Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Francisco Jose Alguacil and Version 2 by Camila Xu.
Ionic liquids (ILs), or room-temperature ionic liquids (RTILs), are a class of chemicals consisting of ions that maintain a liquid state below 100 °C. ILs serve as the basis of deep eutectic solvents (DESs), which formed a new class of chemicals characterized as being formed by mixtures of components of a eutectic, with the resulting product presenting a melting point lower than the values presented by the pure components. The presence of these rare earths in different wastes varies for each element, and it seems to be difficult to establish a fixed concentration for each element.
- rare-earth elements
- ionic liquids
- deep eutectic solvents
- solvometallurgy
1. Introduction
Besides their use as raw materials, metals can also be recovered from different secondary resources. Among these, the processing of industrial wastes, which contain several times the quantities of valuable metals, is of special interest. These industrial wastes include (i) metallurgical wastes (phosphogypsum and red mud), (ii) fly ash, (iii) mining wastes (mine tailings and acid mine drainage byproducts), and (iv) electronic wastes (magnets, NiMH batteries, lithium-ion batteries, and phosphors) [1]. Among these metals are rare-earth elements (REEs), a category that includes 15 lanthanides (LNs), yttrium, and scandium [2][3][2,3]. Moreover, rare-earth elements are commonly divided into two families: (i) light rare earths, which comprise lanthanum, cerium, praseodymium, neodymium, promethium, and samarium, and (ii) heavy rare earths, with europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium being components of this subcategory.
The presence of these rare earths in different wastes varies for each element, and it seems to be difficult to establish a fixed concentration for each element [1][4][1,4]. As a first approach, Table 1 shows REES concentrations in a series of industrial wastes; this variety of composition means that these wastes can be treated via different processing routes, and these routes result in different processing costs, yields, and economic benefits. As shown in Table 1, a common feature of these various wastes is that light rare earths are always present in greater concentrations than those of heavy rare earths.
Table 1.
Presence of REEs in various wastes.
| Waste | La | Nd | Ce | Pr | Tb | Lu | Ho | Yb | Eu | Gd |
|---|---|---|---|---|---|---|---|---|---|---|
| phosphogypsum | 1450 | 899 | 74 | 0.6 | ||||||
| red mud | 99 | 368 | 2.4 | 4.3 | ||||||
| fly ash | 114 | 99 | 1 | 14 | ||||||
| mine tailing | 903 | 906 | 4 | |||||||
| a AMD | 0.01–0.09 | 0.006–0.03 | 16 | 0.0002–0.002 | 0.002–0.03 | |||||
| NdFeBmagnets | 260 | |||||||||
| NiMH batteries | 237 | 36 | 67 | |||||||
| phosphors | 36 | 49 | 2.7 | 2.5 |
Numbers in mg/kg. a Acid mine drainage. Data from [1].
Due to their specific atomic structures, this group of elements presents unique optical, thermal, electrical, and magnetic properties; thus, they are widely used in various fields, including traditional industries, such as glass, agriculture, ceramics, chemicals, etc., as well as high-tech industries such as the energy sector, the automotive sector, healthcare, the nuclear industry, communications, and the military [5][6][7][8][9][5,6,7,8,9].
Against the above, these increasing exploitations and applications have increased the presence of REEs in the environment (in the atmosphere, water, and soil), boosting the potential risk of contamination for humans and other organisms [10].
In view of the relevance of the recovery of these REES for resource conservation, and as a legitimate alternative to the traditional recovery (pyro- or hydrometallurgical) processes, there is an increasing interest in the use of smart recovery processes, with the same efficiency and more environmentally friendly characteristics, and here the concept of solvometallurgy arises.
If the difference between pyrometallurgy and hydrometallurgy lies in the use of high temperatures (pyrometallurgy) versus the use of moderate temperatures, pressure, and aqueous systems (in the case of hydrometallurgical processing), the difference between hydrometallurgy and solvometallurgy is that the latter uses non-aqueous solvents. Most of the unit processes (leaching, solvent extraction, ion exchange, precipitation, and electrolysis) in solvometallurgy are similar to those used in hydrometallurgy, with the main difference, as said above, being that water is replaced by a non-aqueous solvent [11][12][11,12].
2. Ionic Liquids
Ionic liquids (ILs), or room-temperature ionic liquids (RTILs), are a class of chemicals consisting of ions that maintain a liquid state below 100 °C. They are frequently composed of an organic cation (i.e., tetraethyl ammonium, dialkylimidazolium, 1-ethyl-3-methylimidazolium, and phosphonium-based) and an organic or inorganic anion (i.e., chloride, nitrate, bisulfate, chlorate, and thiocyanate). Moreover, ILs have relatively high viscosity and density [13]. The bulky characteristics of the organic moiety of ILs are responsible for these chemicals’ amply liquidous range and thus low volatility. The properties presented by ILs include thermal and radioactive stability, non-volatility, non-flammability, adjustable miscibility in organic diluents, and polarity. Also, these properties can be modified to match a given necessity by changing to the appropriate cation and/or anion to form the more specialized Task-Specific Ionic Liquids (TSILs) family of chemicals [14]. The different applications of ILs and TSILs have enhanced the development of different extraction processes: simplifying analytical methodologies, the removal of environmental contaminants, breaking of azeotropes [15], and purification of fuels [16]. Technologies including liquid–liquid extraction (LLE), solid-phase extraction (SPE), pressurized liquid extraction (PLE), and liquid-phase microextraction (LPME) use these chemicals in a very efficient manner. Properly used, these ILs can avoid the use of chelating agents in the selective extraction of ions [17]. Also, they can bring about mass transfer in novel miniaturized homogeneous LPME models [18]. Other uses of this family of compounds include UV-V spectrophotometric determination of mercury ions from water samples [19], the use of silica as support of ILs phases, to act as SPE adsorbents used in the removal of organic acids, amines, and aldehydes from atmospheric aerosol samples [20], and to remove carbon dioxide from gaseous streams [21]. IL chemicals have been labeled as green compounds due to their properties, though there are also some claims against this green label because there are several concerns about air, water, and terrestrial pollution. Some of these harmful properties, on living organisms, are connected with undesirable effects on cellular walls [22], though this harmfulness varies from one organism to another. Several uses of ILs in the recovery of metals have been recently published [23][24][25][26][27][28][23,24,25,26,27,28].ILs and REEs
Several reviews [29][30][29,30] deal with the application of ILs in the recovery of these strategic elements. These reviews focused on the use of ionic liquids in the recovery of secondary resources such as e-wastes and nickel-metal hydride batteries (NiMHBs). In the case of e-wastes [29], the review included the use of IL extraction, selectivity, and reusability, including several types of TSILs, and the use of diluents in the organic phase. The content of REEs (about 10% wt) in nickel-metal hydride spent batteries are usually dumped, though several approaches to recover La, Ce, Nd, and Pr, from these discarded resources are also into consideration [30]. Since REEs and some of their derivatives have several uses in smart technologies, praseodymium oxide nanoparticles (Pr6O11 nps) are formed by the use as a templating agent of an IL (BMIM-PF6) and an alcohol [31]. The IL inhibited particle growth, whereas ethylene glycol is used as a diluent of the organic phase. The as-synthesized nanoparticles presented anti-cancerogenous properties and antibacterial activity against Gram-negative bacteria K. pneumoniae and Gram-positive bacteria S. aureus. The separation of some REEs with 1,2-hydroxypyridinone grafted ionic liquid (HOPO-IL) has been investigated [32]. Moreover, the performance of this extractant for lanthanide separation in the presence of various ionic liquids (ILs) and organic diluents has also been investigated, revealing better extraction performance in the ILs instead of using 1-octanol. Lanthanide extraction with HOPO-IL was pH-dependent, the metals being extracted by a cation-exchange-based reaction, in which lanthanide elements, in the form of cations, are exchanged with [Cnmim+] from the ionic liquid. With respect to the ILs’ composition, the addition of [NTf2]− had a minor influence on metal loading onto the organic phase, whereas the presence of [Cnmim]+ had an increasing suppressing effect when n increased from 4 to 10 on lanthanide extraction, which supports the idea that the cation exchange mechanism is dominant in this extraction system. The dependence of the extraction of these REEs on the aqueous pH value suggested that the stripping step can be performed by solutions of acidic pH values. A method for recycling permanent magnet waste via betaine hydrochloride ([Hbet]Cl) solution extraction was presented [33]. The next optimum leaching conditions were obtained as a leaching temperature of 200 °C, reaction time of 8 h, [Hbet]Cl concentration of 0.2 mol/L, and solid–liquid ratio of 1:150 (g/mL). The abovementioned experimental values allowed reaching leaching rates of 99.8% Pr, 97.1% Nd, 95.5% Gd, 56.2% Ce, and less than 0.3% in the case of iron, and the residue of the leach operation contained iron oxide. When mineral acids HCl, H2SO4, or HNO3 are used to dissolve the magnet, the present procedure allows for an improvement in the leaching percentage and the selective separation of these elements, the dissolution sequence being in accordance with the properties of these metals. REEs are leached via the next reaction: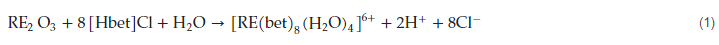 The separation of the various REEs from the leaching solution was not described in the published manuscript.
A method for recycling a real scrap NdFeNi magnet from computer hard disks in order to recover Nd(III) as a marketable salt and other valuable by-products was described [34]. Solvent extraction of Nd(III) and Ni(II) used the synthesized bi-functional ionic liquid (Bif–IL) [AL336][Cy572] in kerosene, based on Aliquat 336 (quaternary ammonium salt) and Cyanex 572 (phosphonic acid). When compared to Cyanex 572 alone, Bif–IL improved not only the extraction percentage of these metals but also the separation factors between Nd(III) and Ni(II), with the highest Nd/Ni separation factor of 26.3 obtained with 0.2 M HCl medium. It was determined that the extracted species were NdCl3(R4NCy)3 and NiCl2(R4NCy)2. The Nd-loaded organic phase was stripped with 0.5 M HCl solution, and from the stripped solution, Nd(III) was precipitated with oxalic acid; furthermore, the resulting solid was calcined to yield Nd2O3.
As part of the downstream technology development efforts in the treatment of acid mine drainage (AMD), several ionic liquids were synthesized and compared in the extraction of REEs, including Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Th, and U [35]. In the investigation, the extractants and their ionic liquids shown in Table 2 were used.
The separation of the various REEs from the leaching solution was not described in the published manuscript.
A method for recycling a real scrap NdFeNi magnet from computer hard disks in order to recover Nd(III) as a marketable salt and other valuable by-products was described [34]. Solvent extraction of Nd(III) and Ni(II) used the synthesized bi-functional ionic liquid (Bif–IL) [AL336][Cy572] in kerosene, based on Aliquat 336 (quaternary ammonium salt) and Cyanex 572 (phosphonic acid). When compared to Cyanex 572 alone, Bif–IL improved not only the extraction percentage of these metals but also the separation factors between Nd(III) and Ni(II), with the highest Nd/Ni separation factor of 26.3 obtained with 0.2 M HCl medium. It was determined that the extracted species were NdCl3(R4NCy)3 and NiCl2(R4NCy)2. The Nd-loaded organic phase was stripped with 0.5 M HCl solution, and from the stripped solution, Nd(III) was precipitated with oxalic acid; furthermore, the resulting solid was calcined to yield Nd2O3.
As part of the downstream technology development efforts in the treatment of acid mine drainage (AMD), several ionic liquids were synthesized and compared in the extraction of REEs, including Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Th, and U [35]. In the investigation, the extractants and their ionic liquids shown in Table 2 were used.
Table 2.
Extractants and ionic liquids used in the extraction of REEs from AMD.
| Extractant | Acronym |
|---|---|
| di-2-ethylhexyl phosphoric acid | D2EHPA |
| tri(hexyltetradecyl phosphonium chloride | C101 |
| 2-ethylhexyl phosphoric acid mono-2-ethylhexyl ester | EHEHPA |
| Mixture of phosphonic acid and phosphinic acids | C572 |
| Derived Ionic liquids | |
| trihexyltetradecylphosphonium and di-2-ethylhexyl phosphate | [C101]+[D2EHP]− |
| trihexyltetradecylphosphonium and 2-ethylhexyl phosphate mono-2-ethylhexyl ester | [C101]+[HEHP]− |
| trihexyltretadecylphosphonium and mixture of phosphate and phosphinate | [C101]+[C572]− |
From [35].
Kerosene was used to dissolve the above-shown reactants in order to reduce their viscosity, and extractions were carried out with solutions of pH 4.4. The results indicated that the ionic liquids [C101]+[D2EHP]− and [C101]+[EHEHP]− were not better extractants for REEs than the single reactants, whereas [C101]+[C572]− compares well with data obtained with single D2EHPA. The presence of zinc and calcium in the solution decreased the extraction of these REEs; thus, there was a necessity to minimize the presence of these two elements in the circuit. No data were given about experimental variables influencing REE extraction or about the stripping stage. In this manuscript, the composition of Cyanex 572 differed from that given in reference [34].
In the next reference [36], Sm and Co electrodeposition and their co-deposition in the [BMP][DCA] (BMP = 1-butyl-1-methylpyrrolidinium, DCA = dicyanamide) ionic liquid with controlled water content via electrochemical methods was investigated. It was shown that both metals can be deposited electrochemically from the corresponding single-component solutions and their deposition potential shifted positively when the water concentration was increased, indicating deposition acceleration. From binary solutions, Sm–Co co-deposition was also observed. In these binary solutions, Sm was co-deposited at much less negative potentials than did the element from a single solution. An increase in the water concentration resulted in inhibition of the process of Sm/Co co-deposition. The addition of water promoted Co and Sm oxidation with the formation of oxides/hydroxides occurring in parallel with electrochemical deposition; the formation of these compounds resulted in fouled Sm/Co co-deposition.
Neodymium was extracted, from aqueous solutions, by trioctylphosphine oxide (TOPO) dissolved in an ionic liquid [1-Butyl-3-methylimidazolium] [Bis (trifluoromethanesulfonyl)imide] ([C4mim][Tf2N]) in small channel contactors [37]. A 1:6 Nd:TOPO stoichiometry was found at high initial Nd concentrations of 0.005 and 0.01 M in a 0.001 M nitric acid medium. The continuous flow extractions were carried out in channels with 0.5 and 1 mm diameter, and at equal phase mixture velocities (0.01 and 0.05 m/s), the flow pattern studies highlighted a plug flow regime, resulting in interfacial areas of up to 4900 and 2500 m2/m3 for 0.5 mm and 1 mm channels, respectively. No stripping data were given in the published manuscript.
The solvent extraction of Nd (III), Sm (III), and Eu (III) by using the bifunctional ionic liquid tri-n-octyl amine-di-2-ethylhexyl phosphate ([TOA-D2]) as an extractant was investigated [38]. The extraction was performed in the presence of a complexing agent such as EDTA. The extraction of the three elements increased with the increase of the pH of the solution from 1 to 2 and tended to stabilize at pH values of 2–3 (Table 3).
Table 3.
Percentages of extraction of REEs at various pH values.
| REE | pH 1 | pH 2 | pH 3 |
|---|---|---|---|
| Nd(III) | 33 | 69 | 69 |
| Sm(III) | 31 | 69 | 60 |
| Eu(III) | 24 | 51 | 55 |
From [38].
In the three above-shown cases, the extraction process was exothermic; thus, the extraction efficiency decreased when the temperature was increased from 25 to 55 °C. The equilibrium data modeling also confirmed the formation of solvated species with one extractant moiety. Stripping of the three elements increased with the increase in the acid concentration (HCl or HNO3) from 0.02 to 0.8 M.
Being that neodymium has similar physicochemical properties to lanthanum, cerium, and praseodymium, it was difficult to achieve the separation (and purification) of these elements; thus, various phosphate-based ionic liquids, including N,N-dimethyloctylamine bis(2-ethylhexyl)phosphate ([N1,1,8,H][DEHP]), N,N-dimethyldecylamine bis(2-ethylhexyl)phosphate ([N1,1,10,H][DEHP]), and N,N-dimethyldodecylamine bis(2-ethylhexyl)phosphate ([N1,1,12,H][DEHP]), were synthesized and investigated for selective separation of Nd(III) from aqueous solution [39]. Under the various experimental conditions, it was found that the extraction efficiency of Nd(III) using [N1,1,8,H][DEHP] was near 100% at a pH of 4. At this pH value, the separation factor (β) values of βNd/La, βNd/Ce, and βNd/Pr using this ionic liquid were 13.8, 6.9, and 3.4, respectively. Neodymium can be stripped from loaded organic phases by the use of HCl solutions, and the stripping efficiency increases from neutral solution to 0.16 M HCl medium.
Two ionic liquids [A336][BTA] (BTA = dibutyl thiodiglycolamate) and [A336][OTA] (OTA= dioctyl thiodiglycolamate), both presenting low viscosities, were formed to extract Nd(III), in a selective form, using waste NdFeB magnets [40]. Better results were obtained with [A336][OTA], though in both cases the extraction increased with the increase in the pH value (1 to 3) and then (3–6) remained constant. Also, the increase in the ionic strength (NaCl addition) from 0 to 0.3 M increased the extraction efficiency. Using both ionic liquids, the extracted species responded to the IL2NdCl3 stoichiometry. Stripping was performed via precipitation with potassium oxalate. This work used kinetic and extraction isotherm models not suitable for liquid–liquid extraction science but for ion exchange and adsorption processing. Thus, this manuscript must not be published with these data.
The next investigation focused on the study of the extraction of neodymium from an aqueous nitrate feed using a bifunctional ionic liquid formed by trihexylamine di-2-ethyl hexyl phosphate ([TAHAH]+[DEHP]−) in kerosene [41]. Several experimental variables including shaking time, salt concentration, aqueous phase pH, diluting agents, metal concentration, and temperature were investigated in order to determine their influence on neodymium extraction. The results indicated that the extraction percentage increased when salt and extractant concentrations increased. The percentage of metal extraction is dependent on the pH value, increasing when the pH shifted from 1 to 3 and reaching 99% at 2 M NaNO3 and pH 3 using a 0.1 M solution of the ionic liquid in the organic diluent. Neodymium extraction responded to the next equilibrium:
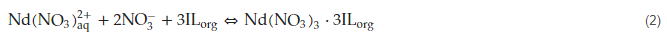 This extraction process showed a negative value of ΔH° (−15 kJ/mol), indicative of heat evolution within the extraction process. The negative value (−11 kJ/mol·K) of ΔS° seemed to be indicative of a decrease in randomness as the metal was extracted into the organic phase. Metal-loaded organic phases can be stripped via the use of very diluted sulphuric acid solutions (2 × 10−3–8 × 10−3 M). There was a continuous decrease in the extraction efficiency under several extraction–stripping cycles.
Yttrium(III) was extracted from nitrate medium by the use of the same bifunctional ionic liquid ([THAH]+[DEHP]−) [42]. The extraction percentage of Y(III) decreased from 97% to 73% as the equilibration time increased, this being attributable to a dissociation of the extracted complex. Again, extraction increased from pH 1 to 3, and the extracted complex presented the Y(NO3)3·2IL stoichiometry. The variation in temperature revealed the endothermic nature of the extraction process. Using 0.1 M nitric acid solution, yttrium was completely stripped from metal-loaded organic phases; however, the increase in the acid concentration up to 0.8 M decreased the stripping efficiency. Similarly to the previous reference, the extraction efficiency decreased after continuous (up to five) extraction–stripping cycles.
Benzyltributylammonium decanedioate ([N444Bn]2[SA]) ionic liquid was used to extract rare earths and enable the separation of Sm(III) contained in waste SmCo magnets. This investigation was carried out in an acetate medium [43]. The experimental results showed that both La(III) and Nd(III) were extracted better in the acetate medium, with an apparent extractability order of acetate > nitrate > chloride, these results being attributable to the low-hydrated nature of acetate ions. REEs were extracted by an ion association mechanism, with stoichiometries dependent on the aqueous medium (Table 4). The stripping results showed that 91% Nd(III) was recovered from organic phases using 1.5 M HOAc. In the case of the magnets and using a simulated synthetic solution, samarium(III) extraction increased from nearly 4% at pH 4 to 98% at pH 5.5, and copper(II) extraction of 87% at this same 5.5 pH value, against cobalt(II), was poorly extracted (14% at pH 5.5). The maximum separation factor Sm(III)/Co(II) reached values of 3078; however, this separation factor decreased to 148 in the treatment of a real solution coming back from the processing of magnets. No stripping data were given in the manuscript in the case of Sm/Co/Cu solutions.
This extraction process showed a negative value of ΔH° (−15 kJ/mol), indicative of heat evolution within the extraction process. The negative value (−11 kJ/mol·K) of ΔS° seemed to be indicative of a decrease in randomness as the metal was extracted into the organic phase. Metal-loaded organic phases can be stripped via the use of very diluted sulphuric acid solutions (2 × 10−3–8 × 10−3 M). There was a continuous decrease in the extraction efficiency under several extraction–stripping cycles.
Yttrium(III) was extracted from nitrate medium by the use of the same bifunctional ionic liquid ([THAH]+[DEHP]−) [42]. The extraction percentage of Y(III) decreased from 97% to 73% as the equilibration time increased, this being attributable to a dissociation of the extracted complex. Again, extraction increased from pH 1 to 3, and the extracted complex presented the Y(NO3)3·2IL stoichiometry. The variation in temperature revealed the endothermic nature of the extraction process. Using 0.1 M nitric acid solution, yttrium was completely stripped from metal-loaded organic phases; however, the increase in the acid concentration up to 0.8 M decreased the stripping efficiency. Similarly to the previous reference, the extraction efficiency decreased after continuous (up to five) extraction–stripping cycles.
Benzyltributylammonium decanedioate ([N444Bn]2[SA]) ionic liquid was used to extract rare earths and enable the separation of Sm(III) contained in waste SmCo magnets. This investigation was carried out in an acetate medium [43]. The experimental results showed that both La(III) and Nd(III) were extracted better in the acetate medium, with an apparent extractability order of acetate > nitrate > chloride, these results being attributable to the low-hydrated nature of acetate ions. REEs were extracted by an ion association mechanism, with stoichiometries dependent on the aqueous medium (Table 4). The stripping results showed that 91% Nd(III) was recovered from organic phases using 1.5 M HOAc. In the case of the magnets and using a simulated synthetic solution, samarium(III) extraction increased from nearly 4% at pH 4 to 98% at pH 5.5, and copper(II) extraction of 87% at this same 5.5 pH value, against cobalt(II), was poorly extracted (14% at pH 5.5). The maximum separation factor Sm(III)/Co(II) reached values of 3078; however, this separation factor decreased to 148 in the treatment of a real solution coming back from the processing of magnets. No stripping data were given in the manuscript in the case of Sm/Co/Cu solutions.
 This extraction process showed a negative value of ΔH° (−15 kJ/mol), indicative of heat evolution within the extraction process. The negative value (−11 kJ/mol·K) of ΔS° seemed to be indicative of a decrease in randomness as the metal was extracted into the organic phase. Metal-loaded organic phases can be stripped via the use of very diluted sulphuric acid solutions (2 × 10−3–8 × 10−3 M). There was a continuous decrease in the extraction efficiency under several extraction–stripping cycles.
Yttrium(III) was extracted from nitrate medium by the use of the same bifunctional ionic liquid ([THAH]+[DEHP]−) [42]. The extraction percentage of Y(III) decreased from 97% to 73% as the equilibration time increased, this being attributable to a dissociation of the extracted complex. Again, extraction increased from pH 1 to 3, and the extracted complex presented the Y(NO3)3·2IL stoichiometry. The variation in temperature revealed the endothermic nature of the extraction process. Using 0.1 M nitric acid solution, yttrium was completely stripped from metal-loaded organic phases; however, the increase in the acid concentration up to 0.8 M decreased the stripping efficiency. Similarly to the previous reference, the extraction efficiency decreased after continuous (up to five) extraction–stripping cycles.
Benzyltributylammonium decanedioate ([N444Bn]2[SA]) ionic liquid was used to extract rare earths and enable the separation of Sm(III) contained in waste SmCo magnets. This investigation was carried out in an acetate medium [43]. The experimental results showed that both La(III) and Nd(III) were extracted better in the acetate medium, with an apparent extractability order of acetate > nitrate > chloride, these results being attributable to the low-hydrated nature of acetate ions. REEs were extracted by an ion association mechanism, with stoichiometries dependent on the aqueous medium (Table 4). The stripping results showed that 91% Nd(III) was recovered from organic phases using 1.5 M HOAc. In the case of the magnets and using a simulated synthetic solution, samarium(III) extraction increased from nearly 4% at pH 4 to 98% at pH 5.5, and copper(II) extraction of 87% at this same 5.5 pH value, against cobalt(II), was poorly extracted (14% at pH 5.5). The maximum separation factor Sm(III)/Co(II) reached values of 3078; however, this separation factor decreased to 148 in the treatment of a real solution coming back from the processing of magnets. No stripping data were given in the manuscript in the case of Sm/Co/Cu solutions.
This extraction process showed a negative value of ΔH° (−15 kJ/mol), indicative of heat evolution within the extraction process. The negative value (−11 kJ/mol·K) of ΔS° seemed to be indicative of a decrease in randomness as the metal was extracted into the organic phase. Metal-loaded organic phases can be stripped via the use of very diluted sulphuric acid solutions (2 × 10−3–8 × 10−3 M). There was a continuous decrease in the extraction efficiency under several extraction–stripping cycles.
Yttrium(III) was extracted from nitrate medium by the use of the same bifunctional ionic liquid ([THAH]+[DEHP]−) [42]. The extraction percentage of Y(III) decreased from 97% to 73% as the equilibration time increased, this being attributable to a dissociation of the extracted complex. Again, extraction increased from pH 1 to 3, and the extracted complex presented the Y(NO3)3·2IL stoichiometry. The variation in temperature revealed the endothermic nature of the extraction process. Using 0.1 M nitric acid solution, yttrium was completely stripped from metal-loaded organic phases; however, the increase in the acid concentration up to 0.8 M decreased the stripping efficiency. Similarly to the previous reference, the extraction efficiency decreased after continuous (up to five) extraction–stripping cycles.
Benzyltributylammonium decanedioate ([N444Bn]2[SA]) ionic liquid was used to extract rare earths and enable the separation of Sm(III) contained in waste SmCo magnets. This investigation was carried out in an acetate medium [43]. The experimental results showed that both La(III) and Nd(III) were extracted better in the acetate medium, with an apparent extractability order of acetate > nitrate > chloride, these results being attributable to the low-hydrated nature of acetate ions. REEs were extracted by an ion association mechanism, with stoichiometries dependent on the aqueous medium (Table 4). The stripping results showed that 91% Nd(III) was recovered from organic phases using 1.5 M HOAc. In the case of the magnets and using a simulated synthetic solution, samarium(III) extraction increased from nearly 4% at pH 4 to 98% at pH 5.5, and copper(II) extraction of 87% at this same 5.5 pH value, against cobalt(II), was poorly extracted (14% at pH 5.5). The maximum separation factor Sm(III)/Co(II) reached values of 3078; however, this separation factor decreased to 148 in the treatment of a real solution coming back from the processing of magnets. No stripping data were given in the manuscript in the case of Sm/Co/Cu solutions.
Table 4.
Species formed in the extraction of Nd(III) from various aqueous media.
| Aqueous Medium | Species |
|---|---|
| Acetate | [N4,4,4,Bm]3Nd[SA]1.5(OAc)3 |
| Nitrate | [N4,4,4,Bm]3Nd[SA]1.5(NO3)3 |
| Chloride | [N4,4,4,Bm]4Nd[SA]2Cl3 |
From [43].
Reference [44] mentioned one of the problems in the use of ionic liquids in the recovery of rare earth elements (and metals in general) that is often neglected by authors. This problem is the same in nature as those of ionic liquids, since, during the extraction process, an ionic liquid would enter the solution in the form of ions, causing the loss of the reactant and increasing the production cost; moreover, this method can also be considered as non-environmentally friendly. At the time of writing and/or publication, the use of these ionic liquids as extractants for REEs was still only in the laboratory research stage.
The abovementioned situation is common in investigations devoted to the use of ILs in the recovery of metals. Practically, the recovery of all metals using ILs has been investigated over time, with recent efforts described in the literature, including the recovery of platinum group metals from spent automotive converters using Cyphos IL101 and IL104 ILs [45], the separation of cobalt–nickel mixtures with the IL formed by the reaction of primary amine Primene JMT and Versatic 10 [46], the extraction of gold(III) from scrap electronic devices using A327H+Cl− IL [47], the recovery of rare earths from electronic scraps [48], the recovery of gold, silver, and precious metals from chloride media [49], and the recovery of plutonium from spent nuclear fuels using ILs [50]. Ionic liquids have been also proposed for the recovery of sulfide from fuels [51]. The abovementioned examples indicate that there is still room to improve the knowledge of IL usage for REEs and other metals recovery in large-scale operations, at larger processing times, and in operational conditions such as counter-current mixers-settlers.
 Deep eutectic solvents were investigated in the recovery of rare earth elements from coal fly ash [62]. Mixtures of choline chloride (ChCl) with lactic acid (LA) and ChCl with para-toluene sulphonic acid monohydrate (pTSA) were used in the investigation. The first results showed better leaching rates when the molar ratios of ChCl:LA and ChCl:pTSA were 1:2 and 1:1, respectively. Both systems showed REE recoveries in the 85–95% range, though the best results were generally obtained with the ChCl:pTSA mixture, indicating the higher acidity provided by pTSA, which contributed to the REEs’ dissolution process. Both DES systems had about 5–8% higher dissolution than single LA, pTSA, and ChCl systems, with this enhancement in the dissolution being even higher, about 35%, when comparing the use of these DES systems with the use of sulphuric acid or another mineral acid (HCl, HNO3). After dissolution, the leached liquor was diluted at 50% with demineralized water and REEs were recovered by direct chemical precipitation with oxalic acid.
The leaching behavior of rare earth (yttrium) carbonate before and after mechanical activation in choline chloride–urea–malonic acid deep eutectic solvents was investigated [63]. Without mechanical activation, yttrium dissolution was about 49%, while the leaching efficiency of yttrium was increased to 85% when the activation time was 60 min, this increase being attributable to the decrease in the particle size and the increased contact area of the reaction when mechanical activation was used. The dissolution process was controlled via chemical reaction and diffusion in the solid product layer. No data were provided regarding what to do with the Y-bearing DES phase.
A strategy for the separation of yttrium from heavy rare earth elements (HREEs) based on ternary hydrophobic deep eutectic solvent (HDES) extraction was proposed [64]. A total of 44 HDESs were prepared with four carboxylic acids serving as hydrogen bond donors (HBDs), bis(2-ethylhexyl) amine (BEA) serving as the hydrogen bond acceptor (HBA), and 1-decanol (DL) serving as the third component of the mixture. The prepared HDESs had the advantages of simple preparation, no purification requirement, low viscosity, low water solubility, and low toxicity. Among the various investigated compounds, the DL:oleic acid (OA):BEA-based HDES with OA as the HBD presented the best extraction ability, higher saturation loading capacity, and better phase separation stability. The separation factors of the DL:OA:BEA (9:1:5) mixture for HREEs (Dy-Lu) and Y in an industrial Y-enriched solution were Dy/Y ≥ 3.05, Ho/Y ≥ 3.37, Er/Y ≥ 4.29, Tm/Y ≥ 6.00, Yb/Y ≥ 10.8, and Lu/Y ≥ 11.2. The extraction of these elements was performed via ion association and partial cation exchange reactions, which took place simultaneously. The loaded HDES can be stripped from the organic phase with 0.2 M sodium oxalate or water.
In order to improve recovery strategies, electric-field-assisted mining has arisen as a technique to extract species from soils using green electrolytes to help in the extraction of metals. Thus, reference [65] evaluated the influence of various types of biodegradable electrolytes, including the use of deep eutectic solvents, in the electromining process. The soil, sampled from the northern region of Brazil, contained cerium(IV), lanthanum(III), and neodymium(III), and the DES-based solutions were prepared by mixing choline chloride and (i) acetic acid, (ii) citric acid, and (iii) oxalic acid in a 1:2 (ChCl:acid) molar ratio. Applying an electric field of 1 V/cm, as was somewhat expected, the use of the various electrolytes resulted in different solubilities. The maximum efficiency using only acetic acid resulted in nearly 70% of cerium(IV) recovery; citric acid removed 63% of lanthanum (III) and oxalic acid extracted 22% of the same rare earth. The results revealed that the use of the abovementioned DES+ acid mixtures did not improve the recovery of these REEs (Table 5); this is attributable to the possible degradation of choline chloride and the increase in the pH in the cathodic region. There was no mention of the recovery of these valuable REEs or what to do with the resulting solutions.
Deep eutectic solvents were investigated in the recovery of rare earth elements from coal fly ash [62]. Mixtures of choline chloride (ChCl) with lactic acid (LA) and ChCl with para-toluene sulphonic acid monohydrate (pTSA) were used in the investigation. The first results showed better leaching rates when the molar ratios of ChCl:LA and ChCl:pTSA were 1:2 and 1:1, respectively. Both systems showed REE recoveries in the 85–95% range, though the best results were generally obtained with the ChCl:pTSA mixture, indicating the higher acidity provided by pTSA, which contributed to the REEs’ dissolution process. Both DES systems had about 5–8% higher dissolution than single LA, pTSA, and ChCl systems, with this enhancement in the dissolution being even higher, about 35%, when comparing the use of these DES systems with the use of sulphuric acid or another mineral acid (HCl, HNO3). After dissolution, the leached liquor was diluted at 50% with demineralized water and REEs were recovered by direct chemical precipitation with oxalic acid.
The leaching behavior of rare earth (yttrium) carbonate before and after mechanical activation in choline chloride–urea–malonic acid deep eutectic solvents was investigated [63]. Without mechanical activation, yttrium dissolution was about 49%, while the leaching efficiency of yttrium was increased to 85% when the activation time was 60 min, this increase being attributable to the decrease in the particle size and the increased contact area of the reaction when mechanical activation was used. The dissolution process was controlled via chemical reaction and diffusion in the solid product layer. No data were provided regarding what to do with the Y-bearing DES phase.
A strategy for the separation of yttrium from heavy rare earth elements (HREEs) based on ternary hydrophobic deep eutectic solvent (HDES) extraction was proposed [64]. A total of 44 HDESs were prepared with four carboxylic acids serving as hydrogen bond donors (HBDs), bis(2-ethylhexyl) amine (BEA) serving as the hydrogen bond acceptor (HBA), and 1-decanol (DL) serving as the third component of the mixture. The prepared HDESs had the advantages of simple preparation, no purification requirement, low viscosity, low water solubility, and low toxicity. Among the various investigated compounds, the DL:oleic acid (OA):BEA-based HDES with OA as the HBD presented the best extraction ability, higher saturation loading capacity, and better phase separation stability. The separation factors of the DL:OA:BEA (9:1:5) mixture for HREEs (Dy-Lu) and Y in an industrial Y-enriched solution were Dy/Y ≥ 3.05, Ho/Y ≥ 3.37, Er/Y ≥ 4.29, Tm/Y ≥ 6.00, Yb/Y ≥ 10.8, and Lu/Y ≥ 11.2. The extraction of these elements was performed via ion association and partial cation exchange reactions, which took place simultaneously. The loaded HDES can be stripped from the organic phase with 0.2 M sodium oxalate or water.
In order to improve recovery strategies, electric-field-assisted mining has arisen as a technique to extract species from soils using green electrolytes to help in the extraction of metals. Thus, reference [65] evaluated the influence of various types of biodegradable electrolytes, including the use of deep eutectic solvents, in the electromining process. The soil, sampled from the northern region of Brazil, contained cerium(IV), lanthanum(III), and neodymium(III), and the DES-based solutions were prepared by mixing choline chloride and (i) acetic acid, (ii) citric acid, and (iii) oxalic acid in a 1:2 (ChCl:acid) molar ratio. Applying an electric field of 1 V/cm, as was somewhat expected, the use of the various electrolytes resulted in different solubilities. The maximum efficiency using only acetic acid resulted in nearly 70% of cerium(IV) recovery; citric acid removed 63% of lanthanum (III) and oxalic acid extracted 22% of the same rare earth. The results revealed that the use of the abovementioned DES+ acid mixtures did not improve the recovery of these REEs (Table 5); this is attributable to the possible degradation of choline chloride and the increase in the pH in the cathodic region. There was no mention of the recovery of these valuable REEs or what to do with the resulting solutions.
3. Deep Eutectic Solvents
The mentioned toxicity, in conjunction with their non-biodegradable properties, precluded the use of ILs as neoteric solvents with green characteristics. ILs serve as the basis of deep eutectic solvents (DESs), which formed a new class of chemicals characterized as being formed by mixtures of components of a eutectic, with the resulting product presenting a melting point lower than the values presented by the pure components [52][53][52,53]. Often, DESs were considered as a subclass of ILs because both share a series of physicochemical properties: low melting points, density, viscosity, low vapor pressure, the absence of or high flash-point values, and chemical and thermal stability; moreover, their properties can be changed to fulfill the requirements of these extractants to be used in specific cases. The difference in the properties of DESs, with respect to those of ILs, is somewhat advantagous in compensating for the shortcomings of ILs; also, since DESs can be non-ionic, these properties are clearly different between these two types of chemicals. They are obtained by the complexation of a compound that acted as a hydrogen bond acceptor (HBA), typically a quaternary ammonium salt, and either a metal salt or an organic compound that acted as a hydrogen bond donor (HBD). Owing to the large combination of HBAs and HBDs, they can be tuned for various uses and are thus considered designer solvents. DESs follow the nomenclature Cation+X−zY, where Cation+ refers to a cation (e.g., ammonium, phosphonium, sulfonium) with X− being a Lewis base serving as a counter ion (often halide anions) and Y being a Lewis or Brønsted acid with z molecules interacting with the anion. With the next general formulae, one can account for five types of DESs: Type I: Cation+X−zMClx, where M = Sn, Fe, Zn, Al, Ga, or In. They are similar to metal halide/imidazolium salt systems. Type II: Cation+X−zMClx·yH2O, where M = Co, Cu, Cr, Ni, or Fe. This type of DES differentiates from I in that it contains hydrated metal halides, which together with their relatively low cost allow compounds with lower melting points. Type III: Cation+X−zRL, where R is an organic radical and L = CONH2, COOH, or OH. This type is the most widely known and applied. Very often, these DESs are used as precursors of choline chloride (ChCl) and HBDs such as amides, carboxylic acids, and alcohols, which make them easily tunable, cheap, and sometimes biodegradable. Type IV: MClx + RL = MClx−1+RZ + MClx+1−, where M = Al or Zn and L = CONH2 or OH. In their composition, inorganic cations, not forming alone with low-melting-point eutectics, are present. ZnCl2 and HBDs, such as urea, acetamide, or different diols, are normally components of this type of DES. Type V. This type of DES was developed from the strong interaction between the acidity difference of phenolic and aliphatic hydroxyl moieties. Clearly different from the other types, the interactions between HBAs and HBDs are non-ionic in nature and, thus, this type of chemical is formed only by molecular compounds, i.e., the case of the thymol–menthol system. Recently, for these types of DESs, different new terms have been added to the nomenclature of these non-aqueous solvents. Natural Deep Eutectic Solvents (NADESs) is among them, which refers to type III compounds combining ChCl, such as HBA, with naturally occurring carboxylic acids, sugars, and aminoacids; also, water was added on some occasions as a third component. Apart from these ChCl-based NADESs, others can be prepared from binary or ternary mixtures of carbohydrates with themselves (e.g., D(−)-fructose:sucrose(1:1) or D-(+)-glucose:D-(−)-glucose:sucrose(1:1:1)) or organic acids with sugar alcohols (e.g., citric acid:D-sorbitol(1:1)), among many others not requiring the presence of ChCl. NADESs were developed to explore the enhancement of the solubility of some intracellular compounds (flavonoid rutin, starch, albumin, etc.) that presented limited solubility in water. Additionally, therapeutic Deep Eutectic Solvents (THEDESs) are another type of chemical, in which active pharmaceutical ingredients are present and show potential in products where solubility problems should be avoided. Mixtures of menthol and ibuprofen have been proposed to enhance topical delivery systems. Several reviews about the use of these DESs on the recovery of metals from various sources have been recently published [54][55][56][57][58][54,55,56,57,58].Deep Eutectic Solvents and Rare Earth Elements
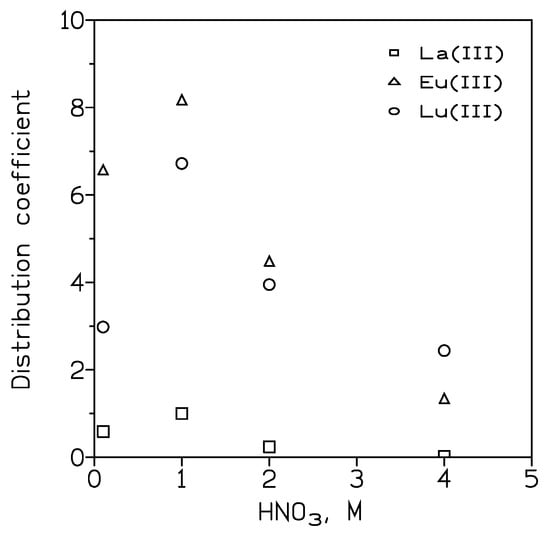
Reference [59] described the use of DESs (and also ILs) to improve the floating characteristics of some minerals, such as rare earth minerals, quartz, and quartz hematite, as well as carbonate asphalt. The amount of DESs used in this field is smaller if compared with traditional flotation agents. One of the various uses of DESs is to provide suitable media for the synthesis of REE-bearing compounds. A DES prepared by mixing choline chloride (ChCl) and urea (1:2) was used as a synthesis medium, via the solvothermal method, of praseodymium vanadate (PrVO4) nanoparticles [60]. These nanoparticles were used as a solely capable electrocatalyst for the detection of furaltadone (FLO). Despite its mutagenic and carcinogenic properties, this FLO is an antibiotic that is commonly used in the growth of aquatic organisms and livestock. The extraction of lanthanides from nitrate media by a non-ideal solvent composed of decanoic acid (C10OOH) and trioctylphosphine oxide (TOPO) was investigated [61]. The maximum distribution of all lanthanides (from La(III) to Lu(III) was observed for an aqueous acidity of 0.5–1 M nitric acid (Figure 1), and the extraction decreased with the increase in the organic acid concentration in the organic phase; thus, TOPO was the key extractant for efficient extraction. No stripping data were available in the published manuscript.
Figure 1. Distribution coefficients of some representative lanthanide elements at various nitric acid concentrations. [Ln]0 = 7.5 mmol/L. Organic phase: C10OOH + TOPO (50% each). O/A ratio: 0.5. T: 25 °C. Data are from [61].
Table 5.
Recovery of REEs using ChCl:acid mixtures in an electromining process.
Similarly to ionic liquids [31], DES can be used as a medium to prepare rare-earth-bearing compounds with further uses. A hydrothermal method to prepare perovskite-type potassium niobate (KNbO3) through a deep eutectic solvent (DES), which can be further used as an electrode material for the determination of bisphenol A (BPA), was investigated [66]. This nitrate was prepared from thymol (C10H14O) and menthol (C10H20O) in a 1:1 ratio.
The cyclic voltammetry of pure CeO2, La2O3, Nd2O3, and PrO2/Pr2O3 in the deep eutectic solvent Ethaline (1:2 mixture of choline chloride and ethylene glycol) was investigated, and the electrochemical activity of these oxides was assessed [67]. The electro-dissolution of pure oxides and water-leached monazite, after high-temperature pretreatment of the mineral, was carried out in a 0.1 mol/L glucose solution in Ethaline and showed a preferential solubility of about 24% for pure Nd2O3 against pure CeO2, La2O3, and PrO2/Pr2O3, which were found to be insoluble. It was also demonstrated that the electrodissolution of the water-leached monazite was not possible because of the inert behavior of the Ce1−xLnxO2−x/2 solid solutions. This compound was formed due to the presence of CeO2 in the product resulting from the high-temperature pretreatment of monazite at low mineral:Na2CO3 ratios. Thus, the formation of cerium oxide must be avoided as much as possible. No information was provided regarding what to do with the resulting solutions.
A deep eutectic solvent composed of isostearic acid (HA) and TOPO diluted in toluene was investigated for the selective recovery of scandium from iron, yttrium, and aluminum [68]. The use of a single isostearic acid or TOPO solutions in toluene did not allow the separation of theses elements; however, the use of this DES solution improved the separation of scandium and yttrium for the other elements. Moreover, undiluted DES improved metal extraction, i.e., a mixture 2:1 (HA:TOPO) allowed for the selective separation of Sc(III) from Y(III) and Fe(III) at pH values near zero. Scandium was extracted via the formation of a Sc(HA)2A3(TOPO)5 complex in the organic phase, releasing three protons to the raffinate. This rare earth can be stripped from loaded organic phases via the use of 2 M HCl or sulphuric acid solutions.
| Electrolyte | Ce(IV) | La(III) | Nd(III) |
|---|---|---|---|
| Acetic acid | 70 | 45 | 37 |
| ChCl:acetic acid | nil | nil | nil |
| Citric acid | 40 | 63 | 35 |
| ChCl:citric acid | 32 | 51 | 30 |
| Oxalic acid | 18 | 22 | 12 |
| ChCl:oxalic acid | nil | 2 | nil |
All the electrolytes at 0.1 M. Data are from [65].
