Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Amílcar Duarte.
Pruning is a common practice in citrus for various reasons. These include controlling and shaping the canopy; improving phytosanitary health, productivity, and fruit quality; and facilitating operations such as harvesting and phytosanitary treatments. Because pruning is an expensive operation, its need is sometimes questioned. However, it has been proven to be particularly important in Mediterranean citriculture, which is oriented towards producing fruits for a high-quality demanding fresh market.
- canopy management
- training system
- formative pruning
- maintenance pruning
- mechanical pruning
1. Introduction
Pruning is a common cultural practice in citrus and one of the most expensive orchard maintenance operations. Even so, technical information on citrus pruning is relatively scarce, especially in peer-reviewed scientific journals.
In the Scopus database, there are only 70 articles on citrus pruning [Search by: TITLE-ABS-KEY “citrus” OR “mandarin” OR “poncirus” AND TITLE “pruning”]. In this database, a yearly average of only four papers were published in the last ten years; none of these was a review. Therefore, a review of the cutting methods and the needs of the main citrus species and cultivars and a critical analysis of pruning are needed to put together and summarize the current knowledge on and technical aspects of citrus pruning.
Citrus fruits are cultivated worldwide, but the technologies that can be used depend on the edaphoclimatic conditions and production objectives. The Mediterranean basin is a subtropical area where citrus have adapted and have significant economic and cultural importance. Moreover, citrus are now an essential part of the Mediterranean landscape and diet [1,2][1][2].
2. General Aspects of Citrus Morphology and Physiology
Pruning allows tree growth management and development using cuts and affects the plant’s physiological behavior [6][3]. Therefore, understanding the general aspects of morphology and physiology is essential for designing appropriate pruning actions.
2.1. Citrus Phenological Cycle
The plant’s phenological cycle must always be considered when planning to prune it; thus, knowing the stages of the citrus plant’s phenological cycle, such as shoot growth, flowering, and fruit development, is fundamental (Figure 1).

Figure 1.
Summarized citrus phenological cycle in subtropical climates (Stages I, II, and III refer to the different fruit development stages).
2.1.1. Shoot Formation and Flowering
In citrus, flowering and vegetative growth depend on the formation of shoots, which arise from apical and axillary buds. Shoots can be of five different types, as illustrated in Figure 2: (a) Multi-flower mixed shoots (MFM), with more than one flower and at least one leaf; (b) single-flower mixed shoots (SFM), with only one terminal flower and at least one leaf; (c) multi-flower generative shoots (MFG), with more than one flower and no leaves; (d) single-flower generative shoots (SFG), with one single flower and no leaves; and (e) vegetative shoots (V), without flowers and only with at least one leaf. Types (a) and (b) can be grouped under the designation of mixed shoots, and types (c) and (d) are both generically called generative shoots.
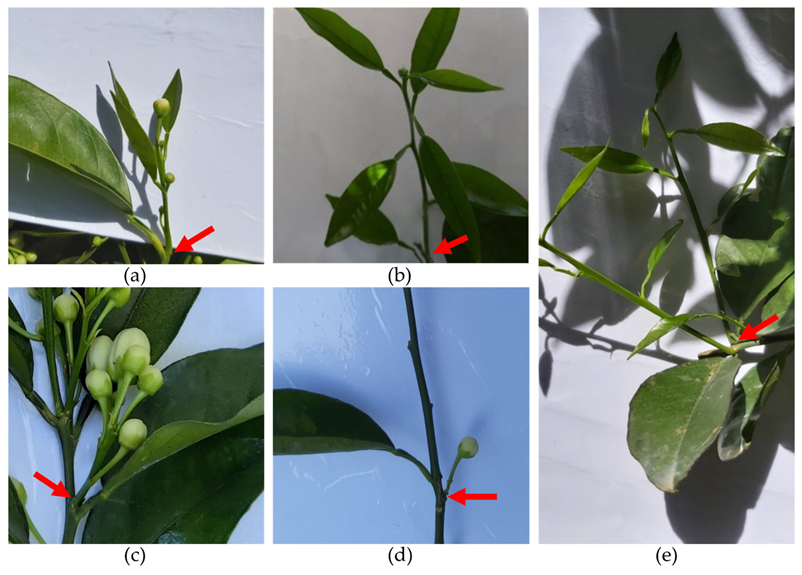
Figure 2. Types of shoots in citrus: (a) Multi-flower mixed shoot (MFM); (b) single-flower mixed shoot (SFM); (c) multi-flower generative shoot (MFG); (d) single-flower generative shoot (SFG); (e) vegetative shoot (V). The arrows mark the shoots’ insertions.
In subtropical climates, there are usually three periods of shoot growth per year: spring, summer, and autumn (Figure 1). Flowering is promoted by the low winter temperatures; in most cultivars, the plant blooms only in spring. In summer and autumn, only vegetative growth occurs. Nonetheless, there are some everblooming cultivars wherein flowering occurs in all shoot growth flushes, such as some lemon and lime cultivars [7,8][4][5]. Pruning may lead to earlier shoot formation [9,10,11][6][7][8]. The application of growth regulators can also affect shoot growth flushes [12][9].
In subtropical climates, flowering and other related aspects can be used to group cultivars according to their bearing habits: (i) single-annual-bearing cultivars (SB), with regular production only once a year, every year; (ii) multiple-annual-bearing cultivars (MB) that produce more than once a year, every year; and (iii) alternate-bearing cultivars (AB) with abundant yields one year and scarce or no yields the next.
The shoots formed in spring develop from the axillary buds of the previous year’s branches that were formed in summer and autumn (Figure 3) [7,13][4][10]. At sprouting, one or more shoots can emerge from each node. The proportion of nodes where the buds remain dormant is higher in the older branches [7,14][4][11].

Figure 3. Shoots formed in different shoot growth flushes: (a) Vegetative shoot formed in autumn and (b) several generative and mixed shoots formed during spring from the axillary buds of shoot (a).
2.1.2. Fruit Development
Fruit development occurs after flowering and fruit set. Fruit development has three main stages (Figure 1) [15][12]: (i) Stage I—intense cell division takes place, and fruit growth is slow; (ii) Stage II—cell expansion, and fruit growth is fast; and (iii) Stage III—lower fruit growth rate, and fruit maturation occurs.
The fruit development period depends on the species and cultivar (Figure 1), which can be of three types: (i) early maturing cultivars, for which the fruit development period takes seven months or less (harvesting is carried out more than four months before the next year’s flowering); (ii) mid-season cultivars, for which the fruit development period is between 8 and 12 months (harvesting for year one is carried out before the flowering in year two, or slightly later when delayed); and (iii) late maturing cultivars, for which the fruit development period is longer than 12 months (harvesting for year one is carried out one to three months after the flowering in year two).
2.2. Shooting Habit
Shooting habit refers to the way trees form new shoots. According to this, citrus cultivars can be classified on the basis of three shooting habits (Figure 4): (a) short multiple shoots (SMSs), (b) intermediate shoots (ISs), and (c) long solitary shoots (LSSs).

Figure 4. Shoots from trees of different shooting habit types: (a) SMSs, short multiple shoots (Citrus clementina); (b) IS, intermediate shooting (Citrus sinensis); (c) LSSs, long solitary shoots (Citrus limon).
SMS cultivars form short and usually multiple shoots (more than one shoot per node) (Figure 4a). Mediterranean mandarin (‘Setubalense’, ‘Avana’, and other cultivars) and the clementine group are examples of SMS-shooting-habit trees. These cultivars tend to form dense canopies with very dense foliage.
LSS shooting cultivars form long, usually solitary shoots (only one shoot per node) (Figure 4c). Lemon cultivars and the satsuma group are examples of LSS cultivars. These cultivars tend to form sparse canopies with scattered foliage.
IS cultivars form shoots that are not as long as in LSSs but are longer than those of SMS cultivars; sometimes, they form more than one shoot per node (Figure 4b). Most orange tree cultivars are IS cultivars. These cultivars have less dense canopies than SMS cultivars and are more compact than LSS cultivars.
There are also cultivars exhibiting characteristics that lie somewhere between these main types of shooting habits, and not all the shoots on a tree are characteristic of that shooting type of cultivar.
2.3. Tree Growth—Formation, Distribution, and Accumulation of Reserves
The roots absorb water and nutrients from the soil and move them up to the leaves, where CO2 is photosynthetically reduced to carbohydrates. Those carbohydrates not readily used by the plant’s cell metabolism are stored, mainly in leaves and branches, with a smaller amount being stored in the roots. The maximum amount of carbohydrates the tree stores is reached just before spring flush [16,17][13][14].
The straighter and more vertical the branch is, the more intense the xylem flow rate is [18][15]. High transpiration rates promote a flow rise in the xylem sap and increase photosynthetic rates, thus boosting carbohydrate synthesis and phloem upload. This favors the development of the tree’s straighter and more vertical branches and upper parts.
On the other hand, phloem sap movement is more difficult in horizontal and tortuous branches. Consequently, phloem sap retention and accumulation in the leaves occurs, promoting flower induction and fruiting [19][16]. This principle is the basis for some complementary operations to pruning [20][17] such as branch girdling and bending. Branch girdling is often practiced to enhance fruit set, fruit size, and citrus trees’ yield, especially in mandarins [21][18]. Based on the same principle, branch bending stimulates the formation of a larger number of flowers and fruits [19,22][16][19]
As previously seen, flower shoots arise from the previous year’s shoots (Figure 3). As new growth builds over previous growth, productive branches are increasingly located on the outer part of the canopy. Over time, this increases the tree size, promoting denser foliage outside the canopy that shades the inside. This way, fruits become located only on the outside of the tree canopy, where they are more exposed to wind and sun damage [23][20].
Cutting off part of the plant (e.g., a branch) removes some reserves [24[21][22],25], but the sap that would go to the removed part is redirected to the remaining parts, increasing the plant’s vegetative vigor. Nevertheless, pruned branches tend to be weakened compared to unpruned ones [26][23].
2.4. Types of Branches
According to their function, branches can be classified into (i) structural and (ii) production branches [27][24].
Structural branches are the largest in diameter, include scaffold branches and secondary and tertiary limbs, and form, with the trunk, the skeleton of the tree, determining its shape [5,27][24][25]. These branches do not produce fruits.
Production branches are small-diameter branches and twigs growing from the structural branches. Compared to the structural branches, they have relatively horizontal growth [27][24]. The fruits grow from these branches or their offshoots.
2.5. Tree Growth Habit
A citrus tree is a low-head tree in which scaffold branches arise from the trunk within a 50–60 cm height from the ground level. The branch insertion (crotch) angle with the main trunk differs according to the growth habit of each cultivar. Based on this tendency, citrus cultivars can be classified into three different groups: (a) upright, (b) spreading, and (c) drooping growth habit (Figure 5).
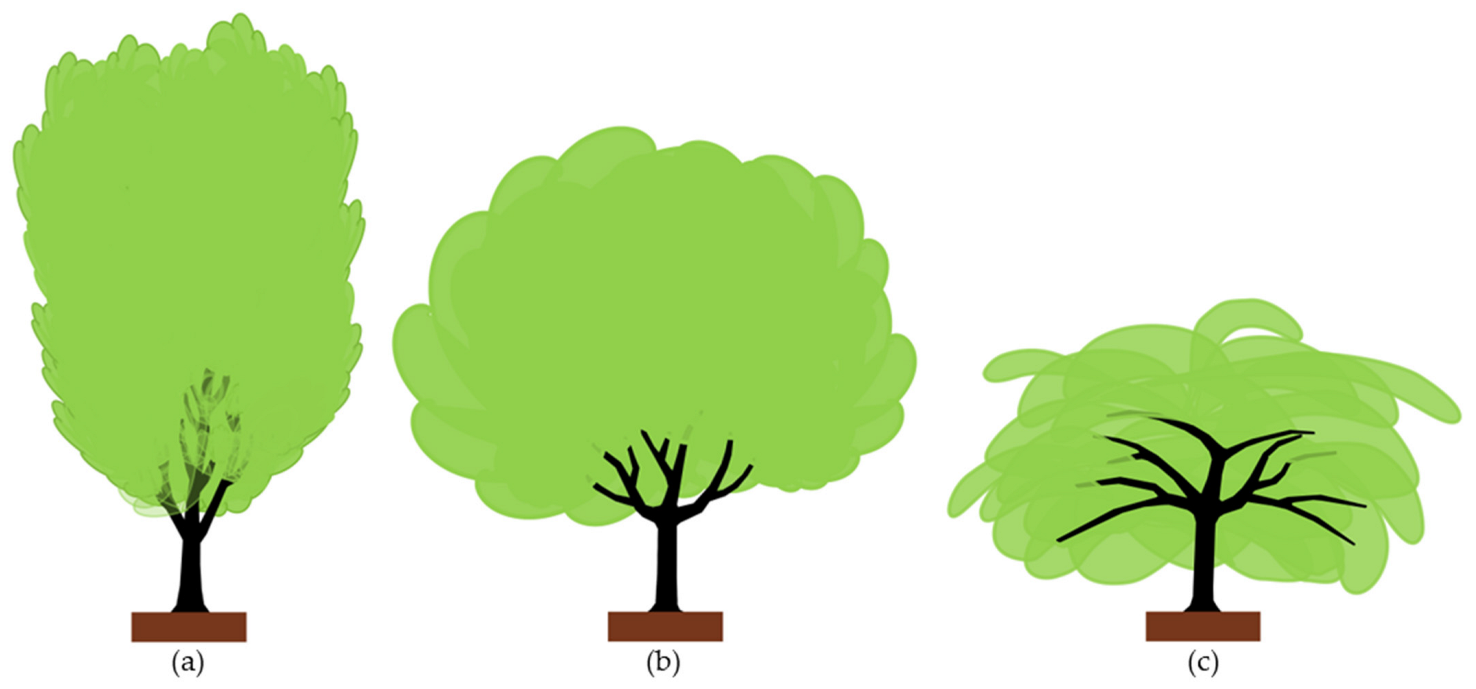
Figure 5.
Types of tree growth habit in citrus: (
a
) Upright; (
b
) spreading; and (
c
) drooping.
Upright cultivars (Figure 5a) form tall vertical branches with acute (narrow) insertion angles. These cultivars tend to form tall canopies. Examples are ‘Marisol’, ‘Salustiana’, and ‘Afourer’ (a.k.a. ‘Nadorcott’) cultivars.
Spreading cultivars (Figure 5b) form lower canopies with less acute branch insertion angles than upright ones. Examples are the “navel” and “blood” orange tree groups, ‘Valencia Late’ orange, and ‘Marsh’ grapefruit.
Drooping cultivars (Figure 5c) form horizontal branches with wider (open) insertion angles, resulting in lower canopies than in upright and spreading cultivars. Examples are the mandarins from the satsuma group, ‘Clemenules’, ‘Fortune’, and ‘Oroblanco’.
2.6. Apical Dominance and Hormonal Relations
Apical dominance refers to the control that the shoot’s terminal bud exerts over lateral bud growth and explains many characteristics of tree growth and response to pruning [28][26].
A branch has two types of buds: (i) the terminal bud, located at the tip of the branch, that controls the longitudinal growth of the branch, and (ii) several lateral buds located in the leaf axils and distributed along the length of the branch. The apical meristem of a branch produces auxins, which migrate downwards, suppressing the new growth of axillary buds [29][27]. This way, while a branch elongates, no lateral shoots appear (except under special conditions). Removing the apex of a branch leads to the breakdown of apical dominance due to the elimination of the auxin source, and new lateral growth can occur as the axillary buds are no longer suppressed [30][28].
If a branch stops growing without terminal bud removal, a new shoot will emerge from the terminal bud at one of the following shoot growth flushes. New side shoots may or may not appear just below (depending on ambient conditions and shooting habits). Some citrus species, such as mandarin trees of the satsuma group, have strong apical dominance.
2.7. Alternate Bearing
Alternate bearing can occur in some cultivars and refers to a cyclical yield in which a high yield with many small-sized fruits in one year (on-year) alternates with a low yield with few big-sized fruits the following year (off-year) [31][29] (Figure 6).
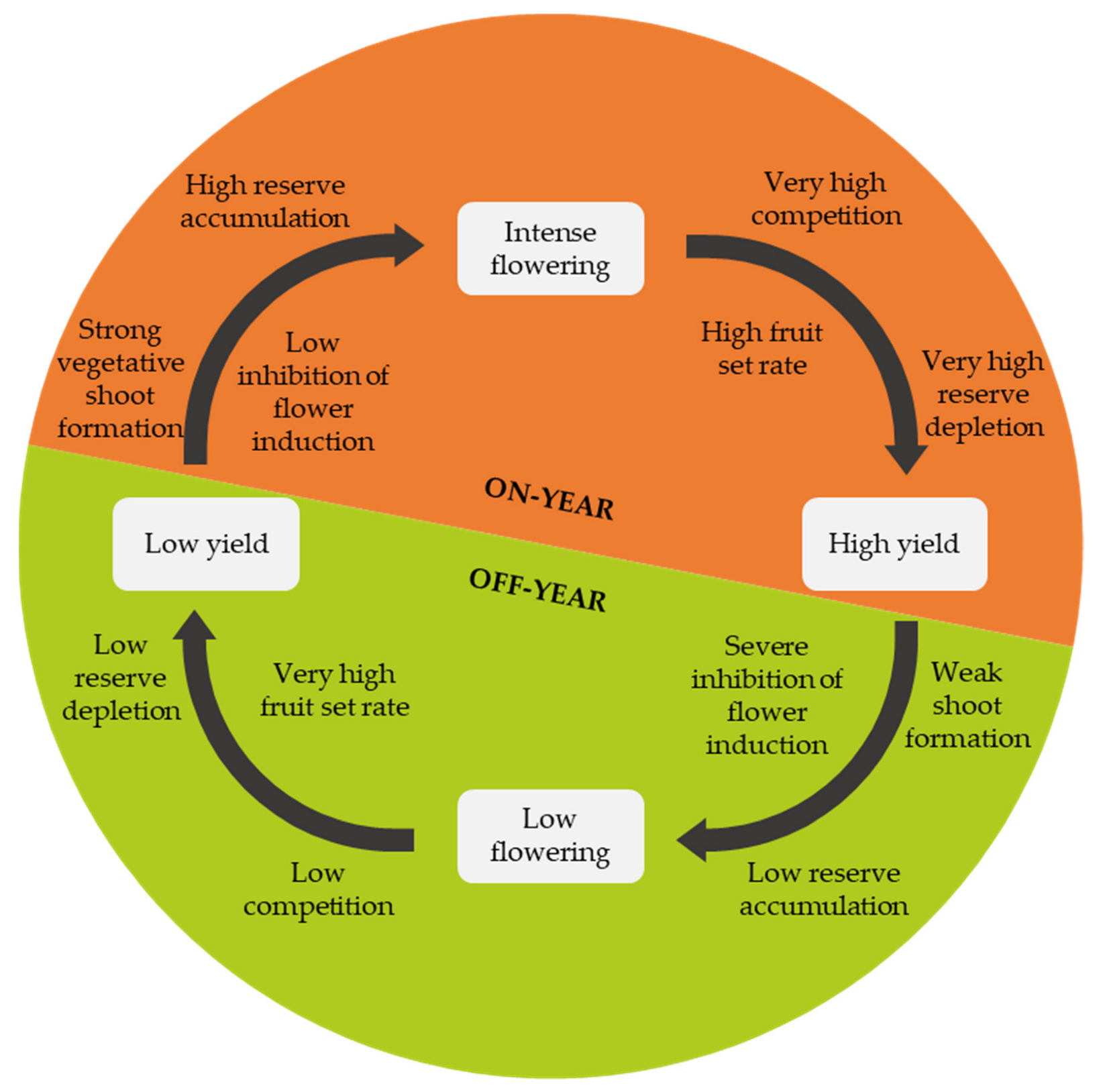
Figure 6.
Citrus alternate bearing cycle.
In alternate-bearing cultivars, a high fruit load during flower induction suppresses the flower-promoting genes [31,32,33,34][29][30][31][32]. Moreover, fruits may inhibit budbreak and, because fruit formation is highly resource-demanding, reserve depletion is high, and carbohydrate availability becomes scarce after an on-year. Consequently, the high fruit load on the tree in an on-year negatively affects the formation of vegetative shoots in summer and autumn, leading to poor flowering in the following spring and a high fruit set [35,36,37][33][34][35]. This results in an off-year with a meagre or almost non-existent yield wherein competition between fruits is low and reserves are consumed only to a small extent [35][33].
After an off-year, the following year’s flower induction is high. Furthermore, after a low yield, the reserve accumulation and the new shoot formation levels are high, leading to abundant flowering. Under these conditions, resource competition is intense, but the fruit set rate is also high, leading again to an on-year [35,36,37][33][34][35].
3. Pruning Fundamentals
3.1. Types of Cuts
There are three types of pruning cuts, depending on the cutting point (Figure 7): (a) heading cuts; (b) reduction or drop-crotch cuts, and (c) thinning cuts.
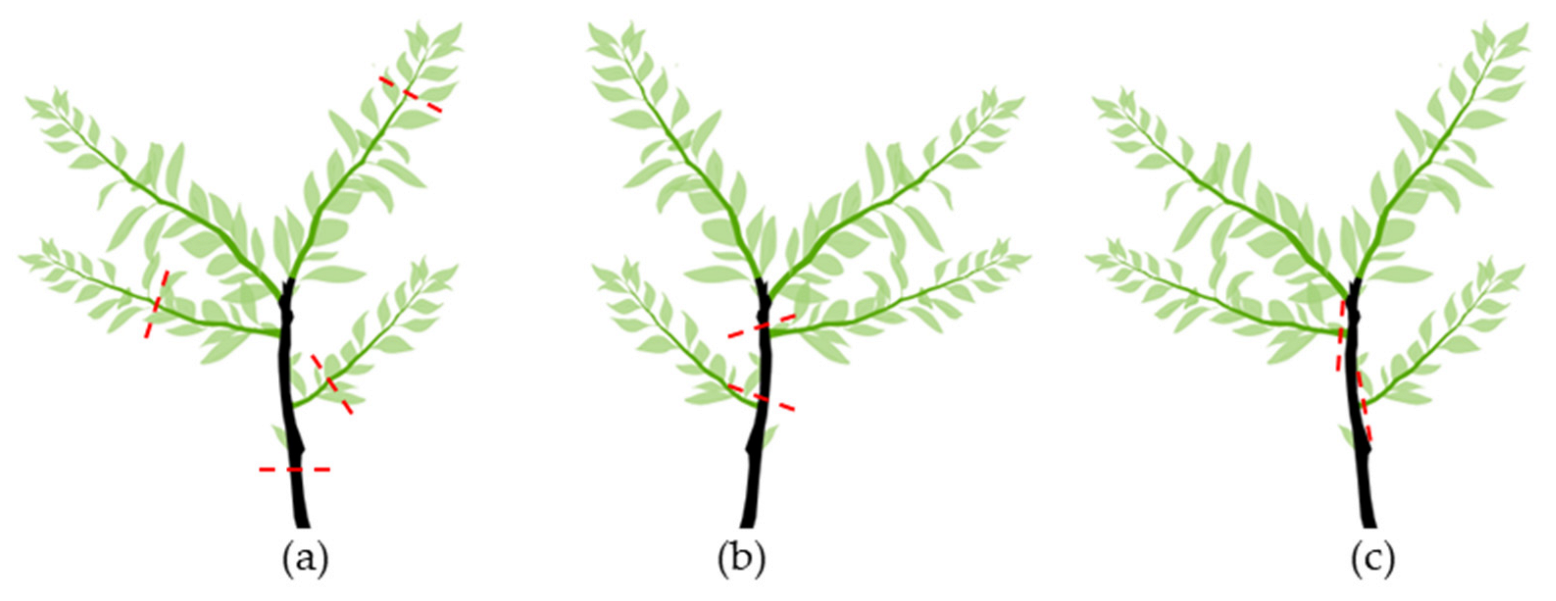
Figure 7.
Types of cuts: (
a
) Heading cuts; (
b
) reduction or drop-crotch cuts; and (
c
) thinning cuts. The red lines mark the place where the cut is done.
A heading cut (Figure 7a) removes the terminal portion of a branch. This way, apical dominance is broken, and branching occurs from the nodes below the cutting. This cut may stimulate branching at a particular place and reduces the sizes of very long branches.
Drop-crotch cuts (Figure 7b) involve cutting a branch back to a lateral branch. A drop-crotch cut aims to stimulate the tree to grow in a particular direction. The lateral branch should be at least 1/3 the size of the cut branch. Otherwise, the cut will promote the development of many water sprouts.
Thinning cuts (Figure 7c) remove the entire branch. They are usually made to reduce competition between too-close branches, remove a branch crossing, and/or rub more desirable branches. Thinning cuts are also used to remove undesirable and bad crotch-angled branches (usually where the angle is less than 45°), where the bark of the two branches often grows down and presses the branches, bark included, instead of forming a small ridge (branch–bark ridge).
3.2. Cutting Operation
3.2.1. Cutting Techniques and Procedures
It is essential to consider the orientation of the cut as it affects the cut surface covering. A cut involving the removal of a branch inserted in a larger branch (thinning cut) should not be parallel to it because this will result in serious injury, as the cut will also eliminate part of the larger branch.
The branch–bark ridge and collar must be identified before the cut (Figure 8). The branch collar is where the larger branch grows with and around the lateral branch; usually, it is a slightly swollen zone at the base of the lateral branch. The cut should be made next to the branch collar without damaging it (Figure 8). Leaving a stub above the branch collar must be avoided as the stub will die and the cut surface will not be covered by bark tissue.
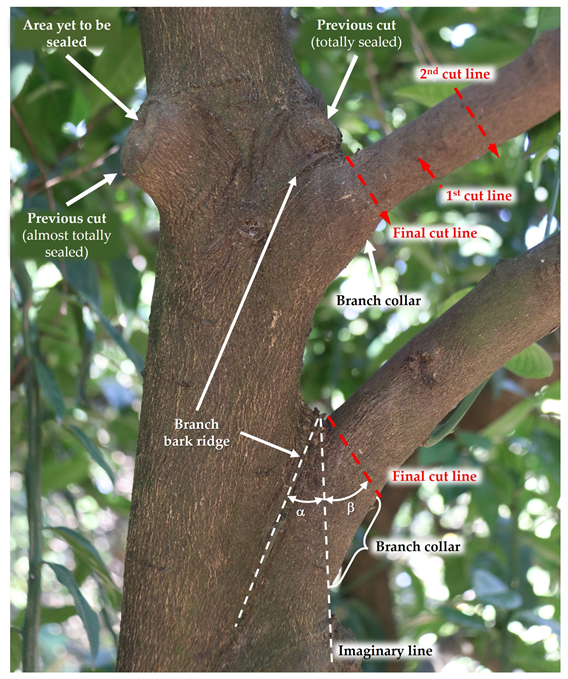
Figure 8.
Cutting techniques.
If the bottom of the branch collar is difficult to identify, the cut angle can be estimated, as illustrated in Figure 8. This is accomplished by visualizing an imaginary line extending from the lower edge of the largest branch. This line forms an angle α with the branch–bark ridge. The final cutting line must form an angle β with the imaginary line, which, in citrus, must be equal to or slightly higher than the angle α.
Thick branches require three cuts for their complete removal to prevent trunk bark tearing (Figure 8). The first cut is made from the underside of the branch, about 40 cm away from the larger branch. The cut should be as deep as possible before the weight of the branch blocks the saw. The second cut is made further forward than the first cut, from top to bottom, so that the branch breaks between the two cuts without tearing the bark. After the second cut, there remains a stub, which is removed by the third cut (final cut line), which should begin on the outside of the tree bark ridge and end just outside of the branch collar, visible by the swelling at the bottom of the branch [38][36].
A reduction or drop-crotch cut must be made above a lateral branch capable of replacing the eliminated branch. For this to be possible, the lateral branch must be at least one-third the diameter of the eliminated branch. A reduction cut should bisect the angle formed by the branch–bark ridge and an imaginary line perpendicular to the stem to be eliminated.
3.2.2. Wound Sealing and Protection
References
- Duarte, A.; Fernandes, J.; Bernardes, J.; Miguel, G. Citrus as a Component of the Mediterranean Diet. J. Spat. Organ. Dyn. 2016, 4, 289–304.
- Matias, P.; Duarte, B.; Duarte, A. Citrinos Na Dieta Mediterrânica: Frutos Com Sumo e Com História. Rev. Assoc. Port. Hortic. 2021, 143, 39–41.
- Germanà, C.; Continella, A.; Tribulato, E. Pruning Influence on Physiological and Productive Behaviour of Young “Lane Late” Orange Trees. In Proceedings of the 10th International Citrus Congress, Agadir, Morocco, 15–20 February 2004; pp. 291–294.
- Agustí, M.; Reig, C.; Martínez-Fuentes, A.; Mesejo, C. Advances in Citrus Flowering: A Review. Front. Plant. Sci. 2022, 13, 868831.
- Davenport, T.L. Citrus Flowering. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 349–408.
- Dhaliwal, H.S.; Banke, A.K.; Kumar, L.; Brar, J.S. Standardization of Pruning Severity for Healthy Bud Production in ‘Kinnow’ (C. Nobilis × C. Deliciosa) Mother Plants. Acta Hortic. 2016, 1130, 311–317.
- Moss, G.I. Regrowth and Flowering in Sweet Orange after Pruning. Aust. J. Agric. Res. 1973, 24, 101–109.
- Lin, S.; Chen, I.; Lai, Y.-T. Heavy Pruning Effects on Flower Buds Formation of Citrus Microcarpa Bunge and Fortunella Margarita Swing. Acta Hortic. 2012, 267–272.
- Duarte, A.M.; García-Luis, A.; Molina, R.V.; Monerri, C.; Navarro, V.; Nebauer, S.G.; Sánchez-Perales, M.; Guardiola, J.L. Long-Term Effect of Winter Gibberellic Acid Sprays and Auxin Applications on Crop Value of “Clausellina” Satsuma. J. Am. Soc. Hortic. Sci. 2006, 131, 586–592.
- Agusti, M.; García-Marí, F.; Guardiola, J.L. The Influence of Flowering Intensity on the Shedding of Reproductive Structures in Sweet Orange. Sci. Hortic. 1982, 17, 343–352.
- Guardiola, I.L.; Monerri, C.; Agusti, M. The Inhibitory Effect of Gibberellic Acid on Flowering in Citrus. Physiol. Plant. 1982, 55, 136–142.
- Bain, J.M. Morphological, Anatomical and Physiological Changes in the Developing Fruit of the Valencia Orange, (Citrus Sinensis (L.) Osbeck). Aust. J. Bot. 1958, 2, 1–23.
- Roccuzzo, G.; Zanotelli, D.; Allegra, M.; Giuffrida, A.; Torrisi, B.F.; Leonardi, A.; Quiñones, A.; Intrigliolo, F.; Tagliavini, M. Assessing Nutrient Uptake by Field-Grown Orange Trees. Eur. J. Agron. 2012, 41, 73–80.
- Agustí, M.; Primo-Millo, E. Flowering and Fruit Set. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 219–244.
- Han, H.-H.; Coutand, C.; Cochard, H.; Trottier, C.; Lauri, P.-É. Effects of Shoot Bending on Lateral Fate and Hydraulics: Invariant and Changing Traits across Five Apple Genotypes. J. Exp. Bot. 2007, 58, 3537–3547.
- Budiarto, R.; Poerwanto, R.; Santosa, E.; Efendi, D. Shoot Manipulations Improve Flushing and Flowering of Mandarin Citrus in Indonesia. J. Appl. Hortic. 2018, 20, 112–118.
- Goren, R.; Huberman, M.; Goldschmidt, E.E. Girdling: Physiological and Horticultural Aspects. In Horticultural Reviews; John Wiley & Sons, Inc.: Oxford, UK, 2010; pp. 1–36.
- Guardiola, J.L.; García-Luis, A. Increasing Fruit Size in Citrus. Thinning and Stimulation of Fruit Growth. Plant Growth Regul. 2000, 31, 121–132.
- Lal, N.; Sahu, N.; Marboh, E.S.; Gupta, A.K.; Patel, R.K. (). A Review on Crop Regulation in Fruit Crops. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 4032–4043.
- Hardy, S.; Barkley, P.; Treeby, M.; Smith, M.; Sanderson, G. Australian Mandarin Production Manual, 1st ed.; Treeby, M., Hardy, S., Eds.; NSW Department of Primary Industries: Camberra, Australia, 2017; ISBN 978-1-76058-057 5.
- Attri, A.; Thakre, M.; Yadav, P.; Verma, M.K.; Singh, B. 14C-Labeling Technique for Discerning Source–Sink Carbon Flow Dynamics in Kinnow (Citrus Nobilis Lour × Citrus Deliciosa Tenora) for Better Crop Management. J. Radioanal. Nucl. Chem. 2018, 317, 1447–1454.
- Barlas, N.T. Pruning Residuals: Their Role in the Micronutrient Budget of Clementine Mandarin (Citrus Reticulata Blanco). J. Agric. Fac. Ege Univ. 2022, 59, 61–66.
- Jacinto, C.; Matias, P.; Oliveira, C.; Duarte, A. Effect of Heading Cuts on Branch Growth of ‘Encore’ Mandarin. Acta Hortic. 2023. submitted.
- Coarite, J. Poda de Cítricos; UNODC: La Asunta, Bolivia, 2017.
- Rodríguez, J.; Villalba, D. Poda de Los Cítricos; Generalitat Valenciana: Valencia, Spain, 1998.
- Cline, M.G. Concepts and Terminology of Apical Dominance. Am. J. Bot. 1997, 84, 1064–1069.
- Leyser, O. Regulation of Shoot Branching by Auxin. Trends Plant Sci. 2003, 8, 541–545.
- Mataa, M.; Cheelo, P.; Lungu, D.; Kinkese, T. Effect of Apical Dominance on Bud Take in Citrus Vegetative Propagation. Int. J. Agric. Res. Innov. Technol. 2017, 7, 64–70.
- Shalom, L.; Samuels, S.; Zur, N.; Shlizerman, L.; Zemach, H.; Weissberg, M.; Ophir, R.; Blumwald, E.; Sadka, A. Alternate Bearing in Citrus: Changes in the Expression of Flowering Control Genes and in Global Gene Expression in ON- versus OFF-Crop Trees. PLoS ONE 2012, 7, e46930.
- Nishikawa, F.; Iwasaki, M.; Fukamachi, H.; Nonaka, K.; Imai, A.; Takishita, F.; Yano, T.; Endo, T. Fruit Bearing Suppresses Citrus FLOWERING LOCUS T Expression in Vegetative Shoots of Satsuma Mandarin (Citrus unshiu Marc.). J. Jpn. Soc. Hortic. Sci. 2012, 81, 48–53.
- Muñoz-Fambuena, N.; Mesejo, C.; González-Mas, M.C.; Primo-Millo, E.; Agustí, M.; Iglesias, D.J. Fruit Load Modulates Flowering-Related Gene Expression in Buds of Alternate-Bearing ‘Moncada’ Mandarin. Ann. Bot. 2012, 110, 1109–1118.
- Goldschmidt, E.E.; Sadka, A. Yield Alternation: Horticulture, Physiology, Molecular Biology, and Evolution. Hortic. Rev. Am. Soc. Hortic. Sci. 2021, 48, 363–418.
- Stander, O.P.J.; Barry, G.H.; Cronjé, P.J.R. Fruit Load Limits Root Growth, Summer Vegetative Shoot Development, and Flowering in Alternatebearing ‘Nadorcott’ Mandarin Trees. J. Am. Soc. Hortic. Sci. 2018, 143, 213–225.
- Soler Aznar, J. Reconocimiento de Variedades de Cítricos En Campo; Generalitat Valenciana, Conselleria de Agricultura, Pesca y Alimentación: Valencia, Spain, 1999; ISBN 8448220889.
- Guardiola, J.L.; Garcia-Marí, F.; Agustí, M. Nutrición Mineral e Improductividd de La Variedad de Naranjo “Navelate”. Rev. Agroquím. Tecnol. Aliment. 1979, 19, 483–497.
- Ferguson, J.J. Your Florida Dooryard Citrus Guide—Pruning. In Series of the Horticultural Sciences Department; University of Florida: Gainesville, FL, USA, 2002; Volume 889, pp. 1–4.
- Machado, F.J.; da Marin, T.G.S.; Canôas, F.; da Silva, G.J., Jr.; Behlau, F. Timing of Copper Sprays to Protect Mechanical Wounds against Infection by Xanthomonas Citri Subsp. Citri, Causal Agent of Citrus Canker. Eur. J. Plant Pathol. 2021, 160, 683–692.
More
