Ion-selective electrodes are a popular analytical tool useful in the analysis of cations and anions in environmental, industrial and clinical samples.
- ion-selective electrode
- potentiometry
- anion ionophore
- nitrates
- solid contact
- liquid contact
1. Introduction
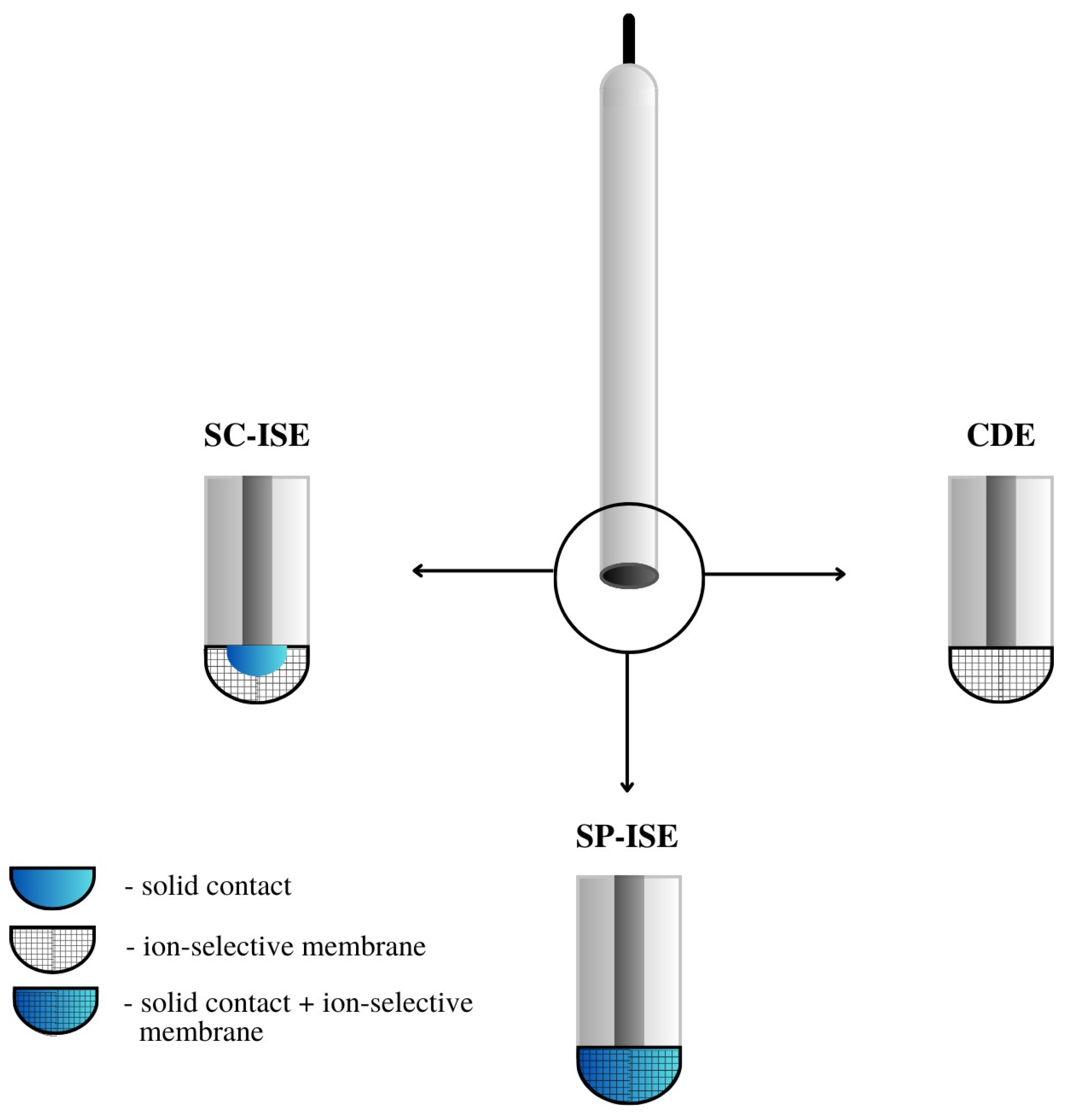
2. Constructional Variants of Anionic Ion-Selective Electrodes
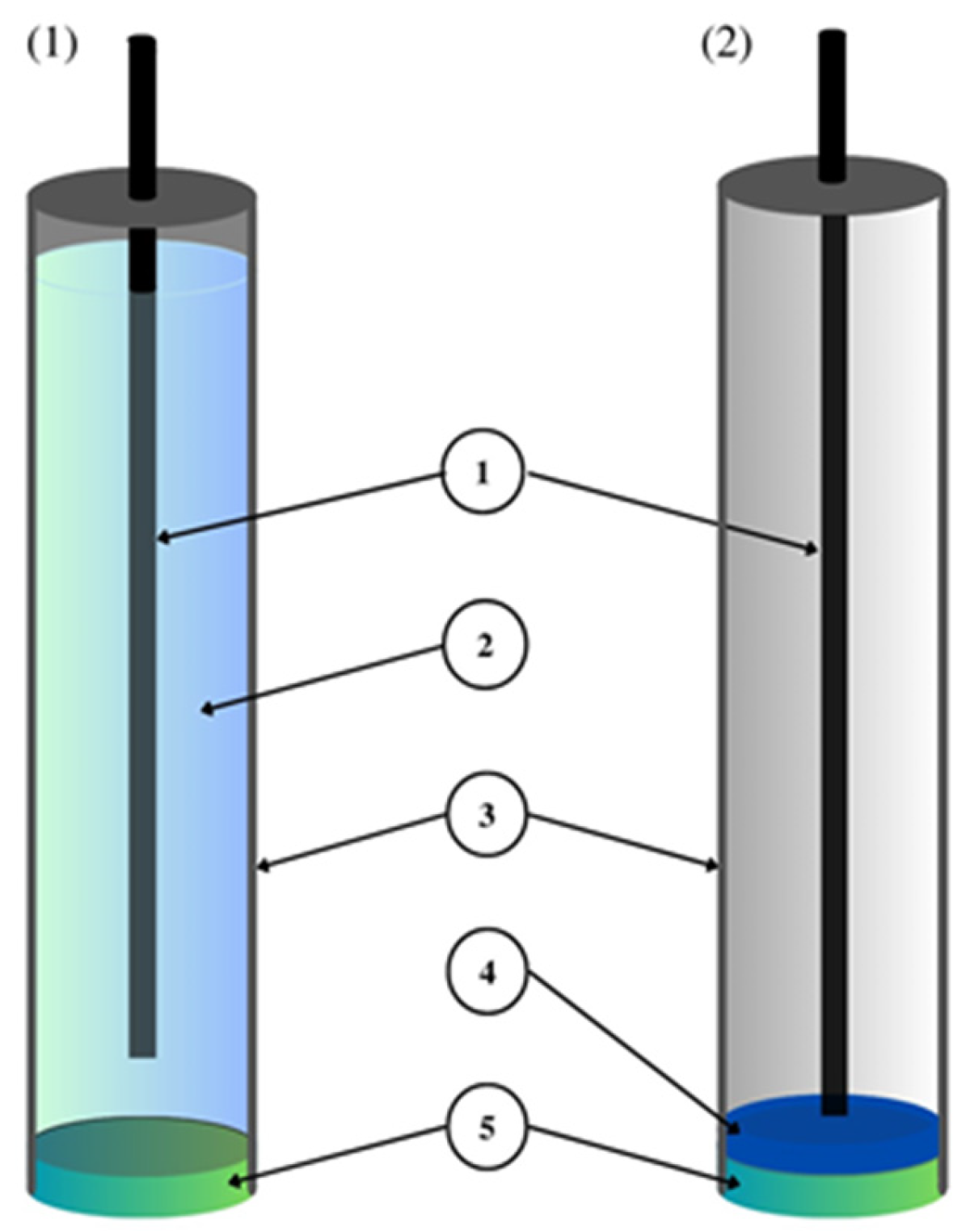
The most important component of any ion-selective electrode is the ion-sensitive membrane. The most important component of the membrane from the point of view of electrode operation is the ionophore, which gives it sensitivity and selectivity to a specific ion. The material used as an ionophore should selectively bind a given ion in the membrane environment. In the case of anion-sensitive electrodes, three types of membranes can be distinguished: polymeric membranes based on PVC, crystalline membranes composed of insoluble salts of the determined ion and composite membranes containing the active ingredient combined in various ways with other materials. Polymeric and crystalline membranes are still used in classic liquid-contact electrodes; however, a much larger group includes a variant of electrodes without internal electrolyte solution called all-solid-state ion-selective electrodes (ASS-ISEs). In recent years, they have become, among ISEs, one of the most studied groups of analytical measuring instruments. Depending on their construction, ASS-ISEs can be classified as follows:- -
-
CWE, CDE—a coated wire/coated disc electrode, which was originally a platinum wire coated with a layer of PVC membrane. CWEs/CDEs are created by applying, for example, a polymer membrane or crystal membrane on a wire/disc used as the inner electrode. Such sensors are now rarely used. In publications, they are studied as a comparative system to assess the effectiveness of solid contact or modification of the membrane composition.
- -
-
SC-ISE—solid contact ISE is a type of electrode in which an intermediate layer of material, generally called solid contact, is introduced between the ion-selective membrane and the inner electrode. Conductive polymers, carbon nanomaterials, metal and metal oxide nanoparticles and composite materials are most often used as intermediate layers.
- -
-
SP-ISE—a single-piece ion-selective electrode with a membrane in which the modifying material is dispersed or dissolved. An ion-selective electrode is created by depositing a membrane cocktail of conductive polymers or other conductive materials, e.g., carbon nanotubes, onto a surface that is usually a glassy carbon electrode [16][17]. Carbon paste ion-selective electrodes can also be included in this group of electrodes in which the active component of the membrane is mixed with graphite powder and binder material, and the mixture is packed in the sensor holder with an internal electrode placed inside (often a copper wire). In order to improve their work, the composition of the paste electrode membrane is also modified by the addition of various materials, which are often carbon nanomaterials. A comparison of the construction of different ASS-ISEs electrodes is shown in Figure 3.
-
-

-
Figure 3. A comparison of the construction of CP-ISE, SP-ISE and SC-ISE electrodes.A comparison of the construction of CP-ISE, SP-ISE and SC-ISE electrodes.
3. Research Methods Used to Assess New ISEs
Newly developed ion-selective electrodes are subjected to numerous tests to determine their parameters and assess their analytical usefulness. The basic measurements carried out in each case include the production of a calibration curve from which the LOD, measuring range and slope of the characteristic are determined. Equally important are the selectivity tests consisting of determining the values of selectivity coefficients towards interfering ions. These measurements are absolutely necessary when a new active substance is introduced into the electrode membrane. The most commonly used methods for determining selectivity coefficients are those recommended by IUPAC, i.e., the separate solution method (SSM) and the fixed interference method (FIM) [19]. In order to check the effectiveness of the introduced electroconductive materials in the form of an intermediate layer or as a membrane component, the potential drift tests are performed: the potential change under zero current conditions and the estimated E0 potential stability in time [20]. Chronopotentiometry and electrochemical impedance spectroscopy measurements also provide valuable information in this area as they make it possible to determine the electrical capacitance of the electrodes and their resistance, which significantly influence the stability of the electrode potential [15][17][21]. Water layer tests are also performed according to the procedure proposed by the Pretsch group [15].4. Ion-Selective Electrodes Sensitive to Nitrates Ions
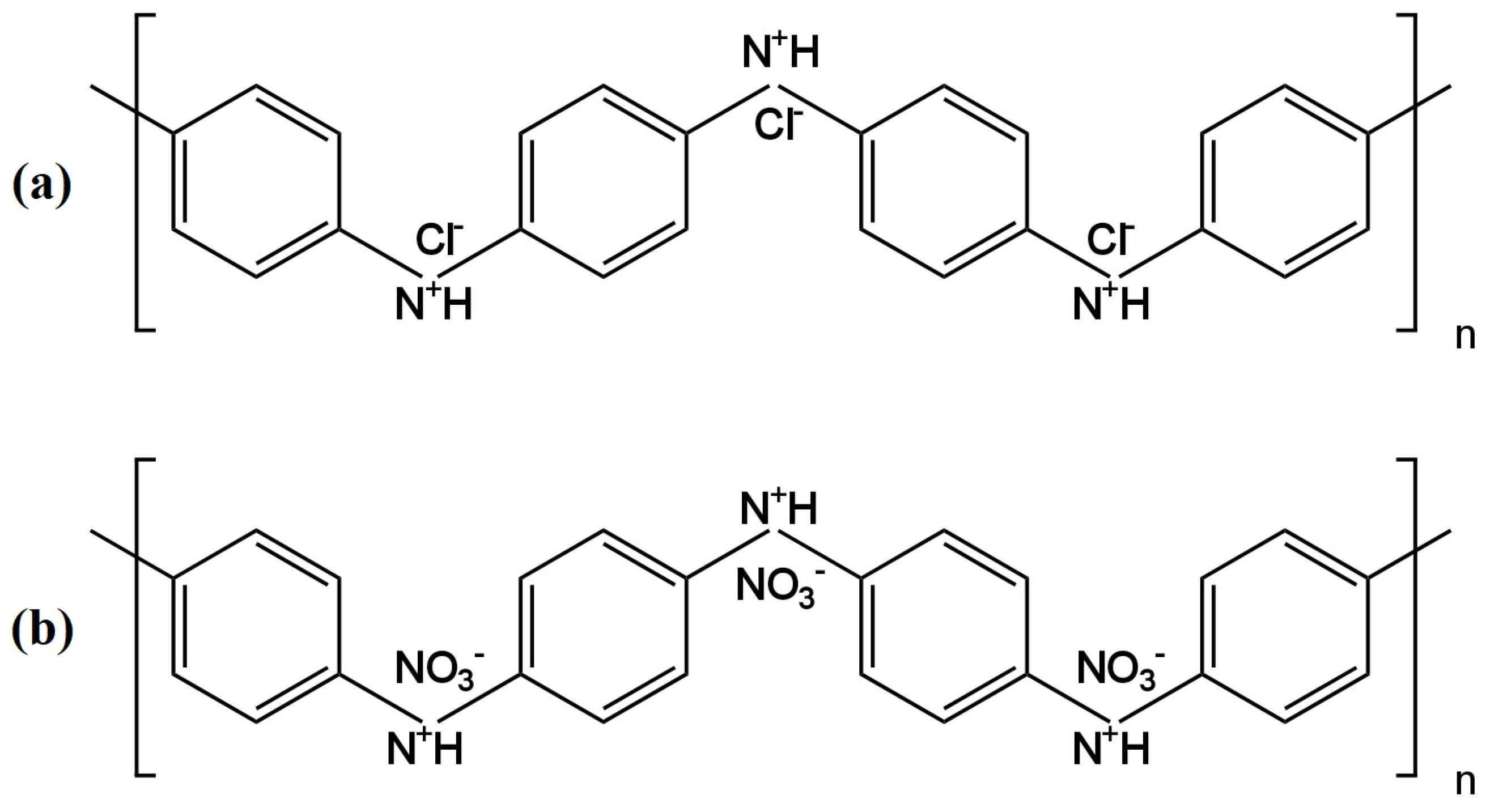
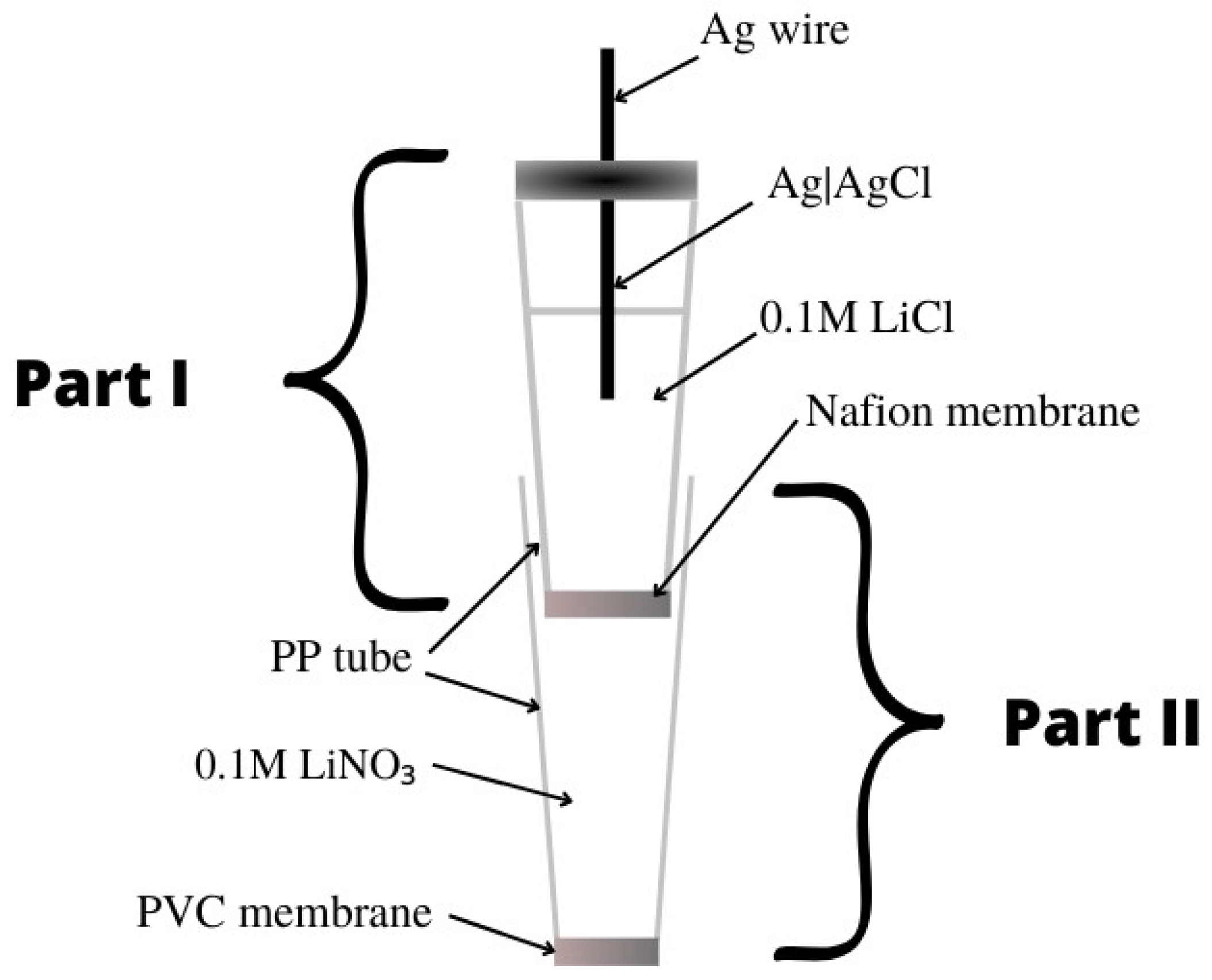
The monitoring of nitrate ions is very important, which is why new tools are being developed to make their identification more accurate and simple. Determining the concentration of nitrate ions is crucial because an excess of nitrates in the human diet is harmful to health and can cause, for example, problems with the cardiovascular system and dysfunction of the intestines and the rest of the gastrointestinal system. It can also lead to pathological conditions such as cancer. WThe researchers have collected 19 nitrate ion-selective electrodes that were developed over the past five years. Almost all of the nitrate electrodes were solid contact electrodes, with the exception of two that had liquid contact. For the determination of nitrates, a few groups of scientists proposed electrodes with a solid contact, which had TDMAN (tridodecylmethylammonium nitrate) as the ionophore. They were different from each other, for example, in the inner electrode and material of the intermediate layer. In article [22], the structure of the electrode was developed by using screen-printing technology, in which the working electrode was a graphite electrode deposited from a graphite-based ink, was described. Graphite was used as the transducer layer due to its stability, as well as its good conductive and hydrophobic properties. The slope of the characteristic of −55.4 mV/decade slightly deviated from the Nernst value, and the obtained linearity range for the tested microelectrode was not very wide (2.9 × 10−4–1.7 × 10−1 M). The limit of detection was quite high, so measurements of low concentrations could be problematic. The advantage of this electrode is its good repeatability. The second electrode with TDMAN as the ionophore was described in the publication [23], where the ISE based on hydrophobic laser-induced graphene (LIG) as an intermediate layer was presented. The hydrophobic LIG was created using a polyimide substrate and a double lasing process. A study using the XPS (X-ray photoelectron spectroscopy) technique was carried out to check the composition of the LIG, and the static contact water angle of 135.5 ± 0.7° was determined. The slope of the characteristic equaled −58.17 mV/decade and was much closer to the Nernst response in comparison to the electrode described as the first. The ranges of the linearity were comparable, but the detection limit extended to micromole values, which was a significantly better result. This electrode was successfully applied in the monitoring of surface water quality. The authors of another work [24] proposed a novel electrode in which a new nanocomposite consisting of poly(3-octylthiophene) and molybdenum disulfide (POT-MoS2) was used as the intermediate layer. A gold electrode (AuE) was used as the basic electrode. The SC-ISE exhibited an over-Nerstian response, while the other parameters were comparable to the ISEs described above. The parameters of the nanocomposite-modified electrodes were not remarkably different from those for ISEs based on MoS2 or POT. Nevertheless, the combination of POT-MoS2 gave the best results, i.e., the slope, LOD or potential stability. An additional advantage of the newly proposed composite is its high hydrophobicity and redox properties. By replacing the membrane, this electrode can be used for the determination of other ions, i.e., potassium, phosphate or sulfate ions. Subsequent electrodes having the same type of ionophore—TDMAN—were presented in the work [25][26]. In the publication [25], the solid contact ion-selective electrodes with different types of transducer layers (TLs) were described. The TL was constituted by polyaniline nanofibers doped with Cl− (PANINFs-Cl−) or NO3− (PANINFs-NO3−) ions (the chemical formulas are shown in Figure 4). Several variations of electrodes were prepared using the polymer conductor—it was used as a suspension dropped directly onto the surface of the inner electrode, which was GCE (glass carbon electrode), and as a component of a membrane cocktail, where it occurred in different amounts (0.5%, 1%, 2%). All polyaniline nanofiber-modified electrodes had better parameters than the unmodified electrode. Electrodes with PANI nanofibers doped with Cl− ions had a wider range of linearity and a lower detection limit than those where PANINFs were doped with NO3−. On the other hand, the second ones exhibited a slope closer to the Nernst slope of −57.8 mV/decade. ISEs with both types of nanofibers did not show potential sensitivity to changes in pH in a very wide range. Furthermore, these types of electrodes showed excellent stability and potential reversibility and also had a lower membrane resistance value and a higher value of double-layer electrical capacitance relative to the unmodified electrode. These electrodes were successfully used to determine nitrate ions in real environmental samples, i.e., drinking, river and groundwater. The same type of ionophore was also presented in the article [26], where a miniaturized version of the ion-selective electrode was presented in which the solid contact was mesoporous black carbon (MCB) and the conductive electrode was a silver wire placed in a glass capillary. MCB provides good ion-electron conductivity and, in addition, has a large specific surface. An additional advantage is its high availability and low price. The addition of MCB significantly stabilized the electrode’s response, ensuring adequate double-layer capacitance and decreasing the resistance more than a hundred times over coated wire electrodes. In comparison to electrodes described in the work of [25], these ISEs had a lower slope of −54.8 mV/decade, an order of magnitude more narrow range of linearity and a similar detection limit. Undoubtedly, the disadvantage of this electrode is its relatively short lifetime oscillating around 20 days. By comparing the electrodes proposed in the publications [25][26], it is clearly preferable to incorporate modifications such as PANINFs rather than MCBs. Also, in the paper [27], for the determination of nitrate ions, an electrode in which TDMAN performed the function of the ionophore was proposed. The inner electrode was a screen-printed carbon electrode (SPCE), on whose surface cobalt (II, III) oxide nanoparticles (Co3O4NPs) were deposited, and acted as an ion-to-electron transducer. The solid contact was examined in terms of morphology and physical properties using XRD, EDS (energy-dispersive X-ray spectroscopy), SEM (scanning electron microscope) and TEM (transmission electron microscopy). Novelization of the electrode structure with hydrophobic Co3O4 nanoparticles prevented the formation of an aqueous layer and improved its response in relation to ISE without a Co3O4NP layer. This electrode also exhibited a good slope of −56.8 mV/decade, as well as a low limit of detection of 1.04 × 10−8 M. According to the EIS spectra, a reduction of membrane resistance was observed for the electrode modified with oxide nanoparticles. As was confirmed by comparing the results of SC-ISEs and spectrophotometer determinations, this electrode is certainly suitable for the analysis of various types of water samples. The subsequent two electrodes were based on a glassy carbon electrode using polypyrrole (PPy) doped with NO3− ions as the ion carrier. The electrodes differed in their solid contact layer, which, in the case of the electrode presented in the publication [28], was a pericarpium granati-derived biochar (PGCP) activated with phosphoric acid. PGCP was applied together with PPy, forming a bilayer membrane. Based on morphological studies PGCP was found to have a porous structure and, on the basis of the EIS measurements, also a low resistance, which translates into the possibility of using it as a transducer material for the charge transfer. In the second article, a double-layer structure consisting of gold nanoparticles (AuNPs) (solid contact) and a conductive polymer PPy-NO3− (ion-selective membrane) was successfully used [29]. AuNPs solid contact layer and nitrate-doped polypyrrole molecularly imprinted polymer membranes were prepared by electrodeposition. Both of these electrodes had a slope that deviated from the ideal Nernstian slope and respectively amounted to −50.86 mV/decade for PGCP-ISE and −50.4 mV/decade for AuNPs-ISE. For the PGCP-modified electrode, the linearity range was in the range of 1.0 × 10−5–5.0 × 10−1 M and the LOD was 4.64 × 10−6 M, which is very similar to the results obtained for the gold nanoparticle-modified electrode, where the LOD was an order of magnitude higher. A positive aspect of the electrode, with solid contact in the form of AuNPs, is the favorable result of the aqueous layer test because no potential drift towards higher values of potentials was observed for this electrode compared to the unmodified AuNPs electrode. Finally, both the PGCP- and AuNPs-modified electrodes exhibited excellent long- and short-term stability and significantly better electrical parameters than the unmodified electrode. The PGCP-ISE was also successfully used for the determination of nitrates in samples from Shenzhen OCT wetland and laboratory wastewater with 4% RSD. In publications [30][31][32] are presented electrodes that were connected by the presence of the same ionophore, which was nitrate ionophore VI. The ISE described by [30] was developed using screen-printing technology by applying inkjet printing gold onto the substrate, thereby forming a screen-printed gold electrode. Afterwards, the entire sensor was secured with Teflon tape and the membrane containing the ionophore was applied. The parameters of this potentiometric sensor were determined, i.e., the slope of the characteristic −54.1 ± 2.1 mV/decade and the linearity range between 1.0 × 10−5 M and 1.0 × 10−1 M. The water layer test was carried out with positive results. Unfortunately, the LOD for the obtained electrode was not included in the publication, and the electrical parameters were not determined. Another article [32] described an SC-ISE that was produced by applying a membrane mixture containing varying amounts of PTFE (poly(tethrafluoroethylene)) (0%, 2.5%, 5%. 7.5%, 10%) onto a screen-printed electrode. The best results were obtained for an ISE in which PTFE constituted 5% of the membrane cocktail. This electrode was characterized by a slope of −58.0 mV/decade, which unfortunately reduced to −35.0 mV/decade after 20 days of measurements, which means that the lifetime of this electrode is not long, and further studies are required to eliminate this drawback. The last discussed electrode using nitrate ionophore VI is an SC-ISE based on the gold electrode (AuE) and thiol-functionalized reduced graphene oxide (TRGO) as solid contact material, which was used for the first time in this role [31]. This electrode was characterized by an almost Nernst response as the slope equaled −60.0 ± 0.5 mV/decade. The range of linearity of the described ISE was similar to electrodes possessing the same type of ionophore. An additional advantage of this SC-ISE is the low detection limit reaching micromole values and the wide working range of pH 2.0–10.0. Moreover, the use of TRGO improved the potential reversibility, which indicates that reduced graphene oxide performs well in the solid contact function due to its good conductivity. In addition, according to the EIS tests, the membrane resistance values of the electrode with TRGO are lower than those for the unmodified electrode. The aqueous layer test came off satisfactorily as no potential drift was observed. After improving the stability of the electrode in real samples, it will be possible to use it for the determination of ions in blood samples. A new ionophore as the active ingredient in an ion-selective membrane used in the construction of nitrate(V) ion-selective electrodes was proposed by outhe researchers' group in the publication [33]. In the construction of SC-ISEs, a cobalt(II) complex with 4,7-diphenyl-1,10-phenanthroline (Co(Bphen)2(NO3)2(H2O)2) was used, which performed very well in this role and cooperated with the Ag|AgCl inner electrode. Trihexyltetradecylphosphonium chloride was selected as the ionic component of the membrane, which provided a constant concentration of chloride ions and represented a reversible redox system with the internal electrode Ag/AgCl/Cl-(silver/silver chloride electrode/chloride). A satisfying electrode response was obtained for this ISE, which was characterized by a slope of −56.34 mV/decade, a linearity range of 1.0 × 10−5–1.0 × 10−1 M and an LOD lower than the micromole value. The electrode exhibited a constant potential over a wide pH range of 5.4–10.6. Furthermore, the reversibility of the potential and its drift were at a very satisfactory level. This electrode was successfully applied to the determination of the concentration of NO3− ions in tap, mineral and river water samples with reproducibility close to 100%. The same ionophore was used in an ion-selective electrode based on a glassy carbon electrode [34]. A nanocomposite consisting of multi-walled carbon nanotubes and the ionic liquid trihexyltetradecylphosphonium chloride (THTDPCl) was proposed as a solid contact and used as a membrane component. Various composites obtained from MWCNTs differing in structure were tested. The electrode with a nanocomposite containing nanotubes characterized by the highest porosity and homogeneity of the structure showed the best performance. In comparison to the previous electrodes with the same ionophore [33] based on Ag/AgCl/Cl, the electrode with a nanocomposite achieved a better slope closer to Nernst’s value, which was −57.1 mV/decade, a lower detection limit of 5.0 × 10−7 M and a linearity range that was an order of magnitude larger, as well as a wider working pH range. Interestingly, the addition of the nanocomposite directly to the membrane did not cause the redox sensitivity of the electrodes. Moreover, this simultaneously increased their hydrophobicity, so that no water film formation was observed between the membrane and the inner electrode in these SC-ISEs. The ionophore in the form of TDANO3 (tetradecylammonium nitrate) was used both in a nitrate ion-selective electrode with a solid contact [35] and a liquid contact [36]. In the solid-contact electrode, the leading electrode was a screen-printed carbon electrode, and the solid-contact layer was a reduced graphite oxide aerogel (rGOA) [35]. A Nernst response of −59.1 mV/decade, a linearity range of 0.1 M to micromoles and a detection limit of 7.59 × 10−7 M were obtained. With success, this electrode was used for the determination of NO3− ions in perilla leaves, where the results achieved by ISE were compared with chromatographic results. In the case of liquid contact, the inner electrode was one of the more commonly selected Ag|AgCl electrodes and the inner electrolyte—solution of 0.01 M KNO3 or 0.001 M KCl [36]. There is no doubt that the team carrying out the study on SC-ISEs succeeded in improving the parameters obtained for the competing electrode with LC. The slope of the characteristic differed from the theoretical value and was −53.7 ± 0.4 mV/decade, and, additionally, the values for linearity range and LOD were less satisfactory, so in this case the solid-contact ISE worked much more effectively. In order to improve the performance of nitrate ion-selective electrodes with solid contact, the application of a nitron–nitrate complex (Nit+/NO3−) that acted as an ionophore was proposed [37]. A novel form of an ion-selective membrane that had the shape of a ‘sandwich membrane’ (bilayer membrane) was presented. This type of membrane was formed by pressing together two previously dried membranes, where one of them contained an ionophore and the other did not. The membrane prepared in this way was then applied onto the surface of the glassy carbon electrode with the non-ionophore side, where the part with the ionophore had direct contact with the testing sample. Multi-walled carbon nanotubes were used as the solid contact. The presented electrode showed a very wide linearity range of 8.0 × 10−8–1.0 × 10−1 M, and a low LOD of 2.8 × 10−8 M. The response of the GCE/MWCNTs/NO3−-ISM electrode was equal to −55.1 ± 2.1 mV/dec. The working pH range of such an electrode was between 3.5 and 10.0 pH and the lifetime was 8 weeks. The proposed ISE exhibited a very high tolerance to the presence of interfering ions, as we could observe from the rather low values of the selectivity coefficients. However, the authors did not report a value of this parameter for highly lipophilic ions, i.e., ClO4− or SCN−, which are well-known interferents for nitrate electrodes. The ISE was successfully used to measure the concentration of nitrate ions in wastewater samples. The article [38] presents liquid-contact nitrate ion-selective electrodes used for the determination of nitrates in hydroponic solutions. A design with two internal solutions and two membranes was used. The internal electrode was a silver chloride electrode placed in a PP tube filled with 0.1 M LiCl, which ended with a Nafion membrane—this constituted part 1. Part 1 was immersed in part 2, which was a second PP tube that ended with a PVC membrane, which, in turn, contained a 0.1 M LiNO3 solution (Figure 5). The ionophore included in the PVC membrane was THANO3 (tetraheptylammonium nitrate), which had already been used in nitrate ion-selective electrodes. On the basis of the calibration curve, a slope of −53.3 ± 0.1 mV/decade was determined, which deviates slightly from the ideal value for monovalent ions. The range of linearity and the detection limit took on average values and did not stand out from other nitrate electrodes. It was concluded that this type of electrode could be used in studies of NO3− ions for more than four weeks in order to determine their concentration in hydroponic solutions. The paper [39] presents a new form of solid contact in the form of TTF-TCNQ (tetrahiafulvalene-tetracyanoquinodimethane) illustrated in Figure 6. Here, the inner electrode was a glassy carbon disc onto which an intermediate layer was applied, which was followed by an ion-selective membrane containing an active ingredient, which was a nitrate ionophore V. The electrode showed great potential stability and was not sensitive to changes in the redox potential. The response of the electrode was almost Nernstian and amounted to −58.47 mV/decade, while the LOD was equal to 1.6 × 10−6 M. The presence of the proposed solid contact improved the selectivity of the nitrate electrodes and their electrical parameters, i.e., decreased the membrane resistance and increased the electrical capacitance.A comparison of the analytical parameters of various nitrate electrodes is presented in Table 1.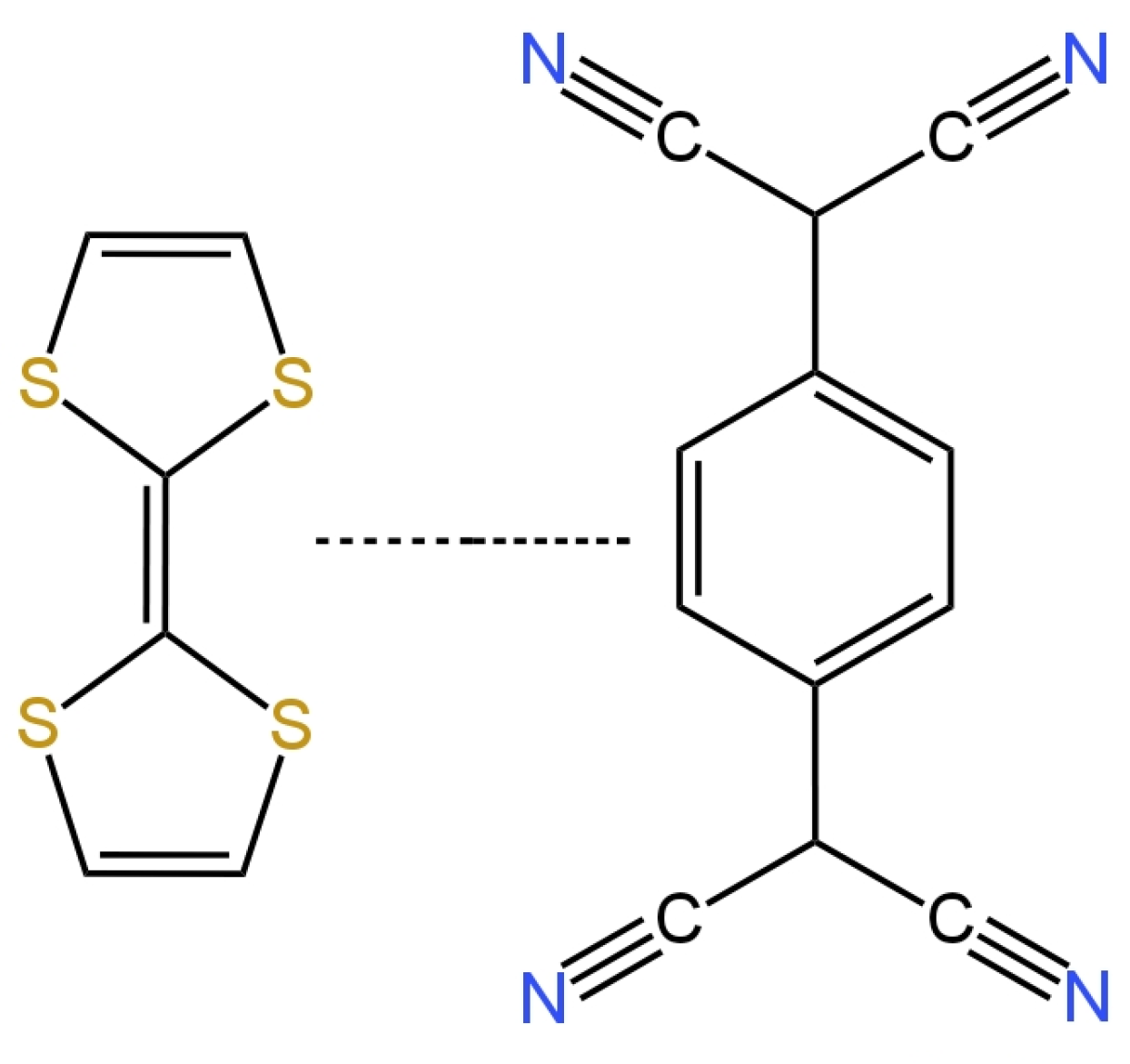 Figure 6.Tetrathiafulvalene 7,7,8,8-tetracyanoquinodimethane TTF-TCNQ salt used as solid contact in nitrate SC-ISE[39].Table 1.Nitrate ion-selective electrodes.
Figure 6.Tetrathiafulvalene 7,7,8,8-tetracyanoquinodimethane TTF-TCNQ salt used as solid contact in nitrate SC-ISE[39].Table 1.Nitrate ion-selective electrodes.E. No
Type of Contact
Ionophore/Ion Carrier
Intermediate/Transducer Layer
Type of Internal Electrode
Slope [mV/decade]
Range of Linearity [M]
Limit of Detection [M]
pH Range
Application/Samples
References
1
SC
TDMAN
Graphite
GrE
−55.4 ± 0.7
2.9 × 10−4–1.7 × 10−1
2.04 × 10−4
4.0–11.0
Industrial and environmental.
[22]
2
SC
TDMAN
LIG
-
−58.2 ± 4.2
5.0 × 10−4–1.0 × 10−1
6.01 × 10−6
6.0–8.0
Agricul-ture and surface water.
[23]
3
SC
TDMAN
Co3O4NPs
SPCE
−56.8
1.0 × 10−7–1.0 × 10−2
1.04 × 10−8
3.0–8.0
Aquaculture, river,
domestic and tap
water.
[27]
4
SC
TDMAN
PANINFs-Cl
GCE
−56.8
1.0 × 10−6–1.0 × 10−1
3.16 × 10−7
4.0–12.5
Environmental
samples.
[25]
5
SC
TDMAN
PANINFs-NO3
−57.8
1.0 × 10−6–1.0 × 10−1
1.12 × 10−6
4.0–11.5
6
SC
TDMAN
MCB
Ag wire
−54.8
5.0 × 10−5–1.0 × 10−1
2.5 × 10−6
-
-
[26]
7
SC
TDMAN
POT-MoS2
AuE
−64.0
7.1 × 10−4–1.0 × 10−1
9.2 × 10−5
-
Soil.
[24]
8
SC
PPy-NO3−
PGCP
GCE
−50.9
1.0 × 10−5–5.0 × 10−1
4.64 × 10−6
3.5–9.5
Environmental and clinical laboratories.
[28]
9
SC
PPy-NO3−
AuNPs
GCE
−50.4
5.3 × 10−5–1.0 × 10−1
5.25 × 10−5
-
Water samples and aqueous solutions of fertilizers.
[29]
10
SC
Nitrate
ionophore VI
-
PAuE
−54.1
5.0 × 10−5–1.0 × 10−1
-
-
Field soils.
[30]
11
SC
Nitrate
ionophore VI
TRGO
AuE
−60.0 ± 0.5
4.0 × 10−5–1.0 × 10−1
4.0 × 10−6
2.0–10.0
Blood.
[31]
12
SC
Nitrate
ionophore VI
PTFE
SPCE
−58.0
-
-
4.0–11.0
Wastewater.
[32]
13
SC
Co(Bphen)2(NO3)2(H2O)2
MWCNTs-THTDPCl
GCE
−57.1
1.0 × 10−6–1.0 × 10−1
5.0 × 10−7
6.0–8.0
-
[34]
14
SC
Co(Bphen)2(NO3)2(H2O)2
Ag/AgCl/Cl−
Ag|AgCl
−56.3
1.0 × 10−5–1.0 × 10−1
3.98 × 10−6
5.4–10.6
Mineral, tap and river water.
[33]
15
SC
TDANO3
rGOA
SPCE
−59.1
1.0 × 10−6–1.0 × 10−1
7.59 × 10−7
-
Plant sap e.g., perilla leaf.
[35]
16
LC
TDANO3
0.01 M KNO3 and 0.001 M KCl
Ag|AgCl
−53.7 ± 0.4
1.0 × 10−5–1.0 × 10−1
1.3 × 10−6
-
-
[36]
17
SC
Nit+/NO3−
MWCNTs
GCE
−55.1 ± 1.0
8.0 × 10−8–1.0 × 10−2
2.8 × 10−8
3.5–10.0
Environmental
samples.
[37]
18
LC
THANO3
0.1 M LiCl and 0.1 M LiNO3
Ag|AgCl
−53.3 ± 1.0
1.0 × 10−5–1.0 × 10−1
1.0 × 10−6
-
Hydroponic solutions.
[38]
19
SC
Nitrate ionophore V
TTF-TCNQ
GCD
−58.5
1.0 × 10−5–1.0 × 10−1
1.6 × 10−6
-
Aqueous samples.
[39]
-
-
-
References
- Morf, W.E. The Principles of Ion-Selective Electrodes and of Membrane Transport; Elsevier: New York, NY, USA, 1981.
- Głąb, S.; Maj-Żurawska, M.; Hulanicki, A. Ion-Selective Electrodes|Glass Electrodes. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013.
- Sohail, M.; De Marco, R. ELECTRODES|Ion-Selective Electrodes. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013.
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128.
- Paczosa-Bator, B.; Cabaj, L.; Piech, R.; Skupień, K. Platinum Nanoparticles Intermediate Layer in Solid-State Selective Electrodes. Analyst 2012, 137, 5272.
- Schwarz, J.; Trommer, K.; Mertig, M. Solid-Contact Ion-Selective Electrodes Based on Graphite Paste for Potentiometric Nitrate and Ammonium Determinations. Am. J. Anal. Chem. 2018, 9, 591–601.
- Wardak, C.; Morawska, K.; Paczosa-Bator, B.; Grabarczyk, M. Improved Lead Sensing Using a Solid-Contact Ion-Selective Electrode with Polymeric Membrane Modified with Carbon Nanofibers and Ionic Liquid Nanocomposite. Materials 2023, 16, 1003.
- Wardak, C.; Pietrzak, K.; Morawska, K.; Grabarczyk, M. Ion-Selective Electrodes with Solid Contact Based on Composite Materials: A Review. Sensors 2023, 23, 5839.
- Wardak, C. Solid Contact Cadmium Ion-Selective Electrode Based on Ionic Liquid and Carbon Nanotubes. Sens. Actuators B Chem. 2015, 209, 131–137.
- Wardak, C.; Pietrzak, K.; Morawska, K. Nanocomposite of Copper Oxide Nanoparticles and Multi-Walled Carbon Nanotubes as a Solid Contact of a Copper-Sensitive Ion-Selective Electrode: Intermediate Layer or Membrane Component–Comparative Studies. Appl. Nanosci. 2023.
- Ma, S.; Wang, Y.; Zhang, W.; Wang, Y.; Li, G. Solid-Contact Ion-Selective Electrodes for Histamine Determination. Sensors 2021, 21, 6658.
- Bobacka, J. Conducting Polymer-Based Solid-State Ion-Selective Electrodes. Electroanalysis 2006, 18, 7–18.
- Lenik, J.; Wesoły, M.; Ciosek, P.; Wróblewski, W. Evaluation of Taste Masking Effect of Diclofenac Using Sweeteners and Cyclodextrin by a Potentiometric Electronic Tongue. J. Electroanal. Chem. 2016, 780, 153–159.
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351.
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; de Rooij, N.F.; Pretsch, E. Potential Drifts of Solid-Contacted Ion-Selective Electrodes Due to Zero-Current Ion Fluxes Through the Sensor Membrane. Electroanalysis 2000, 12, 1286–1292.
- Bobacka, J.; Lindfors, T.; McCarrick, M.; Ivaska, A.; Lewenstam, A. Single-Piece All-Solid-State Ion-Selective Electrode. Anal. Chem. 1995, 67, 3819–3823.
- Wardak, C.; Grabarczyk, M. Single-Piece All-Solid-State Co(II) Ion-Selective Electrode for Cobalt Monitoring in Real Samples. Int. Agrophys 2019, 1, 17–24.
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937.
- Lindner, E.; Umezawa, Y. Performance Evaluation Criteria for Preparation and Measurement of Macro- and Microfabricated Ion-Selective Electrodes (IUPAC Technical Report). Pure Appl. Chem. 2008, 80, 85–104.
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26.
- Bobacka, J.; Lewenstam, A.; Ivaska, A. Equilibrium Potential of Potentiometric Ion Sensors under Steady-State Current by Using Current-Reversal Chronopotentiometry. J. Electroanal. Chem. 2001, 509, 27–30.
- Paré, F.; Visús, A.; Gabriel, G.; Baeza, M. Novel Nitrate Ion-Selective Microsensor Fabricated by Means of Direct Ink Writing. Chemosensors 2023, 11, 174.
- Hjort, R.G.; Soares, R.R.A.; Li, J.; Jing, D.; Hartfiel, L.; Chen, B.; Van Belle, B.; Soupir, M.; Smith, E.; McLamore, E.; et al. Hydrophobic Laser-Induced Graphene Potentiometric Ion-Selective Electrodes for Nitrate Sensing. Microchim. Acta 2022, 189, 122.
- Ali, A.; Wang, X.; Chen, Y.; Jiao, Y.; Mahal, N.K.; Moru, S.; Castellano, M.J.; Schnable, J.C.; Schnable, P.S.; Dong, L. Continuous Monitoring of Soil Nitrate Using a Miniature Sensor with Poly(3-Octyl-Thiophene) and Molybdenum Disulfide Nanocomposite. ACS Appl. Mater. Interfaces 2019, 11, 29195–29206.
- Pietrzak, K.; Wardak, C.; Malinowski, S. Application of Polyaniline Nanofibers for the Construction of Nitrate All-Solid-State Ion-Selective Electrodes. Appl. Nanosci. 2021, 11, 2823–2835.
- Zhang, Z.; Papautsky, I. Miniature Ion-selective Electrodes with Mesoporous Carbon Black as Solid Contact. Electroanalysis 2021, 33, 2143–2151.
- Thuy, N.T.D.; Wang, X.; Zhao, G.; Liang, T.; Zou, Z. A Co3O4 Nanoparticle-Modified Screen-Printed Electrode Sensor for the Detection of Nitrate Ions in Aquaponic Systems. Sensors 2022, 22, 9730.
- Fozia; Zhao, G.; Nie, Y.; Jiang, J.; Chen, Q.; Wang, C.; Xu, X.; Ying, M.; Hu, Z.; Xu, H. Preparation of Nitrate Bilayer Membrane Ion-Selective Electrode Modified by Pericarpium Granati-Derived Biochar and Its Application in Practical Samples. Electrocatalysis 2023, 14, 534–545.
- Zhang, L.; Wei, Z.; Liu, P. An All-Solid-State NO3− Ion-Selective Electrode with Gold Nanoparticles Solid Contact Layer and Molecularly Imprinted Polymer Membrane. PLoS ONE 2020, 15, e0240173.
- Baumbauer, C.L.; Goodrich, P.J.; Payne, M.E.; Anthony, T.; Beckstoffer, C.; Toor, A.; Silver, W.; Arias, A.C. Printed Potentiometric Nitrate Sensors for Use in Soil. Sensors 2022, 22, 4095.
- Liu, Y.; Liu, Y.; Meng, Z.; Qin, Y.; Jiang, D.; Xi, K.; Wang, P. Thiol-Functionalized Reduced Graphene Oxide as Self-Assembled Ion-to-Electron Transducer for Durable Solid-Contact Ion-Selective Electrodes. Talanta 2020, 208, 120374.
- Fan, Y.; Huang, Y.; Linthicum, W.; Liu, F.; Beringhs, A.O.; Dang, Y.; Xu, Z.; Chang, S.-Y.; Ling, J.; Huey, B.D.; et al. Toward Long-Term Accurate and Continuous Monitoring of Nitrate in Wastewater Using Poly(Tetrafluoroethylene) (PTFE)–Solid-State Ion-Selective Electrodes (S-ISEs). ACS Sens. 2020, 5, 3182–3193.
- Pietrzak, K.; Wardak, C.; Łyszczek, R. Solid Contact Nitrate Ion-selective Electrode Based on Cobalt(II) Complex with 4,7-Diphenyl-1,10-phenanthroline. Electroanalysis 2020, 32, 724–731.
- Pietrzak, K.; Wardak, C. Comparative Study of Nitrate All Solid State Ion-Selective Electrode Based on Multiwalled Carbon Nanotubes-Ionic Liquid Nanocomposite. Sens. Actuators B Chem. 2021, 348, 130720.
- Kim, M.-Y.; Lee, J.-W.; Park, D.J.; Lee, J.-Y.; Myung, N.V.; Kwon, S.H.; Lee, K.H. Highly Stable Potentiometric Sensor with Reduced Graphene Oxide Aerogel as a Solid Contact for Detection of Nitrate and Calcium Ions. J. Electroanal. Chem. 2021, 897, 115553.
- Ivanova, A.; Mikhelson, K. Electrochemical Properties of Nitrate-Selective Electrodes: The Dependence of Resistance on the Solution Concentration. Sensors 2018, 18, 2062.
- Hassan, S.S.M.; Eldin, A.G.; Amr, A.E.-G.E.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Improved Solid-Contact Nitrate Ion Selective Electrodes Based on Multi-Walled Carbon Nanotubes (MWCNTs) as an Ion-to-Electron Transducer. Sensors 2019, 19, 3891.
- Fukao, Y.; Kitazumi, Y.; Kano, K.; Shirai, O. Construction of Nitrate-Selective Electrodes and Monitoring of Nitrates in Hydroponic Solutions. Anal. Sci. 2018, 34, 1373–1377.
- Pięk, M.; Piech, R.; Paczosa-Bator, B. TTF-TCNQ Solid Contact Layer in All-Solid-State Ion-Selective Electrodes for Potassium or Nitrate Determination. J. Electrochem. Soc. 2018, 165, B60–B65.