2. Ecological Importance of Tunicates
The tunicates population plays an important role in the marine food web through filter feeding
[4]. Earlier studies have suggested that phytoplankton productivity in a shallow fjord is controlled by the tunicates population
[28]. Tunicates are known to trap the sinking particulate organic matter and generate mucus rich organic matter and fecal pellets with carbohydrates and minerals
[29][30][29,30], thereby triggering the downward biogeochemical flux (e.g., carbon flux) patterns from surface to deep waters
[29][31][32][29,31,32]. Some obligate photosymbiotic tunicates have been suggested to act as environmental stress indicators
[33]. The unknown ecological functions of a few tunicate MNPs
[34] in understanding their ecological role is yet to be understood.
3. Profile of MNPs from Tunicates and Associated Microbes
Tunicates are known to produce a wide range of MNPs with various bioactive properties. These organisms are considered as a rich source of cellulose, which varies with different species
[35]. Alkaloids and peptides are the major chemical constituents observed in tunicates
[36]. Metabolites originated from tunicate hemocytes are also found to be cytotoxic to foreign particles
[37] and various cell lines
[38]. Microorganisms associated with the invertebrate hosts have also been identified as a source of bioactive metabolites
[39]. In fact, bioactive metabolite-producing invertebrate-associated microorganisms have special implications in solving the “supply problem” in the initial steps of drug discovery
[40]. Recently, Chen et al. reviewed the biological and chemical diversity of ascidian-associated microorganisms
[41].
Microbes associated with tunicates have been found to produce potential metabolites showing antimicrobial and anticancer activities (
Figure 1,
Figure 2 and
Figure 3). Tunicate-associated bacteria such as
Bacillus,
Pantoea,
Pseudoalteromonas,
Salinicola,
Streptomyces,
Vibrio and
Virgibacillus have recently been identified with potential antimicrobial activities
[16]. The introduced tunicate species are also reported to harbor diverse host-specific microbial populations
[42][49] that produce species-specific metabolites
[43][50]. In general, tunicate associated bacteria and fungi are known to produce a variety of MNPs with various biological properties
[41]. The chemistry of yellow pigment-producing parasitic bacteria in the interstitial and blood-filled spaces of planktonic tunicates,
Oikopleura vanhoeffeni and
Oikopleura dioica, are yet to be characterized
[44][51].
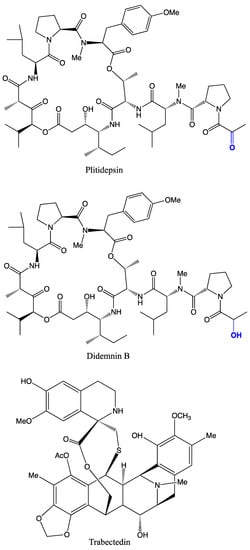
Figure 1.
Important anticancer drugs of tunicates and their associated microbes in clinical trials.
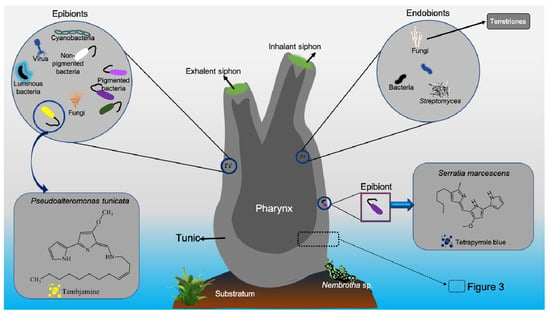
Figure 2.
Tunicate-associated epibiotic and endobiotic symbionts. (the small inserted empty box provides more details in
Figure 3
).
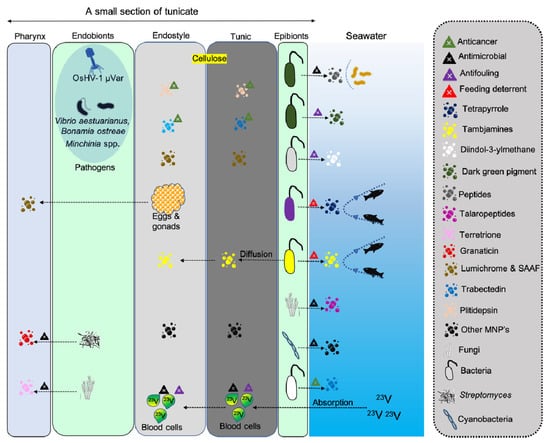
Figure 3.
Illustration depicting various MNPs released from endobiotic and epibiotic microbes associated with tunicate’s endostyle and tunic.
4. Antimicrobial Applications
Tunicates
[45][123], with their associated epi-symbionts
[16][46][16,124] and endosymbionts
[47][125], are prolific producers of antimicrobial and antifungal compounds inhibiting pathogens. The brominated alkaloids
[48][126] and other compounds from tunicates have been reported to possess several biological activities
[25][26][25,26].
Pseudoalteromonas tunicata produces alkaloid tambjamine (425 nm), an antifungal yellow pigment
[49][50][127,128], and violacein (575 nm), a purple pigment with antiprotozoal activity
[51][52][129,130], in addition to a range of bioactive compounds
[51][53][129,131]. Methanol extraction of
Lissoclinum fragile displayed antibacterial, antifungal, hemolytic, and cytotoxic activities
[54][92]. The kuanoniamine A metabolite produced by
Eusynstyela tincta inhibited pathogenic bacteria such as
B. subtilis, E. coli, S. aureus, V. cholerae, and
V. parahaemolyticus and fungi
A. fumigatus and
C. albicans [55][88]. A diffusible 190-kDa protein produced by tunicate
Ciona intestinalis associated bacterium
Pseudoalteromonas tunicata was found to show antibacterial activity against marine isolates
[56][132]. The four α-helical peptides “clavanins A, B, C, and D” isolated from the hemocytes of tunicate
Styela clava showed antibacterial activity against pathogenic
Listeria monocytogenes strain EGD and antifungal activity against
Candida albicans [57][44]. Halocidin, an antimicrobial peptide purified from tunicate
Halocynthia aurantium showed antibacterial activity against methicillin-resistant
Staphylococcus aureus and multidrug-resistant
Pseudomonas aeruginosa [58][47]. Similarly, halocyntin and papillosin peptides isolated from tunicate
Halocynthia papillosa also displayed antibacterial activity against both Gram-positive and Gram-negative marine bacteria
[59][46]. Halocyamine peptides synthesized by the hemocytes of
Halocynthia roretzi showed antimicrobial activity against various bacteria and yeasts
[60][90]. Similarly, Halocyamines produced by
Styela clava also displayed antimicrobial properties
[61][108]. A salt-tolerant peptide isolated from hemocytes of
Ciona intestinalis showed both antibacterial and antifungal activity
[62][133]. Vanadium chloride and vanadyl sulfate also displayed antibacterial activity against various pathogens
[63][95].
An endobiont,
Streptomyces sp., isolated from the tunicate,
Styela canopus, produced antibacterial compounds such as granaticin, granatomycin D, and dihydrogranaticin B
[64][121]. Similarly endosymbiotic fungi associated with the tunicates,
Polycarpa aurata [65][134] and
Rhopalaea crassa [66][135], showed antimicrobial activity. The fungi
Talaromyces sp., isolated from an unidentified tunicate, produced talaropeptides A and B, two antibacterial metabolites that inhibited Gram-positive bacteria,
Bacillus subtilis [24]. The endophytic fungus
Penicillium sp. isolated from
Didemnum sp. produced antifungal and cytotoxic compounds, terretrione C and D
[67][136].
Some tunicates produced antiviral molecules, indicating their chemical defense function against environmental viruses. The Caribbean tunicate,
Trididemnum sp., was found to produce depsipeptides, particularly didemnin A and B, exhibiting antiviral activity against DNA and RNA viruses in vitro
[68][69][111,137]. Another species of Caribbean tunicate,
Eudistoma olivaceum, produced prolific MNPs, such as eudistomins A, D, G, H, I, J, M, N, O, P, and Q, which possessed antiviral activity
[70][83]. The ascidian
Didemnum guttatum was found to produce the cyclodidemniserinol trisulfate compound that showed anti-retroviral activity by inhibiting HIV-1 integrase
[71][72]. The tunicate,
Didemnum molle, released lanthipeptide divamide A that promised to be a potential anti-HIV drug
[72][74].
5. Anticancer and Antitumor Applications
Trabectedin (Ecteinascidin; ET-743; Yondelis
®), an alkaloid extracted from the orange tunicate,
Ecteinascidia turbinata, is approved as a first anticancer drug
[73][138] to treat breast cancer
[74][75][139,140], soft tissue sarcoma
[76][141], and ovarian cancer
[77][78][79][142,143,144]. This molecule is suggested to originate from
E. turbinata symbiotic bacteria,
Candidatus Endoecteinascidia frumentensis [80][145]. However, plitidepsin (Aplidin
®), a depsipeptide isolated from the Mediterranean tunicate,
Aplidium albicans, is in phase II clinical trials
[73][81][138,146] as an anticancer drug against breast cancer
[82][147], human kidney carcinoma cells
[83][52], and multiple myeloma
[84][53]. Didemnin B is also in phase II trials
[85][148], showing anticancer activity against leukaemia P388 cells
[68][111]. Significantly, 60% of the human cervical carcinoma cell lines (HeLa) were inhibited by Eudistomins H extracts (IC
50 0.49 μg/mL) obtained from
E. viride [86]. Clavepictine A and B alkaloids originated from
Clavelina picta demonstrated potential cytotoxic activity (IC
50 12 μg/mL) against murine leukemia and human solid tumor cell lines
[87][62]. Lamellarin sulfates originated from
Didemnum ternerratum [88][78] and polycarpine dihydrochloride, a disulfide alkaloid extracted from an ascidian
Polycarpa clavata, were found to inhibit human colon tumor cell lines
[89][97].
Cystodytins A, B, and C, three teracyclic alkaloids isolated from Okinawa tunicate
Cystodytes dellechiajei, were reported to show antitumor activities
[90][64]. Macrolides isolated from tunicates
Lissoclinum patella (Patellazole C)
[91][94] and
Eudistoma cf.
rigida (Lejimalides A, B, C, and D)
[92][93][149,150] possessed anticancer activity
[94][151]. Diplamine, an orange pigment alkaloid produced by
Diplosoma sp., demonstrated cytotoxic activity against leukemia cells
[95][79]. Halocyamine A and B peptides extracted from
H. roretzi showed anticancer activity against various cell lines
[60][90]. A depsipeptide, dehydrodidemnin B, produced by
Aplidium albicans inhibited Ehrlich carcinoma cells in mice and reduced 80–90% tumor cells
[96][54]. Bryostatins Ecteinascidins products, such as ET-729, 743, 745, 759 A, 759B, and 770, extracted from the Caribbean tunicate
Ecteinascidia turbinata showed immunomodulator activity and antitumor activity against various leukemia cells
[97][152] and breast, lung, ovary, and melanoma cells
[98][153]. The Brazilian ascidian,
Didemnum granulatum, produced G2 checkpoint-inhibiting aromatic alkaloids, granulatimide and isogranulatimide
[99][154]. The ascidian
Cystodytes dellechiajei produced topoisomerase II-inhibiting ascididemin, which has antitumor activity against various tumor cell lines
[100][66]. This marine alkaloid exhibits marked cytotoxic activities against a range of tumor cells. The kuanoniamine A metabolite extracted from
E. tincta displayed 100% inhibition of Dalton’s lymphoma and Ehrlich ascites tumor cell lines
[55][88]. Cynthichlorine, an alkaloid isolated from the tunicate
Cynthia savignyi, showed cytotoxicity against
Artemia salina larva at an LD
50 of 48.5 μg/mL
[101][63]. Siladenoserinols A and B derivatives isolated from didemnid tunicates possessed antitumor activity by inhibiting the interaction of p53-Hdm2
[102][69].
6. Antifouling and Anti-Deterrent Activities
The colonial tunicate,
Eudistoma olivaceum, was found to produce brominated alkaloids, Eudistomins G and H, which acted as antifouling substances and fish antifeedants; thus, the
E. olivaceum surface was completely free from fouling epibionts
[34]. A dark green pigmented bacteria,
Pseudoalteromonas tunicata, isolated from the surface of
Ciona intestinalis, collected originally from off the west coast of Sweden, showed antifouling activity against algal spores, invertebrate larvae, and diatoms
[53][103][104][131,155,156]. The yellow pigmented
Pseudoalteromonas tunicata mutants have demonstrated antifouling activity against algal spore germination, bacterial growth, fungal growth, and invertebrate larvae
[51][129]. Diindol-3-ylmethane products isolated from an unidentified ascidian-associated bacteria,
Pseudovibrio denitrificans, displayed nearly 50% antifouling activity against barnacle
Balanus amphitrite and bryozoan
Bugula neritina [105][118].
Deterring activity of vanadium acidic solutions, such as vanadyl sulfate and sodium vanadate, was observed against
Thalassoma bifasciatum when incorporated into food pellets
[63][106][95,157]. Didemnimides C and D from
Didemnum conchyliatum [107][158], nordidemnin B
[108][102] and didemnin B
[109][159] from
Trididemnum solidum, and granulatamides from
Didemnum granulatum [110][73] displayed antifeedant effects on various fishes in laboratory experiments. The kuanoniamine A molecule from
E. tincta displayed feeding-deterrent activities against carnivore gold fish,
Carassius auratus [55][88]. MNPs isolated from Antarctic tunicates have demonstrated variability in anti-deterrent activities
[111][58]. Both the yellow pigmented tambjamine metabolites and blue tetrapyrrole metabolite released from
Sigillina sp. (i.e.,
Atapozoa sp.) showed feeding-deterrent activity against various carnivore fishes
[112][113][59,160]. The blue tetrapyrrole pigment was suggested to originate from the associated bacteria
Serratia marcescens [114][120]. Tambjamines and tetrapyrrole chemical constituents from both adult and larvae were reported to function as defensive chemicals against predators
[108][102]. Lipophilic crude extracts from Antarctic tunicate,
Distaplia cylindrica [115][161], and polyandrocarpidines from
Polyandrocarpa sp.
[108][116][101,102] demonstrated deterrent activity against certain sea-stars, hermit crabs, and snails.
7. Miscellaneous Applications
The chiton
Mopalia sp. spawned when injected with 1.0 mg/L of gonadotropin releasing hormone (GnRH2) of a tunicate
[117][48]. Lumichrome, a compound extracted from tunic, gonads, and eggs of ascidian,
Halocynthia roretzi, was involved in the larval metamorphosis
[118][89]. Similarly, sperm-activating and attracting factors (SAAF) were isolated from eggs of the ascidians
Ciona intestinalis and
Ascidia sydneiensis [119][162]. Lipids extracted from
H. roretzi have demonstrated the antidiabetic and anti-obese properties in mice models
[120][163]. Two novel alkaloids, mellpaladine and dopargimine, isolated from Palauan tunicate have demonstrated neuroactive behavior in mice
[121][68]. Two new alkaloids, polyaurines A and B, isolated from the tunicate,
Polycarpa aurata, inhibited blood-dwelling
Schistosoma mansoni [122][96]. Lepadin and villatamine alakaloids isolated from
Clavelina lepadiformis [123][61] and lepadins from
Didemnum sp.
[124][71] displayed potential antiparasitic and cytotoxic activities. The ascidian species,
Didemnum psammathodes, collected from the central west coast of India was extracted in organic solvents. These extracts showed antimicrobial and antifouling properties
[125][164].