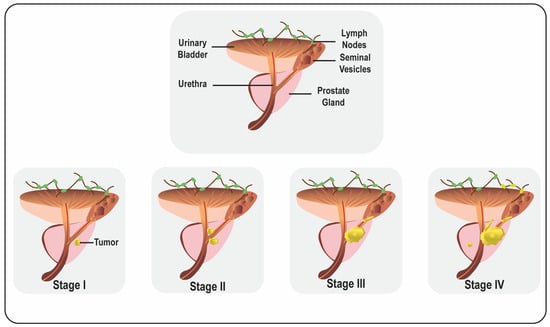
2. Prostate Cancer Progression and Metastasis
CaP is generally divided into localized, locally advanced, and metastatic CaP
[13][19]. At the advanced stage, CaP spreads from the prostate to different body parts (
Figure 1). Usually, metastasis occurs through the lymphatic route to the pelvic and para-aortic lymph nodes and hematogenously spreads to the skeletal system, predominantly affecting the bones. It has also been noticed that visceral metastasis to the liver, lungs, and organs is comparatively rare and connected with intermittent pathology and a poor prognosis
[14][20].
Figure 1.
Representative diagram of male reproductive organs and prostate cancer progression.
The AR signaling pathway is central to prostate carcinogenesis and advances towards androgen-independent states. Almost all primary CaP cases exhibit some level of expression of the AR. Abnormal AR signaling can be associated with a rising serum PSA level, which is treated as the prognostic marker for CaP patients
[15][16][21,22]. Hormone therapy in the form of medical or surgical castration remains the mainstay of systemic treatment for CaP. Despite an initial favorable response to hormone therapy, these tumors will develop androgen independence over time, resulting in death. Although AR is central to the initiation and growth of CaP, various other pathways are also involved in CaP progression and metastasis
[17][18][23,24].
2.1. Prostate Cancer and Androgen Receptor Signaling Pathway
The AR is an androgen-activated steroid hormone receptor belonging to the nuclear receptor superfamily. The function of the AR is dependent on its ligand, and the interaction between the AR and androgens (testosterone and its active form, dihydrotestosterone) is essential for the normal development and function of male reproductive organs. The interaction of the AR and androgens results in the separation of Hsp90 from the AR, facilitating the formation of AR nuclear translocation
[9][19][20][9,25,26]. However, the deregulated expression of the AR or androgens results in CaP development and progression. Early-stage CaP cells are AR-positive, while late-stage CaP cells are undifferentiated and AR-negative
[15][21]. The deregulated AR signaling axis also deregulates the expression of target genes such as PSA, fibroblast growth factor 8 (FGF8), cyclin-dependent kinase 1 (CDK1), transmembrane serine protease 2 (TMPRSS2), and prostate transmembrane protein androgen induced 1 (PMEPA1), resulting in CaP progression, development, and metastasis
[21][27]. The number (19–25 repeats) of Glutamine (CAG) repeats in the N-terminal transactivation domain affects the transcriptional activity of the AR. It was observed that shorter Glutamine repeats result in higher AR transcriptional activity and is associated with an increased CaP risk
[22][28]. Moreover, histone methyltransferase DOT1L was observed to be overexpressed in CaP and upregulates the AR via c-MYC by suppressing c-MYC-regulated E3 ubiquitin ligases like HECTD4 and MYCBP2
[23][29].
Further, the AR helps in CaP cell growth and survival by modulating autophagy-related genes. Studies suggest that autophagy has the central oncogenic role in CaP progression, where transcription factor TEFB and four autophagy genes, such as autophagy-related 4B/4D cysteine peptidase (
ATG4B and
ATG4D) and Unc-51-like autophagy-activating kinases 1 and 2
(ULK1 and
ULK2), play crucial roles in CaP cell survival, proliferation, invasion, and migration
[24][30]. The AR is associated with the co-activator NCOA2 and facilitates CaP cell proliferation and metastasis through the modulation of the PI3K and MAPK/ERK axis. Further, the androgen-dependent activation of ERK1/2 regulates the functional activity of transcription factors independent of AR-DNA binding and increase the level of c-FOS by activating the ETS domain-containing protein Elk-1. Interestingly, the interaction of the AR with p85a induces AKT kinase activity via phosphatidylinositol-3,4,5-triphosphate (PIP3), signaling lipid generation and the activation of the p110 subunit
[22][25][28,31]. Another study reported that FOXA1, GATA2, and OCT1 can increase the AR-DNA interaction and level of FOXA1, positively correlates with CaP stages, Gleason scores, and CaP-specific survival
[26][32]. AR signaling in the stroma of the prostate is also essential for the development of carcinogenesis. During normal prostate development, androgen promotes mesenchymal paracrine secretion; hence, the increased paracrine secretion of stimulatory growth factors due to androgen action might also result in accelerated CaP progression
[27][33]. Further, AR-V1, AR-V7, and AR-V567es are overexpressed in CRPC bone metastasis compared to hormone naïve bone metastasis and are associated with poor survival
[24][30]. Although the depletion of androgens via androgen ablation temporarily represses tumor growth, most CaP patients eventually develop androgen-insensitive tumors, for which no effective therapy exists
[15][21].
2.2. WNT/β-CATENIN Signaling Pathways
WNT/β-CATENIN signaling is essential for organogenesis during embryonic development; however, the alteration of adult cells leads to cancer formation. WNT signaling and β-CATENIN play important roles in the various oncogenic processes that give rise to different human malignancies, including CaP
[28][29][34,35]. WNT/β-CATENIN signaling mediates upsurges in receptor Tyrosine kinase-like orphan receptor 1 (ROR1), increasing noncanonical responses to Wnt5a, which plays an important role in prostate stem cell generation during early development and might be reactivated in the CaP microenvironment. The upregulation of the WNT transporter Wntless (WLS) tumor cells was strongly linked with the activity of the WNT/β-CATENIN pathway in primary CaP along with CRPC
[30][31][36,37]. Protein Kinase D1 (PrKD1) is known to be a unique serine/threonine kinase that causes the phosphorylation of Threonine120 (T120) of β-CATENIN, consequently elevating the nuclear β-CATENIN level in CaP. Moreover, it was observed that β-CATENIN, along with the MYC and MAX heterodimers, bind to the PrKD1 promoter region and repress its activity in CaP cells, suggesting that β-CATENIN/c-MYC/MAX downregulate PrKD1 through an autorepressive feedback loop
[32][38].
Interestingly, WNT/β-CATENIN signaling also drives Cap-mediated bone metastasis. The tumor cell at the primary site usually develops mesenchymal properties, which were reported to be regulated by FZD4, a WNT receptor. WNT signaling, particularly Wnt5A, is very important as the cells flow in the bloodstream. The cells, after reaching the bones, retain the tumorigenic or stem-like property, which is supported by the WNT or β-CATENIN signaling and involves CHD11, CD24, and Wnt5A for CaP-mediated bone metastasis progression
[33][39]. WNT-11 belongs to the WNT protein family and is a part of the non-canonical signaling pathway. It was theorized that WNT-11 is overexpressed in CaP cells and is involved in CaP progression and migration and the secretion of factors such as neurone-specific enolase to differentiate CaP cells in neuroendocrine-like cells
[34][40]. The gene amplification and overexpression of transcription factor SOX-2 plays an important role in cancer progression and the metastasis of various cancers, including CaP. Interestingly, SOX-2 modulates EMT via the WNT/β-CATENIN signaling pathway
[35][41].
2.3. Prostate Cancer and Phosphoinositide 3-Kinase Pathway (PI3K)/AKT
Abnormality in the PI3K pathways is recorded in the different human cancer types, especially in advanced CaP cases. The role of PI3K in CaP has been well recorded, and it was shown that PI3K-C2β, the isoform of class II PI3Ks, regulates the activation of extracellular signal-regulated kinase (ERK1/2) and mitogen-activated protein kinase (MEK1/2) and mediates the invasion and migration of CaP cells
[36][37][42,43]. It was observed that the activation of the serine/threonine kinase, AKT, can lead to CaP cell proliferation through the MAPK pathway. MAPK4 activates AKT via the Caspase 4/5-dependent pathway and represses the degradation of GATA2, which concurrently activates the AR
[38][44]. Moreover, CaP progression has been linked with PTEN loss and AKT-dependent hexokinase, as reprograming the glycolytic pathway can improve the overall outcome
[39][45]. Further, the N-Cadherin-mediated dysregulation of monocyte chemoattractant protein-1 stimulates PI3K/AKT signaling, which targets NF-kB, leading to the stimulation of the bone morphogenetic protein-1 (BMP) signaling cascade. The PI3K/AKT/NF-kB/BMP/SMAD axis results in CaP invasion and bone metastasis
[40][46].
It was reported by Mahmoud et al.
[41][47] that the inhibition of AKT via the increased expression of ULK1 mRNA and the LC3II protein can significantly decelerate CaP progression, suggesting the role of AKT in CaP development. In addition, the inhibition of the key molecules of the PI3K/AKT pathways causes blockage in CaP cell proliferation and metastasis via Cyclin D1 downregulation along with the upregulation of p27. An epithelial cell adhesion molecule (EpCAM) upregulates E-Cadherin, the PI3K/AKT/mTOR signaling axis, p-4EBP1, and p-S6K1 expression to promote CaP proliferation. A decrease in tumor sphere formation and CaP progression was observed in CaP cells when EpCAM was targeted, with a significant decrease in PI3K/AKT/mTOR expression, suggesting that EpCAM can be the therapeutic target to inhibit CaP progression
[42][43][48,49]. Further, hypoxic conditions promote the PI3K/AKT/mTOR pathway in CaP cells for the adaptation of cells to a low O
2 microenvironment via hypoxia-induced factor 1 (HIF-1) expression, suggesting the role of the PI3K/AKT/mTOR axis in CaP during both normoxic and hypoxic conditions
[44][50].
A reduction in the expression of the PLK4 and PI3K signaling molecules in CaP cells can suppress cell proliferation, migration, invasion, and metastasis
[31][37]. c-MYC is the oncoprotein that has been implicated in various cancers, including CaP
[45][51]. It was reported that the level of c-MYC is relatively elevated in metastatic CaP. c-MYC directly promotes CaP development through the upregulated expression of various pro-tumorigenic factors, including ribosome biogenesis, which supports tumor growth and PI3K/AKT/mTOR pathway dysregulation, consequently promoting the survival and growth of CaP cells. The c-MYC/PI3K/AKT/mTOR axis can be targeted to decrease tumor progression
[46][52].
2.4. Prostate Cancer and JAK/STAT Pathway
The JAK/STAT signaling pathways are found to be continuously activated in different types of malignancies, including CaP. This signaling pathway in CaP is associated with tumor growth, upregulated angiogenesis, and metastasis
[36][47][42,53]. It was reported that JAK/STAT pathway activation drives prostate tumor plasticity. Interestingly, the role of cytokines in cell proliferation and migration is well known, especially via cytokines. Within the different cytokines, interleukin-6 (IL-6) greatly impacts CaP development and regulates cell growth and immune response through the activation of the JAK/STAT signaling pathways along with cross-talk with PI3K, MAP kinase, and different cellular pathways
[48][54]. In CaP cells, BRCA1 and the AR can bind to STAT3, leading to the activation of the downstream JAK/STAT signaling pathway and upregulating anti-apoptotic and angiogenic proteins such as Bcl-xl, VEGF, Survivin, BCL-2, and MCL-1
[49][55]. Moreover, it is also well observed that the frequent over-activation of the JAK/STAT pathway in CaP cells can initiate the upregulation of PD-L1. Interestingly, STAT3 plays an important role as a mediator of tumor immune evasion. It was shown that the SOCS3 gene of the adenoviral vector increases the sensitivity of CaP via the over-activation of JAK/STAT3 to NK cells via the decline in PD-L1 expression and the generation of IL-6
[50][56].
2.5. Other Factors Involved in Prostate Cancer
MicroRNAs (miRNAs), small noncoding RNAs, play important roles in CaP progression and metastasis. Numerous studies showed that miRNA acts as either a promoter or inhibitor of metastasis in CaP. The dysregulation of the miRNA-34 family was reported in different cancer types, including CaP. Due to their interaction with the tumor suppressor p53 and their participation in EMT via EMT-TFs, miR-34 family members are known as tumor-suppressive miRNAs. By directly suppressing CD44, miR-34a prevents metastasis
[51][57]. Moreover, the failure of miR-34a expression stimulates the PI3K/AKT, JAK/STAT3, and WNT/β-CATENIN signaling pathways, leading to the progression of CaP
[52][58]. Deregulated miRNA can be used as the novel diagnostic and prognostic tool in CaP as there may be a relationship between the patterns of miRNA expression and the androgen dependence of these tumors, indicating a potential link between miRNA expression and the tumor’s reliance on androgens for growth. Further, the downregulation of miR-34a promotes CaP progression by downregulating the oncoprotein STMN1 via the CtBP1\miR-34a\STMN1\GDF15 axis. Moreover, the MiR-34b plays a critical role in migration and invasion inhibition by regulating the TGF-β pathway
[53][59]. Three urine microRNAs (miR-21-5p, miR-141-3p, and miR-205-5p) were observed to be promising non-invasive diagnostic markers for CaP. Additionally, it was found that miR-21-5p, miR-574-3p, and miR6880-5p were significantly elevated in patients with CRPC, suggesting their potential as prognostic biomarkers for CRPC. Specifically, the increased levels of miR-21-5p were associated with the downregulation of programmed cell death protein 4, a regulator of prostate cancer cell growth and resistance to castration. On the other hand, elevated miR-574-3p levels were linked to the suppression of the Notch signaling pathway, as well as changes in DNA damage and apoptosis
[54][60]. By measuring substances called 8-OHdG and 8-Iso-PGF2α in the urine of prostate cancer patients, researchers have discovered consistent and significant associations between increased levels of oxidative stress indicators and CaP. Additionally, they found that when radical prostate cancer removal surgery is performed, these indicators tend to return to normal levels. Even though these biomarkers are not highly specific for prostate tissue and diagnosis, the findings suggest that assessing 8-OHdG and 8-Iso-PGF2α in urine, both before and after surgery, could serve as a valuable technique for predicting the success of radical prostate cancer surgery and possibly for identifying the risk of local recurrence following the procedure
[55][61].
CaP metastasis involves complex interplay and cross-talk between different molecules. It is noted that a high level of Plectin plays an important role in the development of localized and metastatic human CaP, and its knockdown slows the growth of CaP and impairs aggressive cellular behaviors
[56][62]. Various LNCaP cell subpopulations showed a non-integral copy number and a high mutational load. The DNA mismatch repair genes in all three cell lines had pathogenic mutations, homozygous deletions, harmful alterations affecting the cell cycle, and other vital cellular functions
[57][63]. Moreover, calcium is also important in controlling the molecular factors and signaling pathways involved in CaP metastasis. Calcium channels are generally present at the membrane region of the endoplasmic reticulum (ER), like IP3 receptors, and are reportedly involved in CaP cells’ survival. The dysregulation of the calcium channel and its receptor may also lead to CaP progression and metastasis
[58][64]. Moreover, NF-κB transcription factor members are crucial mediators of CaP progression, migration, and invasion. It was also reported that NF-κB activity is higher in metastatic CaP compared to localized disease
[8][16][8,22]. Studies have shown that the inhibition of IRE1α by MKC8866, an IRE1α RNase-specific inhibitor, strongly inhibits CaP tumor growth. Interestingly, a global transcriptomic analysis has revealed that IRE1α-XBP1s pathway activity is mandatory for c-MYC signaling, which is upregulated in CaP development
[59][65].
Similarly, it was also observed that immunoglobulins are very crucial for the regulation of different cancers, including CaP. The level of IgG1 heavy chain was found to be elevated in CaP patients. The activation of IGHG1 via antibody blocking or genetic knockdown resulted in the suppression of cell growth, the initiation of cell cycle arrest, and, finally, apoptosis. There was a positive correlation between the level of c-MYC and IGHG1. Moreover, MEK/ERK/c-MYC pathways are observed to be downstream of IGHG1 in CaP cells. Further, it was observed that IGHG1 inhibition controlled the tumor growth and inactivation of the MEK/ERK/c-MYC pathway
[60][66]. Gaining a deeper understanding of how different signaling pathways interact and influence the progression of CaP is critically important. This knowledge is pivotal for the development of targeted medications that can inhibit or activate specific molecules, ultimately offering the potential to regulate tumor advancement. Further, many deregulated pathways can serve as diagnostic or prognostic markers for CaP. Therefore, it is essential to have a comprehensive understanding of the signal cascades that underlie the initiation and development of CaP. This knowledge is crucial for the eventual development of effective strategies to combat CaP.