Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Camila Xu and Version 2 by Weidson Sutil.
Soybean production is usually performed on large scales, requiring efficient pest management to be successful. Soybean fields are inhabited by several species of arthropods, demanding constant development of management practices to prevent pest outbreaks.
- hymenoptera
- Scelionidae
- sustainability
- preservation
- insecticide mitigation
1. Introduction
Until recently, chemical control has been farmers’ first line of defense to control pests in the production of soybean (Glycine max (L.) Merrill) [1[1][2],2], the most important source of plant-based protein and vegetable oil worldwide [3]. The stink bug species of the genus Euschistus and Diceraeus (Hemiptera: Pentatomidae) [2] are major pests that damage soybeans and require a greater amount of insecticides in South America, mainly in the central region of Brazil at latitudes between 0° and 23° [4] and in the Southeastern USA [5]. In addition to Euschistus sp. and Diceraeus sp., at least 54 other stink bug species from different genus have been reported attacking soybeans [6]. Feeding directly from soybean pods, those insects can significantly impact the crop, reducing yields by 15% [7] and impairing the physiological and sanitary quality of the seeds when not properly managed [8].
Despite the high potential of damage, insecticides used in an uncoordinated manner and without considering the mechanisms of action can impact human health and the environment [9,10][9][10]. Moreover, the overuse of insecticides can reduce natural biocontrol agents [11] and pollinators [12], induce pest resistance [13], and trigger pest resurgence and/or outbreaks of secondary pest species, in addition to other negative side effects [2]. Consequently, reducing synthetic chemical use in agriculture to produce food inexpensively and sustainably [14] has become a global goal [15]. Thus, more sustainable stink bug management tools are of high theoretical and practical interest and will benefit thousands of soybean farmers. Biological control is the most adopted sustainable pest control strategy available for agricultural use [16].
The three commonly adopted ways to apply (or exploit) biological control in the agroecosystem are (1) conservation biological control (CBC), which consists of preserving or favoring the existing natural enemies; (2) classical biological control, by introducing new natural enemies to establish a permanent population into the new agroecosystem; and (3) augmentative biological control (ABC), which relies on massive and periodic releases of biocontrol agents to rapidly reduce pest population [17]. ABC is drastically increasing worldwide, and the global ABC market is expected to surpass USD 10 billion in 2027 [18]. However, the successful adoption of ABC programs depends on CBC practices, which provide more balanced and equilibrated agroecosystems [2]. The establishment of more resilient agroecosystems requires actions that consider the system as a whole and improve agriculture through what is known as Landscape Architecture (LA) [19]. Originally, the idea of LA represented a form of agriculture that applied science-based agricultural practices while considering the aesthetic dimension of rural landscapes in relation to the necessary food production [20]. Subsequently, the concept of integrated landscape management emerged, combining food production with the conservation of ecosystem services, particularly those supplied by habitat biodiversity [21].
Among LA procedures, CBC is highlighted for its beneficial impact on agroecosystems from both ecological and economic standpoints [2,11][2][11]. CBC is based on preserving and improving natural biological control by maintaining a habitat that can sustain natural enemies [22]. The concept of CBC neither necessarily excludes other types of pest management nor the introduction of other biological control agents via ABC strategies [23]. Instead, CBC helps to provide a more favorable environment for biocontrol agents to survive and prosper, contributing to more effective ABC practices [24]. A variety of management practices are needed to manipulate the agricultural habitat in favor of biocontrol agents of pests in the agroecosystem to enhance their fitness and optimize their impact on pest population growth [25]. Despite negative interactions that can exist among different species of natural enemies [26], ABC and CBC strategies are more likely to be compatible with each other than chemical control [11]. Although, in some cases, multiple field experiments have shown negative impacts of chemical insecticides on biological control [27], some selective insecticides have minimal impact on biocontrol agents due to their physiological selectivity. Furthermore, non-selective insecticides can be sprayed in a way that reduces contact with beneficial organisms and, consequently, has a lower impact on them, i.e., a strategy called ecological selectivity [11].
Stink bugs are considered hard-to-kill insects and are the target of up to 60% of all insecticide applications performed on soybean fields in areas where they occur [28]. It is of great theoretical and practical interest to discuss sustainable alternatives to improve their management. Biological control is of crucial importance to agroecosystem sustainability. Some CBC and ABC practices can be adopted within integrated pest management (IPM) to enhance the impact of biological control in soybeans.
2. The Importance of Biocontrol Agents of Stink Bugs in Soybean Production
Several organisms, such as fungi, viruses, bacteria, and arthropods, can act as biocontrol agents of soybean pests, infecting, preying on, or parasitizing pests in their different developmental stages [17]. The capacity of those beneficial organisms to reduce pest population, known as biological control, usually plays an important role in limiting the densities of pests in agriculture. Biocontrol is usually safer for the environment and more specific against the target species than chemicals [11]. Thus, knowledge about the species of biocontrol agents and their specific potential to reduce the population of each economically important stink bug species is critical for the correct adoption of CBC or ABC strategies, aiming at the benefits from the potential of those biocontrol agents in sustainably reducing stink bug population in soybean fields [17]. A large and diverse complex of natural enemies and stink bug pests has been recorded in the most important soybean-producing countries (Brazil and the USA). Stink bug eggs, late nymphal stages, and adults are most commonly attacked by parasitoids (Table 1), whereas predators prefer eggs and early nymph stages [5].Table 1.
Stink bug parasitoids recorded in Brazil or the USA.
| Species | 1 Host Species | 2 Stage(s) Attacked | Parasitism Rate | Country | Reference |
|---|---|---|---|---|---|
| Anastatus mirabilis (Walsh & Riley) (Hymenoptera: Eupelmidae) | E | E | 0.8% | USA | [29] |
| Anastatus reduvii (Howard) (Hymenoptera: Eupelmidae) | Ch | E | 44% | USA | [30] |
| Aridelus rufotestaceus Tobias (Hymenoptera: Braconidae) | E; Nv | N | No information | USA | [31,32][31][32] |
| Gryon obesum Masner (Hymenoptera: Scelionidae) | E | E | 1.4% to 2.4% | USA | [33] |
| E | E | 2.6% | USA | [29] | |
| Hexacladia hilaris Burks (Hymenoptera: Encyrtidae) | Ch; Nv | N; A | No information | Brazil and USA | [34,[35,3436,37]][35][36][37] |
| Hexacladia smithii Ashmead (Hymenoptera: Encyrtidae) | E; Eh | A | 0.6% to 90% | Brazil and USA | [34,,41][35,34][36,3538,39,][36][38][39]40[40][41] |
| Em | A | No information | Brazil | [4] | |
| Ooencyrtus anasae (Ashmead) (Hymenoptera: Encyrtidae) | Nv; Pz | E | No information | Brazil | [42] |
| Ooencyrtus submetalicus (Howard) (Hymenoptera: Encyrtidae) | Nv | E | No information | USA | [34] |
| Em | E | No information | Brazil | [43] | |
| Telenomus edessae Brèthes (Hymenoptera: Scelionidae) | Em | E | 0.4% | Brazil | [39] |
| Telenomus podisi Ashmead (Hymenoptera: Scelionidae) | Nv | E | No information | USA | [34] |
| Nv | E | 11.5% to 100% | USA | [33] | |
| Ch | E | 3.4% | Brazil | [39] | |
| Dm | E | 25% to 50% | Brazil and USA | [39,44][39][44] | |
| Eh | E | 43.4% | Brazil | [39] | |
| Eh | E | 59.3% to 62.5% | Brazil | [45] | |
| E | E | 77.8% | USA | [46] | |
| E | E | 69% to 100% | USA | [33] | |
| Pz | E | 20.9% | Brazil and USA | [39,47][39][47] | |
| Pz | E | 23.8% to 39.5% | Brazil | [45] | |
| Pz | E | No information | Brazil | [42] | |
| Trissolcus basalis (Wollaston) (Hymenoptera: Scelionidae) | Nv | E | 74.5 | USA | [46] |
| Nv | E | 25% to 100% | USA | [33] | |
| Nv | E | 53.8% | Brazil and USA | [39,47][39][47] | |
| Dm | E | 16.7% | |||
| Eh | E | 10.6% | |||
| Ch | E | 24% | |||
| Pz | E | 22.8% | |||
| Th | E | 23.1% | |||
| E | E | 3% to 100% | USA | [33] | |
| E | E | 18.6% | USA | [46] | |
| Trissolcus brochymenae (Ashmead) (Hymenoptera: Scelionidae) | E | E | 7.4% | USA | [48] |
| Trissolcus edessae Fouts (Hymenoptera: Scelionidae) | E | E | 19.3% | USA | [29] |
| E | E | 6.6% | USA | [46] | |
| E | E | 3.1% | USA | [29] | |
| Ch | E | 35% | USA | [30] | |
| Trissolcus elimatus (Johnson) (Hymenoptera: Scelionidae) | Em | E | No information | Brazil | [43] |
| Trissolcus euschisti (Ashmead) (Hymenoptera: Scelionidae) | E | E | 20% | USA | [46] |
| E | E | 3.4% | USA | [48] | |
| E | 5.3% to 20% | USA | [33] | ||
| E | E | 3.6% | USA | [29] | |
| Em | E | No information | Brazil | [43] | |
| Trissolcus teretis Johnson (Hymenoptera: Scelinonidae) | Pz | E | No information | Brazil | [42] |
| Trissolcus thyantae Ashmead (Hymenoptera: Scelionidae) | E | E | 3.4% | USA | [48] |
| E | E | 1.1% to 5.9% | USA | [48] | |
| E | E | 5.9% | USA | [29] | |
| Nv | E | 1.4% to 8.3% | USA | [33] | |
| Trissolcus urichi (Crawford) (Hymenoptera: Scelionidae) | Em | E | 14.2% | Brazil | [39] |
| Em | E | No information | Brazil | [43] | |
| Nv; Eh; Pz | E | No information | Brazil and USA | [42,47][42][47] | |
| Eh | E | No information | Brazil | [49] |
1 Stink bug species abbreviations: Ch: Chinavia sp., Dm: Diceraeus melacanthus, Em: Edessa meditabunda, E: Euschistus sp., Eh: Euschistus heros, Nv: Nezara viridula, Pz: Piezodorus guildinii, Th: Thyanta sp. 2 E: egg, N: nymph, and A: adult.
Many microhymenopteran species are parasitoids of stink bugs and are mostly egg parasitoids. Twenty-three species of stink bug egg parasitoids have already been identified in soybeans [50], and these natural enemies are considered the most important biocontrol agents for this group of pests [17,51,52][17][51][52]. They are often responsible for naturally keeping stink bug populations below economic injury levels (without needing to adopt any additional control strategy) [50].
In the Neotropical region, Nezara viridula (Linnaeus, 1758) (Pentatomidae) was the most abundant species until 1999, accounting for 44% of the stink bugs recorded in soybean areas [53]. In the surveys performed in that era, T. basalis was the most important egg parasitoid species, responsible for more than 90% of the natural parasitism on N. viridula eggs [54].
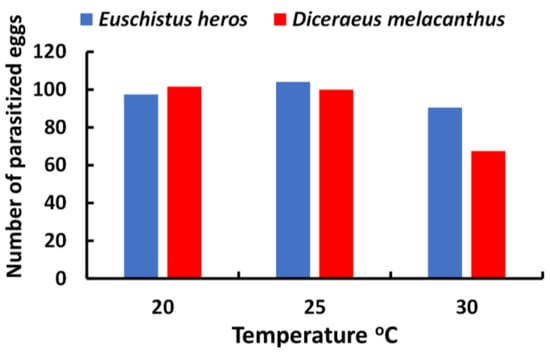
More recently, a significant change in the composition of the pentatomid fauna in soybeans resulted in a reduction in N. viridula and an increase in Euschistus heros (Fabricius, 1798) (Hemiptera: Pentatomidae) in Brazil (Figure 1). Thus, T. podisi gained more importance as an egg parasitoid, being responsible for more than 80% of the parasitism recorded in E. heros eggs [55], as this is its preferred host. The high parasitism capacity of T. podisi on eggs of different stink bug species (Figure 2) led this species to be used in Brazil as a biocontrol agent in ABC of stink bugs in soybean crops [50]. In an agroecosystem such as soybean, where different stink bug species can occur, CBC practices that preserve the diversity of the natural enemy communities are highly desirable to keep the stink bug complex under control [56].
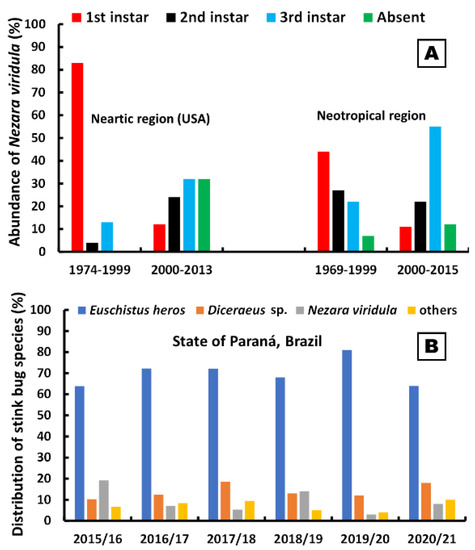
 Agricultural practices that preserve a richer community of biocontrol agents are important to establish stronger and more balanced agroecosystems, which are less susceptible to pest outbreaks [11]. Understanding how the adoption of sustainable IPM practices can help to increase or preserve biological control, an ecosystem service valued at USD 4.5 billion annually, is important to shape agriculture practices, especially for stink bugs in soybeans [1]. Biocontrol agents are particularly vulnerable [67,68][65][66] in large-scale crops such as soybean, which might receive a high number of insecticides every growing season if IPM is not properly adopted [1,2][1][2].
Despite the efficiency and importance of natural biocontrol agents, the logistic complexity of farm operations in large-scale crops such as soybeans can sometimes discourage farmers from integrating different pest management tools [2,69][2][67]. Of the wide range of impediments to the broad adoption of CBC practices among other sustainable IPM strategies in soybeans, the most important are usually related to economic considerations [2]. Concerns about the practicability, complexity, and costs of CBC or ABC practices caused by difficulties in their timing and implementation, as well as the lack of incentives, are reported as the major challenges to greater IPM adoption [70][68].
Agricultural practices that preserve a richer community of biocontrol agents are important to establish stronger and more balanced agroecosystems, which are less susceptible to pest outbreaks [11]. Understanding how the adoption of sustainable IPM practices can help to increase or preserve biological control, an ecosystem service valued at USD 4.5 billion annually, is important to shape agriculture practices, especially for stink bugs in soybeans [1]. Biocontrol agents are particularly vulnerable [67,68][65][66] in large-scale crops such as soybean, which might receive a high number of insecticides every growing season if IPM is not properly adopted [1,2][1][2].
Despite the efficiency and importance of natural biocontrol agents, the logistic complexity of farm operations in large-scale crops such as soybeans can sometimes discourage farmers from integrating different pest management tools [2,69][2][67]. Of the wide range of impediments to the broad adoption of CBC practices among other sustainable IPM strategies in soybeans, the most important are usually related to economic considerations [2]. Concerns about the practicability, complexity, and costs of CBC or ABC practices caused by difficulties in their timing and implementation, as well as the lack of incentives, are reported as the major challenges to greater IPM adoption [70][68].


Figure 2.
References
- Bueno, A.F.; Colmenarez, Y.C.; Carnevalli, R.A.; Sutil, W.P. Benefits and perspectives of adopting soybean-IPM: The success of a Brazilian programme. Plant Health Cases 2023, 1–16.
- Bueno, A.F.; Panizzi, A.R.; Hunt, T.E.; Dourado, P.M.; Pitta, R.M.; Gonçalves, J. Challenges for adoption of integrated pest management (IPM): The soybean example. Neotrop. Entomol. 2021, 50, 5–20.
- Qin, P.; Wang, T.; Luo, Y. A review on plant-based proteins from soybean: Health benefits and soy product development. J. Sci. Food Agric. 2022, 7, 100265.
- Panizzi, A.R.; Corrêa-Ferreira, B.S. Dynamics in the insect fauna adaptation to soybean in the tropics. Trends Entomol. 1997, 1, 71–88.
- Ademokoya, B.; Athey, K.; Ruberson, J. Natural Enemies and Biological Control of Stink Bugs (Hemiptera: Heteroptera) in North America. Insects 2022, 13, 932.
- Panizzi, A.R.; Slansky, F., Jr. Review of phytophagous pentatomids (Hemiptera: Pentatomidae) associated with soybean in the Americas. Fla. Entomol. 1985, 68, 184–203.
- Bueno, A.F.; Bortolotto, O.C.; Pomari-Fernandes, A.; França-Neto, J.B. Assessment of a more conservative stink bug economic threshold for managing stink bugs in Brazilian soybean. Crop Prot. 2015, 71, 132–137.
- Corrêa-Ferreira, B.S.; Azevedo, J. Soybean seed damage by different species of stink bugs. Agric. For. Entomol. 2002, 4, 145–150.
- Jacquet, F.; Jeuffroy, M.H.; Jouan, J.; Le Cadre, E.; Litrico, I.; Malausa, T.; Reboud, X.; Huyghe, C. Pesticide-free agriculture as a new paradigm for research. Agron. Sustain. Dev. 2022, 42, 8.
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106.
- Torres, J.B.; Bueno, A.F. Conservation biological control using selective insecticides: A valuable tool for IPM. Biocontrol 2018, 126, 53–64.
- Kuldna, P.K.; Peterson, H.; Poltimäe, J. An application of DPSIR framework to identify issues of pollinator loss. Ecol. Econ. 2009, 69, 32–42.
- Sosa-Gómez, D.R.; Corrêa-Ferreira, B.S.; Kraemer, B.; Pasini, A.; Husch, P.E.; Delfino Vieira, C.E.; Reis Martinez, C.B.; Negrao Lopes, I.O. Prevalence, damage, management and insecticide resistance of stink bug populations (Hemiptera: Pentatomidae) in commodity crops. Agric. For. Entomol. 2020, 22, 99–118.
- Maciel, R.M.A.; Bueno, A.F. The Role of Integrated Pest Management for Sustainable Food Production: The Soybean Example. In Biodiversity, Functional Ecosystems and Sustainable Food Production; Galanakis, C.M., Ed.; Springer Nature: Cham, Switzerland, 2022; pp. 117–139.
- Lee, R.; den Uyl, R.; Runhaar, H. Assessment of policy instruments for pesticide use reduction in Europe; learning from a systematic literature review. Crop Prot. 2019, 126, 104929.
- van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59.
- Bueno, A.F.; Sosa-Gómez, D.R.; Côrrea-Ferreira, B.S.; Moscardi, F.; Bueno, R.C.O.F. Soja: Manejo Integrado de Insetos e Outros Artrópodes-Praga; Hoffmann-Campo, C.B., Côrrea-Ferreira, B.S., Moscardi, F., Eds.; Embrapa: Brasília, Brazil, 2012; pp. 493–630.
- Borsari, A.C.P.; Vieira, L.C. Mercado e perspectivas dos bioinsumos no Brasil. In Bioinsumos na Cultura da Soja, 1st ed.; Meyer, M.C., Bueno, A.F., Mazaro, S.M.M., Silva, J.C., Eds.; Embrapa: Brasília, Brazil, 2022; pp. 38–52.
- Martin, E.A.; Dainese, M.; Clough, Y.; Báldi, A.; Bommarco, R.; Gagic, V.; Garratt, M.P.D.; Holzschuh, A.; Kleijn, D.; Kovács-Hostyánszki, A.; et al. The interplay of landscape composition and configuration: New pathways to manage functional biodiversity and agroecosystem services across Europe. Ecol. Lett. 2019, 22, 1083–1094.
- Rogers, E.B. Landscape Design: A Cultural and Architectural History; Harry, N., Ed.; Abrams: New York, NY, USA, 2001.
- Patterson, E.L. Agriculture, Landscape Architecture, & Ecological Design: A Foundation for Collaboration between Ecologists and Landscape Architects. Ph.D. Thesis, The University of Georgia, Athens, Georgia, 2004.
- Vargas, G.; Rivera-Pedroza, L.F.; Luis, F.; García, L.F.; Jahnke, S.M. Conservation Biological Control as an important tool in the Neotropical region. Neotrop. Entomol. 2022, 30, 134–151.
- Norris, R.F.; Caswell-Chen, E.P.; Kogan, M. Concepts in Integrated Pest Management; Prentice Hall: Hoboken, NJ, USA, 2003.
- Jonsson, M.; Wratten, S.D.; Landis, D.A.; Gurr, G.M. Recent advances in conservation biological control of arthropods by arthropods. BioControl 2008, 45, 172–175.
- Ehler, L. Conservation Biological Control: Past, Present, and Future; Academic Press: Cambridge, MA, USA, 1998.
- Straub, C.S.; Finke, D.L.; Snyder, W.E. Are the conservation of natural enemy biodiversity and biological control compatible goals? BioControl 2008, 45, 225–237.
- Janssen, A.; van Rijn, P. Pesticides do not signifcantly reduce arthropod pest densities in the presence of natural enemies. Ecol. Lett. 2021, 24, 2010–2024.
- Carnevalli, R.A.; Oliveira, A.B.; Gomes, E.C.; Possamai, E.J.; Silva, G.C.; Reis, E.A.; Roggia, S.; Prando, A.M.; Lima, D. Resultados do Manejo Integrado de Pragas da Soja na Safra 2021/2022 no Paraná; Embrapa Soja: Londrina, Brazil, 2022; Documentos 448; p. 43.
- Tillman, P.G. Diversity of Stink Bug (Hemiptera: Pentatomidae) Egg Parasitoids in Woodland and Crop Habitats in Southwest Georgia, USA. Fla. Entomol. 2016, 99, 286–291.
- Tillman, P.G.; Cottrell, T.E.; Grabarczyk, E.E. Black cherry as a host plant for stink bugs (Hemiptera: Pentatomidae) in agroecosystems in Georgia, USA. Fla. Entomol. 2022, 105, 79–86.
- Ruberson, J.R.; Olson, D.M.; Thompson, M.D.; Ottens, R.J.; Toews, M.D.; Jones, S.; Mills, W.A. Importance of natural enemies for stink bug control. In Cotton Research-Extension Report; University of Georgia: Athens, GA, USA, 2010; pp. 126–135.
- Ruberson, J.R.; Ottens, J.R.; Thompson, M.D.; Shaw, S.R.; Olson, D.M.; Brown, S.; Edwards, P.; Harrison, E.; McGriff, E. Importance of Natural Enemies for Stink Bug Control. In Cotton Research-Extension Report; University of Georgia: Athens, GA, USA, 2011; pp. 92–97.
- Tillman, P.G. Natural Biological Control of Stink Bug (Heteroptera: Pentatomidae) Eggs in Corn, Peanut, and Cotton Farmscapes in Georgia. Environ. Entomol. 2011, 40, 303–314.
- Buschman, L.L.; Whitcomb, W.H. Parasites of Nezara viridula (Hemiptera: Pentatomidae) and other Hemiptera in Florida. Fla Entomol. 1980, 63, 154–162.
- Jones, W.A.; Shepard, B.M.; Sullivan, M.J. Incidence of parasitism of pentatomid (Heteroptera) pests of soybean in South Carolina with a review of studies in other states. J. Agric. Entomol. 1996, 13, 243–263.
- Krombein, K.V.; Hurd, P.D.J.; Smith, D.R.; Burks, B.D. Catalog of the Hymenoptera in America North of Mexico; Volume 1: Symphyta and Apocrita (Parasitica); Smithsonian Institution Press: Washington, DC, USA, 1979.
- Marsaro Júnior, A.L.; Costa, V.A.; Panizzi, A.R. First record of Hexacladia hilaris Burks (Hymenoptera: Encyrtidae) in Brazil and association with Chinavia erythrocnemis (Berg) (Heteroptera: Pentatomidae). EntomoBrasilis 2020, 13, e927.
- Barakat, M.C.; Aquino, D.A.; Cingolani, M.F. Potential of Hexacladia smithii (Hymenoptera: Encyrtidae) to parasitize Piezodorus guildinii (Hemiptera Pentatomidae) adults. Bull. Insectology 2022, 75, 177–182.
- Corrêa-Ferreira, B.S.; Moscardi, F. Seasonal occurrence and host spectrum of egg parasitoids associated with soybean stink bugs. BioControl 1995, 5, 196–202.
- Nunes, M.C. Efeito do parasitismo de Hexacladia smithii Ashmead (Hymenoptera: Encyrtidae) na Capacidade Reprodutiva e no Dano de Euschistus Heros (Fabricius) (Hemiptera: Pentatomidae) Causado a Soja. Master’s Dissertation, Universidade Federal do Paraná, Curitiba, Brazil, 2000.
- Turchen, L.M.; Golin, V.; Favetti, B.M.; Butnariu, A.R.; Costa, V.A. Natural parasitism of Hexacladia smithii Ashmead (Hymenoptera: Encyrtidae) on Euschistus heros (F.) (Hemiptera: Pentatomidae): New record from Mato Grosso State, Brazil. Arq. Inst. Biol. 2015, 82, 1–3.
- Paz-Neto, A.A.; Querino, R.B.; Margaría, C. Egg parasitoids of stink bugs (Hemiptera: Coreidae and Pentatomidae) on soybean and cowpea in Brazil. Fla. Entomol. 2015, 1, 929–932.
- Golin, V.; Loiacono, M.S.; Margaría, C.B.; Aquino, D.A. Natural incidence of egg parasitoids of Edessa meditabunda (F.) (Hemiptera: Pentatomidae) on Crotalaria spectabilis in Campo Novo do Parecis, MT, Brazil. Neotrop. Entomol. 2011, 40, 617–618.
- Carvalho, E.S.M. Dichelops melacanthus (Dallas, 1851) (Heteroptera: Pentatomidae) no Sistema Plantio Direto no sul de Mato Grosso do Sul: Flutuação Populacional, Hospedeiros e Parasitismo. Master’s Dissertation, Universidade Federal da Grande Dourados, Dourados, Brazil, 2007.
- Godoy, K.B.; Galli, J.C.; Ávila, C.J. Parasitismo em ovos de percevejos da soja Euschistus heros (Fabricius) e Piezodorus guildinii (Westwood) (Hemiptera: Pentatomidae) em São Gabriel do Oeste, MS. Cienc. Rural 2005, 35, 455–458.
- Orr, D.B.; Russin, J.S.; Boethel, D.J.; Jones, W.A. Stink Bug (Hemiptera: Pentatomidae) Egg Parasitism in Louisiana Soybeans. Environ. Entomol. 1986, 15, 1250–1254.
- Cingolani, M.F.; Greco, N.M.; Liljesthröm, G.G. Egg parasitism of Piezodorus guildinii and Nezara viridula (Hemiptera: Pentatomidae) in soybean, alfalfa and red clover. Rev. Fac. Cienc. Agrar. Univ. Nac. Cuyo 2014, 46, 15–27.
- Tillman, P.G. Parasitism and predation of stink bug (Heteroptera: Pentatomidae) eggs in Georgia corn fields. Environ. Entomol. 2010, 39, 1184–1194.
- Sousa, K.K.A.; Silva, N.N.P.; Querino, R.B.; Silva, P.H.S.; Grazia, J. Diversity, seasonality, and egg parasitism of hemipteran (Coreidae and Pentatomidae) from a cowpea crop in northeastern Brazil. Fla. Entomol. 2019, 102, 29–35.
- Bueno, A.F.; Parra, J.R.P.; Colombo, F.C.; Colmenarez, Y.C.; Narde, B.V.F.; Pereira, F.F. Manejo de pragas com parasitoides. In Bioinsumos na Cultura da Soja, 1st ed.; Meyer, M.C., Bueno, A.F., Mazaro, S.M.M., Silva, J.C., Eds.; Embrapa: Brasília, Brazil, 2022; pp. 417–434.
- Koppel, A.L.; Herbert, D.A., Jr.; Kuhar, T.P.; Kamminga, K. Survey of stink bug (Hemiptera: Pentatomidae) egg parasitoids in wheat, soybean, and vegetable crops in southeast Virginia. Environ. Entomol. 2009, 38, 375–379.
- Laumann, R.A.; Moraes, M.C.; Silva, J.P.; Vieira, A.M.; Silveira, S.D.; Borges, M. Egg parasitoid wasps as natural enemies of the neotropical stink bug Dichelops melacanthus. Pesqui. Agropecu. Bras. 2010, 45, 442–449.
- Panizzi, A.R.; Lucini, T. What happened to Nezara viridula (L.) in the Americas? Possible reasons to explain populations decline. Neotrop. Entomol. 2016, 45, 619–628.
- Foerster, L.A.; Queiroz, J.D. Incidência natural de parasitismo em ovos de pentatomídeos da soja no centro-sul do Paraná. An. Soc. Entomol. Bras. 1990, 19, 221–232.
- Pacheco, D.J.P.; Corrêa-Ferreira, B.S. Parasitismo de Telenomus podisi Ashmead (Hymenoptera: Scelionidae) em populações de percevejos pragas da soja. An. Soc. Entomol. Bras. 2000, 29, 295–302.
- Shields, M.W.; Johnson, A.C.; Pandey, S.; Cullen, R.; González-Chang, M.; Wratten, S.D.; Gurr, G.M. History, current situation and challenges for conservation biological control. BioControl 2019, 131, 25–35.
- Conte, O.; Oliveira, F.T.; Harger, N.; Corrêa-Ferreira., B.S.; Roggia., S.; Prando, A.M.; Seratto, C.D. Resultados do Manejo Integrado de Pragas da Soja na safra 2015/16 no Paraná; EMBRAPA-CNPSo: Londrina, Brazil, 2016; Documentos 375; p. 59.
- Conte, O.; Oliveira, F.T.; Harger, N.; Corrêa-Ferreira., B.S.; Roggia., S.; Prando, A.M.; Seratto, C.D. Resultados do Manejo Integrado de Pragas da Soja na safra 2016/17 no Paraná; EMBRAPA-CNPSo: Londrina, Brazil, 2017; Documentos 394; p. 70.
- Conte, O.; Oliveira, F.T.; Harger, N.; Corrêa-Ferreira., B.S.; Roggia., S.; Prando, A.M.; Seratto, C.D. Resultados do Manejo Integrado de Pragas da Soja na safra 2017/18 no Paraná; EMBRAPA-CNPSo: Londrina, Brazil, 2018; Documentos 402; p. 66.
- Conte, O.; Oliveira, F.T.; Harger, N.; Corrêa-Ferreira, B.S.; Roggia, S.; Prando, A.M.; Possmai, E.J.; Reis, E.A.; Marx, E.F. Resultados do Manejo Integrado de Pragas da Soja na safra 2018/19 no Paraná; Embrapa Soja: Londrina, Brazil, 2019; Documentos 416; p. 63.
- Conte, O.; Possamai, E.J.; Silva, G.C.; Reis, E.A.; Gomes, E.C.; Corrêa-Ferreira, B.S.; Roggia, S.; Prando, A.M. Resultados do Manejo Integrado de Pragas da Soja na safra 2019/20 no Paraná; Embrapa Soja: Londrina, Brazil, 2020; Documentos 431; p. 66.
- Oliveira, A.B.; Gomes, E.C.; Possamai, E.J.; Silva, G.C.; Reis, E.A.; Roggia, S.; Prando, A.M.; Conte, O. Resultados do Manejo Integrado de Pragas da Soja na Safra 2020/2021 no Paraná; Embrapa Soja: Londrina, Brazil, 2022; Documentos 443; p. 68.
- Silva, G.V.; de Freitas Bueno, A.; Favetti, B.M.; Neves, P.M. Selectivity of chlorantraniliprole and lambda-cyhalothrin to the egg parasitoid Telenomus podisi (Hymenoptera: Platygastridae). Semin. Cien. Agrar. 2018, 39, 549–563.
- Taguti, E.A.; Gonçalves, J.; Bueno, A.F.; Marchioro, S.T. Telenomus podisi parasitism on Dichelops melacanthus and Podisus nigrispinus eggs at different temperatures. Fla. Entomol. 2019, 102, 607–613.
- Kreuss, A.; Tscharntke, T. Habitat fragmentation, species loss, and biological control. Science 1994, 264, 1581–1584.
- Duffy, J.E. Biodiversity and ecosystem function: The consumer connection. Oikos 2002, 99, 201–219.
- Bueno, A.F.; Batistela, M.J.; Bueno, R.C.O.F.; França-Neto, J.B.; Nishikawa, M.; Filho, A.L. Effects of integrated pest management, biological control and prophylactic use of insecticides on the management and sustainability of soybean. Crop Prot. 2011, 30, 937–945.
- Lane, D.E.; Walker, T.J.; Grantham, D.G. IPM adoption and impacts in the United States. J. Integr. Pest Manag. 2023, 14, 1–6.
More
