorded in fossil feathers, suggesting the importance of evolutionary aspects [69][61].
In general, microbes possess innate pigment traits, but some non-pigmented microbes acquire pigment traits from pigmented microbes (see the Section below: Horizontal Gene Transfer). For this reason, microbial pigments are classified as innate pigments and acquired pigments. Often, pigmented microbes release diffusible and non-diffusible pigments in culture media. However, rarely, some pigments are water-insoluble, for instance, blue pigment indigoidine [70][62], red pigment [71][63], and violacein [72][64]. Some pigments even do not dissolve in solvents; in such incidents, resin extraction can be employed to extract pigments.
 Bacteriochlorophylls are photosensitizers (light harvesters) in photosynthetic bacteria but absent in non-photosynthetic bacteria [91][83]. Non-photosynthetic bacteria may utilize a self-photosensitization mechanism [92][84]. In photosynthetic and non-photosynthetic bacteria, carotenoids, the accessory photosynthetic pigments act as photoprotectants and antioxidants, thus protecting cells from damage due to UV and sunlight illumination [91,93,94][83][85][86]. Bacterial communities in the air-water interface did produce more pigmentation to tolerate sunlight and are relatively drug-resistant compared to non-pigmented bacteria [95][87]. The extremophilic bacteria isolated from salt lakes [96][88] and cold environments like Antarctica [97,98][89][90] adopt environmental stress with carotenoids and other pigments. The yellow pigment of Thermus was proposed as a photoprotectant [99][91]. Carotenoids of archaea [100][92], yeasts [101[93][94],102], cyanobacteria, and algae [103][95] also function as photoprotectants. Marennine, a blue pigment produced by diatom Haslea is involved in greening on oysters [104][96], and displayed a prophylactic effect [105,106][97][98].
Bacteriochlorophylls are photosensitizers (light harvesters) in photosynthetic bacteria but absent in non-photosynthetic bacteria [91][83]. Non-photosynthetic bacteria may utilize a self-photosensitization mechanism [92][84]. In photosynthetic and non-photosynthetic bacteria, carotenoids, the accessory photosynthetic pigments act as photoprotectants and antioxidants, thus protecting cells from damage due to UV and sunlight illumination [91,93,94][83][85][86]. Bacterial communities in the air-water interface did produce more pigmentation to tolerate sunlight and are relatively drug-resistant compared to non-pigmented bacteria [95][87]. The extremophilic bacteria isolated from salt lakes [96][88] and cold environments like Antarctica [97,98][89][90] adopt environmental stress with carotenoids and other pigments. The yellow pigment of Thermus was proposed as a photoprotectant [99][91]. Carotenoids of archaea [100][92], yeasts [101[93][94],102], cyanobacteria, and algae [103][95] also function as photoprotectants. Marennine, a blue pigment produced by diatom Haslea is involved in greening on oysters [104][96], and displayed a prophylactic effect [105,106][97][98].
2. Classification of Pigments
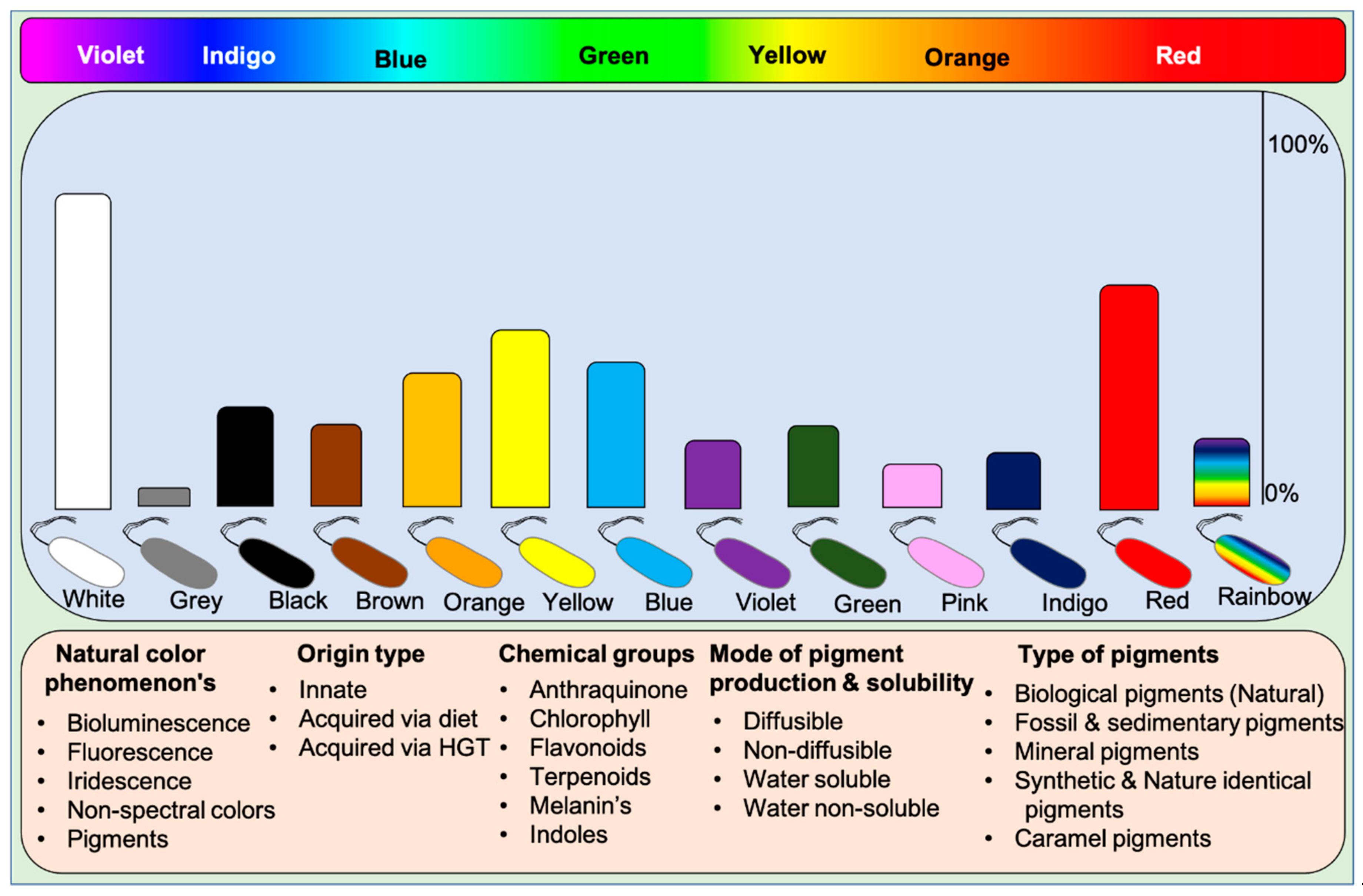
Microbes display all kinds of color hues such as black, blue, bronze, brown, cream, grey, green, orange, purple, indigo, pink, red, yellow, metallic green, red, yellow, and rainbow. These pigments can be classified into various categories based on their visual, chemical, and spectral properties and source of origin (based on mobile genes) [9]. Based on visual appearance, prokaryotes and eukaryotes display monochromatic to polychromatic pigment combinations within the Munsell color system. Some higher organisms like dragonfish [46,47][38][39] and hummingbirds [20] exceptionally display or see colors beyond our visible spectrum and near-infrared spectrum. These incidents suggest that humans lack nonspectral cones to perceive colors existing beyond the visible spectrum. Visually, pigments represent the following phenomena on earth: (1) Natural pigments, (2) Bioluminescence, (3) Fluorescence, and (4) Iridescence (structural colors), and (5) Non-spectral colors. Humans can perceive all the color phenomena except non-spectral colors. Functionally, five different types of pigments are found in nature: (1) Biological pigments, (2) Fossil and sedimentary pigments, (3) Mineral pigments, (4) Synthetic & identical natural pigments, and (5) Caramel pigments (Figure 1). Biological pigments are derived from live microbes, plants, and animals. In contrast, fossil pigments are indeed biologically originated but preserved in fossils for millions of years, acting as evolutionary evidence [48,49,50,51,52,53][40][41][42][43][44][45]. In rare cases, fossil pigments can be of synthetic origin [54][46]. Mineral pigments are inorganic insoluble pigments used in artistic, cosmetic, archeological, and evolutionary studies [55,56,57,58,59,60][47][48][49][50][51][52]. In contrast, synthetic colorants are synthesized in the laboratory for food colorants and dyeing applications [61][53]. Dozens of synthetic colorants are being used in food and beverages [61,62][53][54]. Caramel pigments are natural sugar-based colorants used in a variety of food and beverage products. These caramel colors are classified into Caramel I, II, III, and IV classes to fulfill the requirement of food systems [63][55]. Solvatochromicity of these pigments varies according to the extraction solvent.
Figure 1. A wide array of pigmented microbes seen in nature. The abundance of the type of pigmented bacteria is depicted in bars based on the available literature. Rainbow bacteria are iridescent. Classification of pigments based on various aspects of biochromes. Chlorophyll pigments are not included in the data as they are ubiquitous. HGT: Horizontal gene transfer.
3. Functions of Microbial Pigments
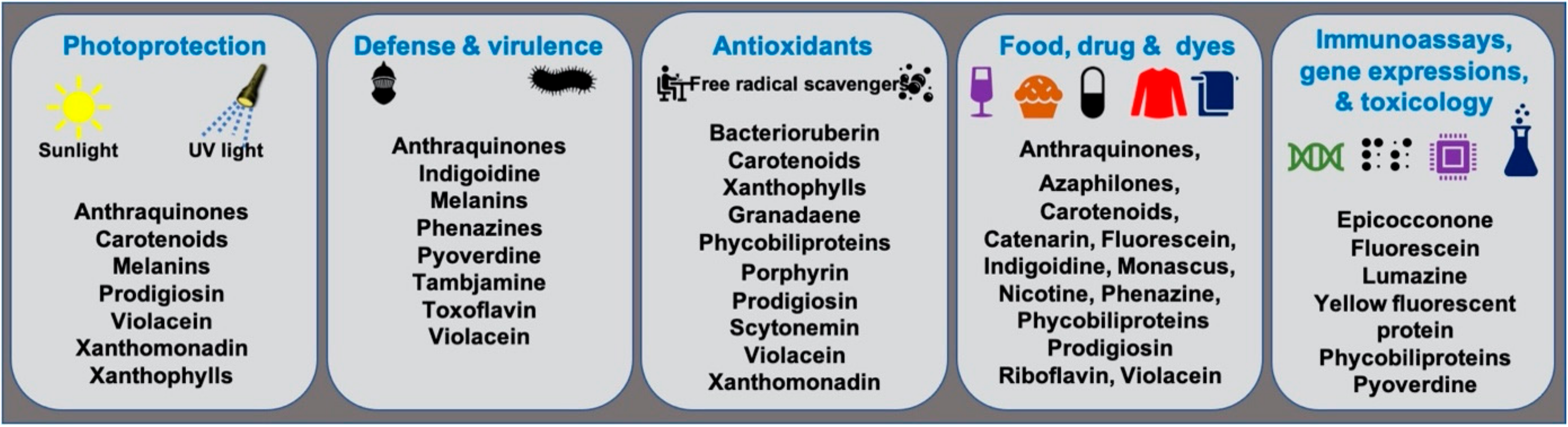
Microbial pigments are known to play a variety of ecological functions in their milieus. (Figure 2). Antioxidant properties of different microbial pigments are detailed in the supplementary file provided in the previous review published in 2019 (see supplementary file) [9]. Prodigiosin pigment produced by some strains of Vibrio sp. function as photoprotectants against UV light [73][65]. Violacein pigment of Janthinobacterium lividum and Chromobacterium violaceum demonstrated antipredator activity against bacterivorous nanoflagellates, indicating its defensive function [74][66]. J. lividum associated with the skins of some frogs and salamanders, secretes violacein pigment to protect them from pathogenic fungi, Batrachochytrium dendrobatidis [75,76,77][67][68][69]. Phenazine compounds produced by bacteria play multiple functions, including chemical signaling, biofilm formation, survival, and virulence [78][70]. Pyoverdine, a fluorescent yellow-green pigment, regulates iron transport and virulence functions in Pseudomonas fluorescens [79][71]. Tambjamine, a yellow pigment produced by Pseudoalteromonas tunicata [80][72], is suggested to help its host prevent other predatory fouling organisms [81][73]. Likewise, indigoidine, a blue pigment produced by Phaeobacter strains, is suggested to inhibit competing bacteria in the environment [82][74]. Bacterial melanin pigments act as photoprotectants [83,84,85,86,87][75][76][77][78][79]. For instance, Vibrio cholerae melanins serve as survival fitness factors when physico-chemical factors become unfavorable [88][80]. Some endophytic fungi releases anthraquinones, to protect the host plant from damage due to insects and microbes [89][81]; while, fungal melanins demonstrate multiple functions [90][82].
Figure 2.
Ecological functions and other applications of important microbial pigments.
References
- Scholes, G.D.; Mirkovic, T.; Turner, D.B.; Fassioli, F.; Buchleitner, A. Solar light harvesting by energy transfer: From ecology to coherence. Energy Environ. Sci. 2012, 5, 9374–9393.
- Croce, R.; van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501.
- Béja, O.; Spudich, E.N.; Spudich, J.L.; Leclerc, M.; DeLong, E.F. Proteorhodopsin phototrophy in the ocean. Nature 2001, 411, 786–789.
- Frigaard, N.U.; Martinez, A.; Mincer, T.J.; DeLong, E.F. Proteorhodopsin lateral gene transfer between marine planktonic Bacteria and Archaea. Nature 2006, 439, 847–850.
- Kamio, M.; Grimes, T.V.; Hutchins, M.H.; van Dam, R.; Derby, C.D. The purple pigment aplysioviolin in sea hare ink deters predatory blue crabs through their chemical senses. Anim. Behav. 2010, 80, 89–100.
- Derby, C.D. Escape by Inking and Secreting: Marine Molluscs Avoid Predators Through a Rich Array of Chemicals and Mechanisms. Biol. Bull. 2007, 213, 274–289.
- Derby, C.D. Cephalopod Ink: Production, Chemistry, Functions and Applications. Mar. Drugs 2014, 12, 2700–2730.
- Santos, J.C.; Coloma, L.A.; Cannatella, D.C. Multiple, recurring origins of aposematism and diet specialization in poison frogs. Proc. Natl. Acad. Sci. USA 2003, 100, 12792–12797.
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Multifaceted applications of microbial pigments: Current knowledge, challenges and future directions for public health implications. Microorganisms 2019, 7, 186.
- Davy, S.H. Essays on Heat, Light, and the Combinations of Light, with a New Theory of Respiration. On the Generation of Oxygen Gas, and the Causes of the Colors of Organic Beings; 1799.
- Friedrich, A.C. A Treatise on Adulterations of Food, and Culinary Poisons: Exhibiting the Fraudulent Sophistications of Bread, Beer, Wine, Spiritous Liquors, Tea, Coffee, Cream, Confectionery, Vinegar, Mustard, Pepper, Cheese, Olive Oil, Pickles, and Other Articles Employed in Domestic Economy and Methods of Detecting Them; Ab’m Small: Philadelphia, PA, USA, 1820.
- Vandewalle, G.; Schmidt, C.; Albouy, G.; Sterpenich, V.; Darsaud, A.; Rauchs, G.; Berken, P.-Y.; Balteau, E.; Degueldre, C.; Luxen, A.; et al. Brain Responses to Violet, Blue, and Green Monochromatic Light Exposures in Humans: Prominent Role of Blue Light and the Brainstem. PLoS ONE 2007, 2, e1247.
- Kurt, S.; Osueke, K.K. The Effects of Color on the Moods of College Students. SAGE Open 2014, 2014, 1–12.
- Spence, C. On the psychological impact of food colour. Flavour 2015, 4, 21.
- Piqueras-Fiszman, B.; Giboreau, A.; Spence, C. Assessing the influence of the color of the plate on the perception of a complex food in a restaurant setting. Flavour 2013, 2, 24.
- Spence, C.; Levitan, C.A.; Shankar, M.U.; Zampini, M. Does food color influence taste and flavor perception in humans? Chemosens. Percept. 2010, 3, 68–84.
- Crameri, F.; Shephard, G.E.; Heron, P.J. The misuse of colour in science communication. Nat. Commun. 2020, 11, 5444.
- Olson, J.A. Biological actions of carotenoids. J. Nutr. 1989, 119, 94–95.
- Downham, A.; Collins, P. Colouring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22.
- Stoddard, M.C.; Eyster, H.N.; Hogan, B.G.; Morris, D.H.; Soucy, E.R.; Inouye, D.W. Wild hummingbirds discriminate nonspectral colors. Proc. Natl. Acad. Sci. USA 2020, 117, 15112–15122.
- Caves, E.M.; Green, P.A.; Zipple, M.N.; Bharath, D.; Peters, S.; Johnsen, S.; Nowicki, S. Comparison of categorical color perception in two Estrildid finches. Am. Nat. 2020.
- Newsome, A.G.; Murphy, B.T.; Van Breemen, R. Isolation and characterization of natural blue pigments from underexplored sources. ACS Symp. Ser. 2013, 1138, 105–125.
- Venil, C.K.; Dufossé, L.; Devi, P.R. Bacterial Pigments: Sustainable Compounds With Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 100.
- Dufossé, L. Research, Development, and Production of Microalgal and Microbial Biocolorants. In Bioprocessing for Biomolecules Production; Molina, G., Gupta, V.K., Singh, B.N., Gathergood, N., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2020.
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R. Marine pigmented bacteria: A prospective source of antibacterial compounds. J. Nat. Sci. Biol. Med. 2019, 10, 104–113.
- Nawaz, A.; Chaudhary, R.; Shah, Z.; Dufossé, L.; Fouillaud, M.; Mukhtar, H.; ul Haq, I. An overview on industrial and medical applications of bio-pigments synthesized by marine bacteria. Microorganisms 2021, 9, 11.
- Rajapaksha, G.K.M.; Wansapala, M.A.J.; Silva, A.B.G. Detection of Synthetic Colours in Selected Foods & Beverages Available in Colombo District, Sri Lanka. Int. J. Sci. Res. 2017, 6, 801–808.
- Saleem, N.; Umar, Z.N.; Khan, S.I. Survey on the use of synthetic Food Colors in Food Samples procured from different educational institutes of Karachi city. J. Trop. Life. Sci. 2013, 3, 1–7.
- Okafor, S.N.; Obonga, W.; Ezeokonkwo, M.A.; Nurudeen, J.; Orovwigho, U.; Ahiabuike, J. Assessment of the Health implications of Synthetic and Natural Food Colourants-A Critical Review. UK J. Pharm. Biosci. 2016, 4, 1–11.
- Babitha, S. Microbial pigments. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Nigam, P.S., Pandey, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 147–162. ISBN 9781402099410.
- Burrows, A.J.D. Palette of our palates: A brief history of food coloring and its regulation. Compr. Rev. Food Sci. Food Saf. 2009, 8, 394–408.
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290.
- Saini, R.D. Textile organic dyes: Polluting effects and elimination methods from textile waste water. Int. J. Chem. Eng. Res. 2017, 9, 121–136.
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711.
- Jamee, R.; Siddique, R. Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118.
- Roy, A.K.C. Eco-friendly dyes and dyeing. Adv. Mat. Technol. Environ. 2018, 2, 145–176.
- Puvaneswari, N.; Muthukrishnan, J.; Gunasekaran, P. Toxicity assessment and microbial degradation of azo dyes. Indian J. Exp. Biol. 2006, 44, 618–626.
- Kenaley, C.P. Comparative Innervation of Cephalic Photophores of the Loosejaw Dragonfishes (Teleostei:Stomiiformes:Stomiidae): Evidence for Parallel Evolution of. J. Morphol. 2010, 271, 418–437.
- Kenaley, C.P.; DeVaney, S.C.; Fjeran, T.T. The complex evolutionary history of seeing red: Molecular phylogeny and the evolution of an adaptive visual system in deep-sea dragonfishes (Stomiiformes: Stomiidae). Evolution 2014, 68, 996–1013.
- Roy, A.; Pittman, M.; Saitta, E.T.; Kaye, T.G.; Xu, X. Recent advances in amniote palaeocolour reconstruction and a framework for future research. Biol. Rev. 2020, 95, 22–50.
- Lindgren, J. Fossil pigments. Curr. Biol. 2016, 26, R445–R460.
- Colleary, C.; Dolocan, A.; Gardner, J.; Singh, S.; Wuttke, M.; Rabenstein, R.; Habersetzer, J.; Schaal, S.; Feseha, M.; Clemens, M.; et al. Chemical, experimental, and morphological evidence for diagenetically altered melanin in exceptionally preserved fossils. Proc. Natl. Acad. Sci. USA 2015, 112, 12592–12597.
- Blumer, M. Pigments of a fossil echinoderm. Nature 1960, 188, 1100–1101.
- Zhang, F.; Kearns, S.L.; Orr, P.J.; Benton, M.J.; Zhou, Z.; Johnson, D.; Xu, X.; Wang, X. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 2010, 463, 1075–1078.
- Babarovic, F.; Puttick, M.N.; Zaher, M.; Learmonth, E.; Gallimore, E.J.; Smithwick, F.M.; Mayr, G.; Vinther, J. Characterization of melanosomes involved in the production of non-iridescent structural feather colours and their detection in the fossil record. J. R. Soc. Interface 2019, 16, 20180921.
- Pérez-Diez, S.; Fernández-Menéndez, L.J.; Morillas, H.; Martellone, A.; De Nigris, B.; Osanna, M.; Bordel, N.; Caruso, F.; Madariaga, J.M.; Maguregui, M. Elucidation of the chemical role of the pyroclastic materials on the state of conservation of mural paintings from Pompeii. Angew. Chemie 2020.
- Siddall, R. Mineral pigments in archaeology: Their analysis and the range of available materials. Minerals 2018, 8, 201.
- Reiche, I. Mineral pigments: The colourful palette of nature. Eur. Mineral. Union Notes Mineral. 2019, 20, 283–322.
- Barnett, J.R.; Miller, S.; Pearce, E. Colour and art: A brief history of pigments. Opt. Laser Technol. 2006, 38, 445–453.
- Zilhão, J.; Angelucci, D.E.; Badal-García, E.; d’Errico, F.; Daniel, F.; Dayet, L.; Douka, K.; Higham, T.F.G.; Martínez-Sánchez, M.J.; Montes-Bernárdez, R.; et al. Symbolic use of marine shells and mineral pigments by Iberian Neandertals. Proc. Natl. Acad. Sci. USA 2010, 107, 1023–1028.
- Aubert, M.; Lebe, R.; Oktaviana, A.A.; Tang, M.; Burhan, B.; Hamrullah; Jusdi, A.; Abdullah; Hakim, B.; Zhao, J.X.; et al. Earliest hunting scene in prehistoric art. Nature 2019, 576, 442–445.
- Kurniawan, R.; Kadja, G.T.M.; Setiawan, P.; Burhan, B.; Oktaviana, A.A.; Rustan; Hakim, B.; Aubert, M.; Brumm, A.; Ismunandar. Chemistry of prehistoric rock art pigments from the Indonesian island of Sulawesi. Microchem. J. 2019, 146, 227–233.
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15.
- Smith, J.; Hong-Shum, L. Food Additives Data Book, 2nd ed.; Blackwell Publishing Ltd: Hoboken, NJ, USA, 2011.
- Sengar, G.; Kumar, H. Food caramels: A review. J. Food. Sci. Technol. 2014, 51, 1686–1696.
- Velmurugan, P.; Venil, C.K.; Veera Ravi, A.; Dufossé, L. Marine bacteria is the cell factory to produce bioactive pigments: A prospective pigment source in the ocean. Front. Sustain. Food Syst. 2020, 4, 589655.
- Yutin, N.; Koonin, E.V. Proteorhodopsin genes in giant viruses. Biol. Direct 2012, 7, 34.
- Slamovits, C.H.; Okamoto, N.; Burri, L.; James, E.R.; Keeling, P.J. A bacterial proteorhodopsin proton pump in marine eukaryotes. Nat. Commun. 2011, 2, 183.
- Cao, W.; McCallum, N.C.; Ni, Q.Z.; Li, W.; Boyce, H.; Mao, H.; Zhou, X.; Sun, H.; Thompson, M.P.; Battistella, C.; et al. Selenomelanin: An abiotic selenium analogue of pheomelanin. J. Am. Chem. Soc. 2020, 142, 12802–12810.
- Kientz, B.; Luke, S.; Vukusic, P.; Péteri, R.; Beaudry, C.; Renault, T.; Simon, D.; Mignot, T.; Rosenfeld, E. A unique self-organization of bacterial sub-communities creates iridescence in Cellulophaga lytica colony biofilms. Sci. Rep. 2016, 6, 19906.
- Vinther, J.; Briggs, D.E.G.; Clarke, J.; Mayr, G.; Prum, R.O. Structural coloration in a fossil feather. Biol. Lett. 2010, 6, 128–131.
- Buchan, A.; Neidle, E.L.; Moran, M.A. Diverse organization of genes of the β-ketoadipate pathway in members of the marine Roseobacter lineage. Appl. Environ. Microbiol. 2004.
- Afra, S.; Makhdoumi, A.; Matin, M.M.; Feizy, J. A novel red pigment from marine Arthrobacter sp. G20 with specific anticancer activity. J. Appl. Microbiol. 2017, 123, 1228–1236.
- Choi, S.Y.; Lim, S.; Cho, G.; Kwon, J.; Mun, W.; Im, H.; Mitchell, R.J. Chromobacterium violaceum delivers violacein, a hydrophobic antibiotic, to other microbes in membrane vesicles. Environ. Microbiol. 2020, 22, 705–713.
- Borić, M.; Danevčič, T.; Stopar, D. Prodigiosin from Vibrio sp. DSM 14379; a new UV-protective pigment. Microb. Ecol. 2011, 62, 528–536.
- Matz, C.; Deines, P.; Boenigk, J.; Arndt, H.; Eberl, L.; Kjelleberg, S.; Jürgens, K. Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl. Environ. Microbiol. 2004, 70, 1593–1599.
- Brucker, R.M.; Harris, R.N.; Schwantes, C.R.; Gallaher, T.N.; Flaherty, D.C.; Lam, B.A.; Minbiole, K.P.C. Amphibian chemical defense: Antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. J. Chem. Ecol. 2008, 34, 1422–1429.
- Harris, R.N.; Brucker, R.M.; Walke, J.B.; Becker, M.H.; Schwantes, C.R.; Flaherty, D.C.; Lam, B.A.; Woodhams, D.C.; Briggs, C.J.; Vredenburg, V.T.; et al. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 2009, 3, 818–824.
- Becker, M.H.; Brucker, R.M.; Schwantes, C.R.; Harris, R.N.; Minbiole, K.P.C. The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl. Environ. Microbiol. 2009, 75, 6635–6638.
- Pierson, L.S.; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670.
- Visca, P.; Imperi, F.; Lamont, I.L. Pyoverdine siderophores: From biogenesis to biosignificance. Trends Microbiol. 2007, 15, 22–30.
- Franks, A.; Haywood, P.; Holmström, C.; Egan, S.; Kjelleberg, S.; Kumar, N. Isolation and structure elucidation of a novel yellow pigment from the marine bacterium Pseudoalteromonas tunicata. Molecules 2005, 10, 1286–1291.
- Egan, S.; James, S.; Holmström, C.; Kjelleberg, S. Correlation between pigmentation and antifouling compounds produced by Pseudoalteromonas tunicata. Environ. Microbiol. 2002, 4, 433–442.
- Cude, W.N.; Mooney, J.; Tavanaei, A.A.; Hadden, M.K.; Frank, A.M.; Gulvik, C.A.; May, A.L.; Buchan, A. Production of the antimicrobial secondary metabolite indigoidine contributes to competitive surface colonization by the marine roseobacter Phaeobacter sp. strain Y4I. Appl. Environ. Microbiol. 2012, 78, 4771–4780.
- Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-protective compounds in marine organisms from the southern ocean. Mar. Drugs 2018, 16, 336.
- Plonka, P.M.; Grabacka, M. Melanin synthesis in microorganisms—Biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443.
- Kotob, S.I.; Coon, S.L.; Quintero, E.J.; Weiner, R.M. Homogentisic acid is the primary precursor of melanin synthesis in Vibrio cholerae, a Hyphomonas strain, and Shewanella colwelliana. Appl. Environ. Microbiol. 1995, 61, 1620–1622.
- Ivanova, E.P.; Kiprianova, E.A.; Mikhailov, V.V.; Levanova, G.F.; Garagulya, A.D.; Gorshkova, N.M.; Yumoto, N.; Yoshikawa, S. Characterization and identification of marine Alteromonas nigrifaciens strains and emendation of the description. Int. J. Syst. Bacteriol. 1996, 46, 223–228.
- Kahng, H.Y.; Chung, B.S.; Lee, D.H.; Jung, J.S.; Park, J.H.; Jeon, C.O. Cellulophaga tyrosinoxydans sp. nov., a tyrosinase-producing bacterium isolated from seawater. Int. J. Syst. Evol. Microbiol. 2009, 59, 654–657.
- Coyne, V.E.; Al-Harthi, L. Induction of melanin biosynthesis in Vibrio cholerae. Appl. Environ. Microbiol. 1992, 58, 2861–2865.
- Gessler, N.N.; Egorova, A.S.; Belozerskaya, T.A. Fungal anthraquinones. Appl. Biochem. Microbiol. 2013, 49, 85–99.
- Cordero, R.J.B.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112.
- Mathews, M.M.; Sistrom, W.R. Function of carotenoid pigments in non-photosynthetic bacteria. Nature 1959, 184, 1892–1893.
- Sakimoto, K.K.; Wong, A.B.; Yang, P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 2016, 351, 74–77.
- Krinsky, N.I. Non-photosynthetic functions of carotenoids. Philos. Trans. R. Soc. London B 1978, 284, 581–590.
- Agogué, H.; Joux, F.; Obernosterer, I.; Lebaron, P. Resistance of marine bacterioneuston to solar radiation. Appl. Environ. Microbiol. 2005, 71, 5282–5289.
- Hermansson, M.; Jones, G.W.; Kjelleberg, S. Frequency of antibiotic and heavy metal resistance, pigmentation, and plasmids in bacteria of the marine air-water interface. Appl. Environ. Microbiol. 1987, 53, 2338–2342.
- Mandelli, F.; Miranda, V.S.; Rodrigues, E.; Mercadante, A.Z. Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J. Microbiol. Biotechnol. 2012, 28, 1781–1790.
- Dieser, M.; Greenwood, M.; Foreman, C.M. Carotenoid pigmentation in Antarctic heterotrophic bacteria as a strategy to withstand environmental stresses. Arctic, Antarct. Alp. Res. 2010, 42, 396–405.
- Marizcurrena, J.J.; Cerdá, M.F.; Alem, D.; Castro-Sowinski, S. Living with Pigments: The Colour Palette of Antarctic Life. In The Ecological Role of Micro-Organisms in the Antarctic Environment; Springer: Cham, Switzerland, 2019; pp. 65–82.
- Albuquerque, L.; Da Costa, M.S. The family thermaceae. In The Prokaryotes: Other Major Lineages of Bacteria and The Archaea; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 955–987. ISBN 9783642301230.
- Rodrigo-Baños, M.; Garbayo, I.; Vílchez, C.; Bonete, M.J.; Martínez-Espinosa, R.M. Carotenoids from Haloarchaea and their potential in biotechnology. Mar. Drugs 2015, 13, 5508–5532.
- Moliné, M.; Flores, M.R.; Libkind, D.; Del Carmen Diéguez, M.; Farías, M.E.; Van Broock, M. Photoprotection by carotenoid pigments in the yeast Rhodotorula mucilaginosa: The role of torularhodin. Photochem. Photobiol. Sci. 2010, 9, 1145–1151.
- Moliné, M.; Libkind, D.; del Carmen Diéguez, M.; van Broock, M. Photoprotective role of carotenoids in yeasts: Response to UV-B of pigmented and naturally-occurring albino strains. J. Photochem. Photobiol. B Biol. 2009, 95, 156–161.
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. UV photoprotectants from algae-synthesis and bio-functionalities. In Algal Green Chemistry: Recent Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 17–38. ISBN 9780444640413.
- Gastineau, R.; Hardivillier, Y.; Leignel, V.; Tekaya, N.; Morançais, M.; Fleurence, J.; Davidovich, N.; Jacquette, B.; Gaudin, P.; Hellio, C.; et al. Greening effect on oysters and biological activities of the blue pigments produced by the diatom Haslea karadagensis (Naviculaceae). Aquaculture 2012, 368–369, 61–67.
- Falaise, C.; James, A.; Travers, M.A.; Zanella, M.; Badawi, M.; Mouget, J.L. Complex relationships between the blue pigment marennine and marine bacteria of the genus Vibrio. Mar. Drugs 2019, 17, 160.
- Turcotte, F.; Mouget, J.L.; Genard, B.; Lemarchand, K.; Deschênes, J.S.; Tremblay, R. Prophylactic effect of Haslea ostrearia culture supernatant containing the pigment marennine to stabilize bivalve hatchery production. Aquat. Living Resour. 2016, 29, 401.
More
