Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Jessie Wu and Version 2 by Jessie Wu.
Considerable changes have been observed in surface waters’ quality. They include an increase in dissolved organic carbon (DOC) concentrations, as well as a shift of natural organic matter (NOM) composition in favor of low molecular weight (LMW), and they are expected to occur on a wider scale in the future.
- NOM
- surface water
- drinking water treatment
- coagulation
1. Introduction
A significant number of water supply systems providing potable water for local communities globally are fed from surface water sources.
In Europe, between 1990 and 2017, around 75% of water for potable water supply purposes was abstracted from surface water sources such as rivers and artificial reservoirs [1]. Similarly, in the United States, more than 60% of drinking water produced in 2015 originated from surface water sources [2], and in Canada over 85% of potable water processed by Drinking Water Treatment Plants (DWTPs) in 2019 was abstracted from surface water sources [3]. The quality of surface water is important, among other concerns, due to its widespread use as the main potable water source for numerous communities worldwide.
While specific targets for surface water treatment may vary depending on source water quality and regulatory standards, the parameters that typically require reducing are the same for most surface waters and include turbidity, color, and the content of natural organic matter (NOM).
Current surface water purification methods include adsorption, membrane filtration, ion exchange, advanced oxidation processes (AOPs), and biological processes [4][5]. However, traditional coagulation is still the most prevalent [5][6]. The conventional surface water treatment system consists of coagulation followed by sedimentation, filtration, and oftentimes activated carbon adsorption (Figure 1) [6].
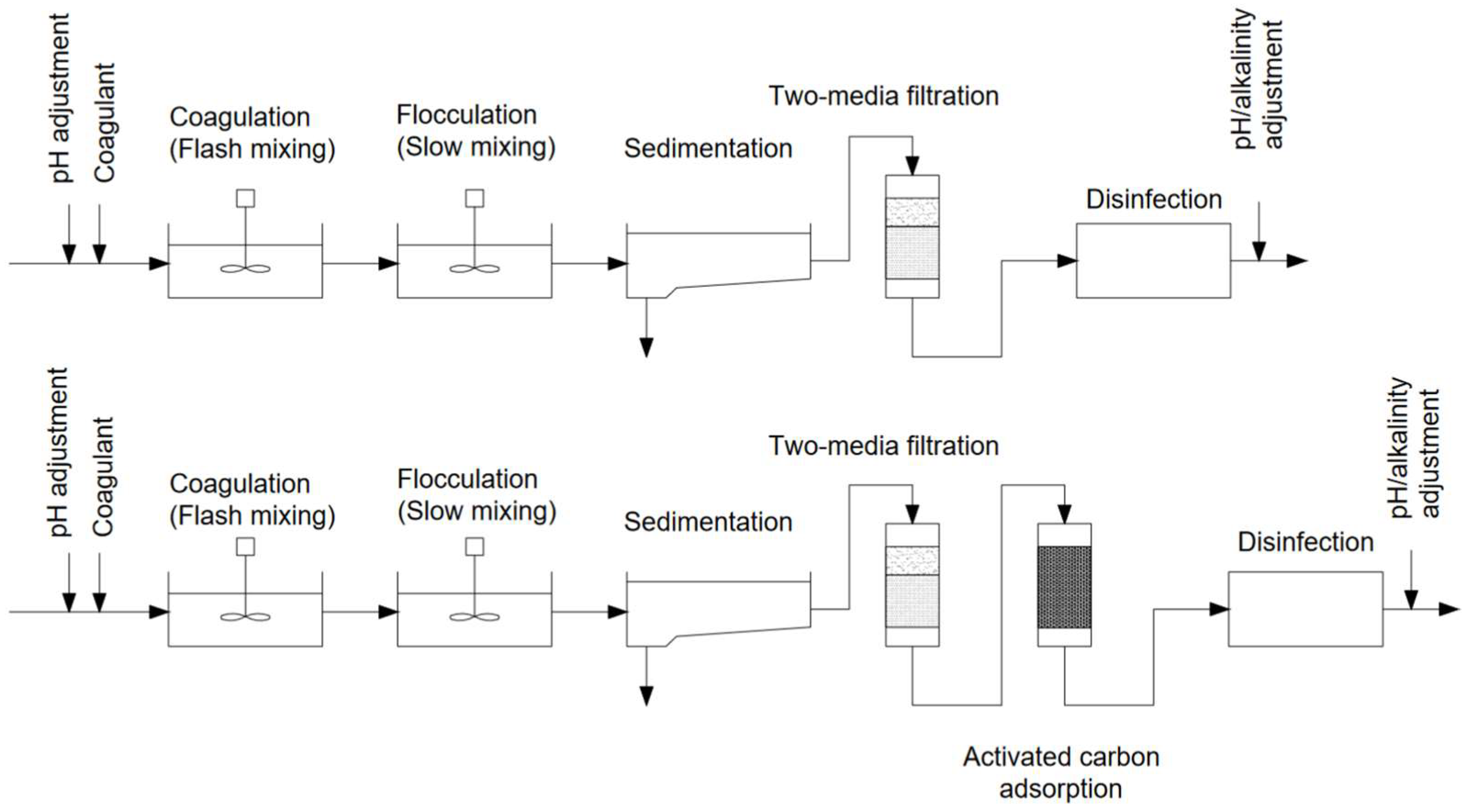
Figure 1. Conventional surface water treatment system solutions (adapted from Ghernaout D. [6]).
Although coagulation has been applied as the main surface water treatment process in DWTPs across the world for decades, there are still a number of issues that researchers investigate. They include the application of ecologically friendly biocoagulants, the development of novel hybrid coagulants, and the possibility of increasing the efficiency of surface water purification by combining coagulation with different unit water treatment processes [5][7][8][9]. Such research is necessary not only for the purpose of overcoming known limitations and disadvantages of conventional coagulation, but also to ensure its continuous applicability in the context of rising challenges [7].
The most pressing problems associated with water treatment via coagulation appear to be the vulnerability of the process to changing NOM concentration and composition, and the consequences of employing metallic salts as coagulants [7][10][11]. Revisiting the topic of surface water purification via coagulation with consideration of both the current and anticipated challenges is necessary.
2. Characteristics of Natural Organic Matter
Understanding NOM background and composition plays an important part in potable water treatment. Characterization of NOM compounds is important because their diverse properties condition their treatability by different unit water purification processes. A better understanding of NOM, its concentration, composition, and character is therefore crucial for identifying the process most suitable for NOM removal [4][5][12].
The term “natural organic matter” refers to a complicated mixture of organic compounds naturally occurring in both surface and ground waters. Its presence in natural waters is a direct result of hydrological, geological, and biological cycles [4][5][13].
Assessing the characteristics of NOM in detail is a difficult task, mostly due to its broad range of molecular weight distribution, complex structure, and chemistry of its components [12].
Its compounds differ in polarity, acidity, charge density, as well as molecular mass, and can be broadly classified based on their polarity into two main groups: hydrophilic and hydrophobic components [4][5][6][12][13]. The hydrophilic fractions of NOM primarily comprise nitrogenous compounds and aliphatic carbon, while hydrophobic NOM mostly consists of humic substances, and typically accounts for over half of the total organic carbon in raw water [4][5].
Natural organic matter can also be classified based on its origin. Autochthonous NOM refers to natural organic matter originating from within the water source through biological activity, while allochthonous NOM is generated in a distant place and is introduced to the water source through drainage within watersheds [4][5][14].
NOM is a structure of high complexity and heterogeneity which is why numerous studies focus on the classification of NOM and methods of its characterization in reference to potable water purification [14][15]. Procedures currently used for NOM characterization include resin adsorption, size exclusion chromatography (SEC), nuclear magnetic resonance (NMR), spectroscopy, and fluorescence spectroscopy [12].
A summary of the major characteristic classes of NOM can be found in the work by Adusei-Gyamfi et al. [14].
3. Natural Organic Matter-Related Problems in Water Treatment
NOM is not inherently toxic, although its presence in drinking water sources is undesirable due to its negative effect on the organoleptic properties of water such as color, taste, and odor. Moreover, it affects the quality of water by acting as a carrier of toxic pollutants such as pesticides and radionuclides. It may also influence the concentrations of dissolved oxygen, nitrogen, phosphorus, and sulfur [4][5][14]. Furthermore, the biodegradable portion of NOM can have a negative impact on the biological stability of water, potentially inducing bacterial regrowth in the water distribution systems [16].
Even more importantly, NOM present in the surface water is a known precursor to the formation of highly detrimental disinfection by-products (DBPs), among which trihalomethanes (THMs) are most prevalent. Organics present in raw water react with disinfectants, particularly chlorine, giving rise to the formation of DBPs [4][5][17]. The reactivity of organic pollutants with disinfectants largely depends on the size, molecular weight, and hydrophobicity of NOM components, although hydrophilic compounds typically produce fewer DBPs than hydrophobic fractions. All molecular weight fractions of NOM can cause DBP formation, although it is mostly hydrophilic. LMWow molecular weight (LMW) constituents of NOM account for a major proportion of DBPs, including THMs, in conventionally treated waters [18][19].
The presence of THMs is highly undesirable. Several studies have observed a correlation between the consumption of chlorinated drinking water and an increased risk of cancer. Therefore, THMs are currently the most widely investigated DPBs regarding their adverse effects on human health [17][20][21].
Considering the carcinogenic potential of THMs, increasing concentrations of DPBs precursors in surface water are a matter of concern, especially since, as Kumari et al. [17] point out, reducing THMs formation during the treatment of source water abundant in NOM is not an easy task.
Cool et al. [22] studied the impact of possible future variations in temperature and precipitation associated with climate change on the probability of total trihalomethanes (TTHM) concentrations exceeding a threshold in drinking water in Quebec, Canada. Results of the study showed a low, although considerable, increase in the probability of TTHM concentrations exceeding the threshold over time in the province of Quebec (up to 4.7%).
Given that the progressing climate change significantly influences the quantity and quality of NOM present in potable water sources, and that it is more likely to have an increasingly adverse effect on human health if not properly addressed, the matter of efficient NOM removal is particularly pressing.
References
- European Environment Agency. Use of Freshwater Resources in Europe. 2019. Available online: https://www.eea.europa.eu/ims/use-of-freshwater-resources-in-europe-1 (accessed on 9 January 2023).
- Center for Sustainable Systems, University of Michigan. U.S Water Supply and Distribution Factsheet. 2022. Available online: https://css.umich.edu/publications/factsheets/water/us-water-supply-and-distribution-factsheet (accessed on 9 January 2023).
- Statistics Canada. Table 38-10-0992-01 Potable Water Volumes Processed by Drinking Water plants, by Source Water Type. 2021. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=3810009201 (accessed on 13 August 2023).
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76.
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71.
- Ghernaout, D. Water treatment coagulation: Dares and trends. Open Access Libr. J. 2020, 7, 1–18.
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.C.; Assadi, A.A.; Amrane, A.; Mouni, L. Comparison of Four Plant-Based Bio-Coagulants Performances against Alum and Ferric Chloride in the Turbidity Improvement of Bentonite Synthetic Water. Water 2022, 14, 3324.
- Chen, S.; Yuan, Z.; Hanigan, D.; Westerhoff, P.; Zhao, H.; Ni, J. Coagulation behaviors of new covalently bound hybrid coagulants (CBHyC) in surface water treatment. Sep. Purif. Technol. 2017, 192, 322–328.
- Marais, S.; Ncube, E.J.; Msagati, T.A.M.; Mamba, B.B.; Nkambule, T. Comparison of natural organic matter removal by ultrafiltration, granular activated carbon filtration and full scale conventional water treatment. J. Environ. Chem. Eng. 2018, 6, 6282–6289.
- Anderson, L.; DeMont, I.; Dunnington, D.; Bjorndahl, P.; Redden, D.; Brophy, M.; Gagnon, G. A review of long-term change in surface water natural organic matter concentration in the northern hemisphere and the implications for drinking water treatment. Sci. Total Environ. 2023, 858, 159699.
- Slavik, I.; Kostrowski, D.; Uhl, W. Effect of solar radiation on natural organic matter composition in surface waters and resulting impacts on drinking water treatment. Environ. Technol. 2023, 44, 1549–1565.
- Loganathan, P.; Gradzielski, M.; Bustamante, H.; Vigneswaran, S. Progress, challenges, and opportunities in enhancing NOM flocculation using chemically modified chitosan: A review towards future development. Environ. Sci. Water Res. Technol. 2019, 6, 45–61.
- Ghernaout, D. Enhanced coagulation: Promising findings and challenges. Open Access Libr. J. 2020, 7, 1–19.
- Adusei-Gyamfi, J.; Ouddane, B.; Rietveld, L.; Cornard, J.P.; Criquet, J. Natural organic matter-cations complexation and its impact on water treatment: A critical review. Water Res. 2019, 160, 130–147.
- Sciscenko, I.; Arques, A.; Micó, P.; Mora, M.; García Ballesteros, S. Emerging applications of EEM-PARAFAC for water treatment: A concise review. Chem. Eng. J. Adv. 2022, 10, 100286.
- He, H.; Li, T.; He, C.; Chen, J.; Chu, H.; Dong, B. Removal of natural organic matter in full-scale conventional and advanced water treatment plants: Assimilable organic carbon and its precursors. Chem. Eng. J. Adv. 2021, 8, 100183.
- Kumari, M.; Gupta, S.K. Cumulative human health risk analysis of trihalomethanes exposure in drinking water systems. J. Environ. Manag. 2022, 321, 115949.
- Beauchamp, N.; Bouchard, C.; Dorea, C.; Rodriguez, M. Ultraviolet absorbance monitoring for removal of DBP-precursor in waters with variable quality: Enhanced coagulation revisited. Sci. Total Environ. 2020, 717, 137225.
- Wang, P.; Ding, S.; Xiao, R.; An, G.; Fang, C.; Chu, W. Enhanced coagulation for mitigation of disinfection by-product precursors: A review. Adv. Colloid Interface Sci. 2021, 296, 102518.
- Diana, M.; Felipe-Sotelo, M.; Bond, T. Disinfection byproducts potentially responsible for the association between chlorinated drinking water and bladder cancer: A review. Water Res. 2019, 162, 492–504.
- Evlampidou, I.; Font-Ribera, L.; Rojas-Rueda, D.; Gracia-Lavedan, E.; Costet, N.; Pearce, N.; Vineis, P.; Jaakkola, J.J.K.; Delloye, F.; Makris, K.C.; et al. Trihalomethanes in drinking water and bladder cancer burden in the European Union. Environ. Health Perspect. 2020, 128, 17001.
- Cool, G.; Delpla, I.; Gagnon, P.; Lebel, A.; Sadiq, R.; Rodriguez, M.J. Climate change and drinking water quality: Predicting high trihalomethane occurrence in water utilities supplied by surface water. Environ. Model. Softw. 2019, 120, 104479.
More
