1. Introduction
Heart failure (HF) is a severe and harmful syndrome and although many diagnostic and therapeutic efforts have been made, effective, holistic management has not yet been achieved. The reported data suggest an HF prevalence ranging from 1% to 2% in adults
[1][2][3][1,2,3] and the HF incidence seems to be higher and increases with age, exceeding 10% for those >70 years old
[3][4][3,4]. Importantly, the mortality rate is high
[5][6][5,6] and is expected to increase further due to the increased population, aging, senescence, coexisting morbidities, and probably the lack of holistic prevention and management
[7]. Thus, although HF is common, its morbidity and mortality rates remain high
[8]. Of note, despite the fact that the major determinants of syndrome severity, namely, prolonged activation of neurohormonal systems, inflammation, and free radical production, have been recognized and the relevant treatments have been implemented, there are still several important issues to be resolved. Indeed, when referring to the HF process, diverse additional factors adversely affecting body homeostasis should be considered
[9][10][9,10]. It has long been recognized
[11][12][11,12] that HF by inducing gut ischemia and congestion may alter the gut microbiota (the community of gut micro-organisms themselves) and intestinal permeability, stimulating immune and inflammatory processes
[13][14][13,14] and leading to a further deterioration of cardiac function
[15][16][15,16]. Moreover, as the gut microbiota regulates the energetic function of several organs, including the heart, its derangement may be associated with multiorgan dysfunction
[17][18][19][17,18,19].
2. Gut Microbiota as a Diagnostic Marker
According to the National Institute of Health (NIH), a biomarker is defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”
[20][102]. Alterations in gut flora have been linked to several human diseases, including gastrointestinal disorders
[21][103], ischemic stroke
[22][104], allergies
[23][24][105,106], inflammation
[25][26][27][107,108,109], cancer
[28][29][30][31][110,111,112,113] and cardiovascular disease
[32][33][34][114,115,116]. For example, gut microbiota derangement is linked to ST-elevation myocardial infarction
[35][117] and can be used in the setting of a relevant prediction model
[36][118]. Thus, it is reasonable to search for gut microbiota alterations per se or its products as diagnostic disease biomarkers
[37][38][39][119,120,121].
As previously mentioned, there is a relationship between gut microbiota, neuro-hormonal activity, inflammation, and free oxygen production, the steadfast underpinnings of HF
[40][41][42][35,36,38]. It is reasonable, therefore, based on this relationship, to test microbiota-based biomarkers in the diagnosis and management of HF.
TMAO is the most studied microbiota biomarker, showing a correlation with the HF functional class
[43][44][45][46][79,80,81,122] and with the B-type natriuretic peptide, with mortality either in chronic
[46][122] or acute HF
[47][84]. Interestingly, the correlation between TMAO and mortality remains even after adjustment for the natriuretic peptide levels
[43][48][79,123]. Additionally, TMAO can be used as an index of mortality/hospitalization risk in HF patients with a preserved ejection fraction presenting with restrictive physiology patterns
[43][47][49][50][51][79,84,124,125,126]. Finally, it seems that TMAO can be used as an index of cardiac fibrosis and contractility, platelet reactivity, and endothelial function
[13].
It has been suggested that short-chain fatty acids augment mitochondrial DNA protection and regulate ATP concentration, thus, controlling the energetic needs of several organs, including the heart
[17][50][52][53][54][17,125,127,128,129]. They are inversely correlated with the outcomes in HF patients with reduced ejection fraction
[40][35] and can be used as markers of cardiac fibrosis and hypertrophy
[55][56][57][46,130,131], vascular tone
[55][56][57][46,130,131], gut barrier function
[58][132], and insulin sensitivity
[59][133]. Given their efficiency and the observation that the enzymatic machinery for oxidation of short-chain fatty acids is up-regulated in the failing hearts of both animals and humans, targeting this unexplored source of energy for therapy for patients with HF could be a promising area of future clinical studies
[60][134]. Nevertheless, their effects always depend on which receptor and in which tissue/cell type they activate each time. FFAR3 and FFAR2 are mainly short-chain fatty acid receptors
[61][135]. Contrary to FFAR2, whose role in cardiovascular homeostasis is virtually unknown, FFAR3’s involvement in cardiovascular function regulation has become increasingly clear over the past decade. FFAR3 has been implicated in the mechanism of lipolysis, while also exerting vasodilating properties resulting in hypotension. On the other hand, FFAR3 promotes neuronal firing and norepinephrine synthesis and release in sympathetic neurons
[62][136] and increases heart rate and cardiac inflammation
[61][135]. Lipopolysaccharides consisting of a hydrophobic domain known as lipid A (or endotoxin), a non-repeating “core” oligosaccharide, and a distal polysaccharide (or O-antigen) are elevated in decompensated HF
[63][137] and play a crucial role in gut barrier function, inflammation, cardiac contractility, insulin resistance, and endothelial function.
Phenylacetyl glutamine (PAGln) along with phenylacetylglycine (PAGly) are gut microbiota metabolites that act through G-protein coupled receptors and are involved in platelet function and thrombosis, leading, therefore, to cardiovascular disease
[64][65][34,138]. Their presence in blood samples is related to increased reactive oxygen production and apoptosis, decreased cell viability and myocardial contraction, and high rates of thrombotic events
[65][66][67][138,139,140]. The increased free radical production activates the enzyme calmodulin kinase II (CaMKII) and the ryanodine receptor 2 (RyR2), inducing a proarrhythmic status characterized by cardiomyocyte apoptosis and electrical remodeling
[68][69][141,142]. Indeed, a recently conducted study demonstrated that plasma PAGln levels are significantly elevated in atrial fibrillation, suggesting that PAGln may be a promising therapeutic target in this clinical setting
[67][140].
Based on the above, it is tempting to suggest the use of gut microbiota or their metabolites either in feces or in blood samples as biomarkers of cardiovascular involvement. However, there are several limitations, mainly because the normal microbiota has not been adequately defined
[42][38]. Additionally, both gut microbiota composition and its products as well as HF are influenced by age
[70][143]. Moreover, there is a database limitation for studying the human gut microbiome
[71][144] and the coupling of taxonomy and function in the microbiome is not well defined. It is hoped that these discrepancies could be resolved
[72][145] by using artificial intelligence,
16S rRNA gene sequencing, or even whole metagenome shotgun sequencing
[73][74][146,147].
3. Gut Microbiota and Medications
There is a bidirectional relationship between the gut microbiota and drugs since microbiota can be altered by drug action and, conversely, the microbiota can modify the pharmacokinetic properties of drugs. Resistance to aspirin
[75][148], along with other platelet aggregation inhibitors, due to microbiota action has also been documented
[76][149]. The use of proton pump inhibitors has been associated with an increase in typically oral bacteria in the gut
[77][78][150,151]. Metformin, an antidiabetic drug, has been associated with changes in the gut microbiome composition both in vivo and in mice
[79][80][152,153]. An in vitro analysis of more than 1000 marketed drugs revealed that non-antibiotic drugs can also inhibit the growth of gut bacterial strains
[81][154]. Further, most of the drugs used in HF, including β-blockers, angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers, statins, and the more recently introduced SGLT2 inhibitors
[82][155], can alter the gut microbiota composition
[76][83][84][149,156,157], which in turn may modify drug action and ultimately affect HF management. An interesting multi-drug meta-analysis of three independent Dutch cohorts (
N = 2396 individuals) reported that the administration of proton pump inhibitors, laxatives, and antibiotics had the largest effect on gut microbiome composition
[85][158]. Although there is a well-documented bidirectional relationship between gut microbiota and medications, the exact mechanisms underlying this interaction have not been delineated. However, there is some evidence to suggest that lifestyle modifications including exercise and a Mediterranean diet, along with the use of pre- or probiotics, might beneficially alter the gut microbiota environment
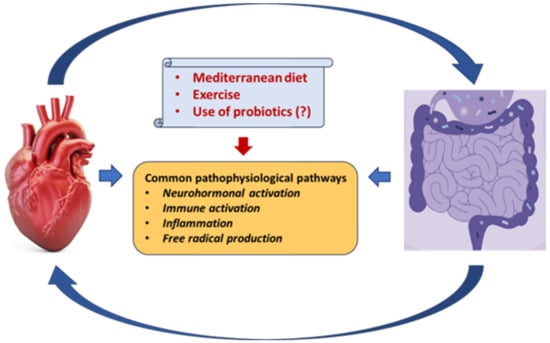
[46][86][87][88][122,159,160,161] (
Figure 12). Current evidence, however, is insufficient, and new paths of research are required to explore new approaches for treatment optimization. Machine learning prediction tools have been developed for investigating the possibility of drug degradation by gut microbes
[89][162]. For example, a machine learning model, trained on over 18,600 drug-bacteria interactions, has been recently proposed to predict (Area Under the Receiver Operating Curve of 0.857) whether drugs would impair the growth of 40 gut bacterial strains
[90][163]. Sequencing the gut microbial genome could also be an option, but it is still under investigation
[35][50][91][92][117,125,164,165]. In the meantime, antibiotics, bile acid sequestrants, non-lethal microbial inhibitors, fecal microbiota transplantation, etc.
[93][94][37,166] might be used along with necessary lifestyle changes.
Figure 12. Common pathophysiological pathways between the gut microbiota and the heart in heart failure. A Mediterranean diet, exercise, and possibly the use of probiotics may attenuate these dangerous interactions.
4. Gut Microbiota, Aging, Diet, Exercise Training, and Supplements
Aging, an inevitable evolution in all species, is characterized by the progressive functional deterioration of multiple organs that leads to dysfunctional tissues, with the cardiovascular system being no exception. Several studies, which have been performed in order to find an approach to extending life span, suggest that life duration depends on the type of diet, exercise, working environment, and pharmacological intervention
[95][96][167,168]. It is well known that adherence to a Mediterranean diet provides a positive trajectory toward healthy successful aging, with major potential benefits for mental and cognitive health
[97][169]. A study that included 153 subjects following the Mediterranean diet reported an increase in the level of fecal short-chain fatty acids, indicating a close relationship between this type of diet and a beneficial gut microbiota profile
[98][170]. The effect of diet on microbiota and health was also demonstrated in another study that included 178 elderly subjects (>65 years); it was reported that the fecal microbiota composition was significantly associated with measures of frailty and comorbidity, as well as markers of inflammation
[99][171]. In the same work, the individual microbiota of people in long-stay care was less diverse compared with that of community dwellers, and the loss of community-associated microbiota was related to increased frailty. Finally, an experimental study in mice showed that a high-fat, high-sugar diet promoted metabolic disease by depleting Th17-inducing microbes, and recovery of commensal Th17 cells restored protection
[100][172]. Thus, a diet with moderate protein consumption, low glycemic index, and abundance of foods rich in fibers and polyphenols, may promote normal gut symbiosis and, hence, healthy aging.
Along with a healthy diet, several studies have suggested the beneficial effect of exercise on the intestinal flora
[101][173]. Indeed, it has been shown that the gut microbiota affects the exercise capacity both of trained and not trained individuals, being a regulatory factor of the physiological function of skeletal muscles
[102][174]. Further, regular exercise training beneficially affects the human lipid profile, metabolic status, and immune activity, reducing the risk for cardiovascular diseases
[101][103][104][173,175,176]. Concerning HF, there are diverging data regarding the effect of diet on cardiac function
[105][106][177,178]. Although there is a large number of studies that recommend the use of a healthy diet, exercise training, and, in some cases, the use of supplements, the evidence is not robust enough to strongly recommend this approach. However, it is a fact that, whereas the consumption of non-refined fiber-rich foods, vegetables, fruits, etc. promotes short-chain fatty acid production, which is considered cardioprotective, meat consumption leads to TMAO production, which is considered harmful for various systems, including the cardiovascular system
[47][86][84,159]. Importantly, a relation between gut microbiota and mitochondria has been documented
[107][179], indicating that the gut environment regulates cell death by toxin secretion, targeting the mitochondria and host innate immune system and leading to chronic inflammation that, in turn, promotes the dysfunction of various systems, including the cardiovascular
[17]. In this respect, by maintaining the gut microbiota “keeper” on track, the control of mitochondrial function and minimization of harmful effects might be achieved. To answer important questions on these issues the PROMOTe (PROtein and Muscle in Older Twins, NCT04309292) study was designed
[108][180]. This is a double-blinded, randomized, placebo-controlled, dietary intervention study in which volunteers are enrolled in twin pairs from the TwinsUK cohort. Each pair is randomized to either receive protein supplementation plus placebo or protein supplementation plus a gut microbiome modulator and the intervention period will last 12 weeks. Clinical and biochemical measures will be collected at 0 and 12 weeks, with two monthly contacts where the gut microbiota composition will be examined, together with a battery of physical assessments. The primary outcome will include the muscle function estimated utilizing the chair-rise time.
A recent meta-analysis of 15 randomized controlled trials examining the differences in the gut microbiome composition between patients on antibiotic therapy with and without additional probiotic supplementation revealed no significant differences between the probiotic-supplemented and control groups
[109][181]. Therefore, the authors concluded that probiotics have only a minor, not permanent effect on the composition of the gut microbiome during antibiotic therapy and are not appropriate for preventing dysbiosis due to antibiotics
[109][181].