Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Laurent Dufossé.
Natural pigments, especially fungal pigments, are receiving more attention and seem to be in high demand worldwide. The immense advantages of fungal pigments over other natural or synthetic pigments have opened new avenues in the market for a wide range of applications in different industries. In addition to coloring properties, other beneficial attributes of fungal pigments, such as antimicrobial, anticancer, antioxidant, and cytotoxic activity, have expanded their use in different sectors.
- color
- natural pigments
- fungal pigments
1. Introduction
Color has always played an important role in the life of all organisms on Earth. Human life has become truly “colorful” due to the use of colors in all its aspects, including clothes, food, and furniture. Much archaeological evidence has shown that the use of pigments as coloring agents has been practiced since ancient times [1]. Pigments, especially synthetic ones, have occupied the entire market due to their wide range of applications in different industries since their discovery in the 19th century. Different attributes such as low production costs, ease of production, and superior coloring properties have largely contributed to the establishment of synthetic pigments in the market. However, the use of synthetic colors has been found to be detrimental to human health and the environment because of their many adverse impacts [2,3,4,5,6,7][2][3][4][5][6][7]. Many disadvantages of synthetic pigments, such as poor degradation, longer persistence, potential to cause cancers/allergies, etc., have increased the demand for natural, organic, and eco-friendly pigments in the current era.
The global response, as well as the demand for eco-friendly natural pigments, has significantly increased in recent decades due to their advantages over hazardous synthetic pigments. They are used as colorants, color intensifiers, additives, antioxidants, etc., in many industries including the textile, pharmaceutical, cosmetic, painting, food, and beverage industries [1,8][1][8].
2. Natural Pigments
Natural pigments are naturally derived pigments synthesized mainly by plants, animals, and microbes [5,9][5][9]. Most of the natural pigments used for different purposes since ancient times are produced from plants, such as annatto, grapes, indigo, beetroot, turmeric, madder, saffron, etc. [10,11][10][11]. However, the process of pigment production from plants may not be a good option because of various problems, such as season dependency, loss of vulnerable plant species due to their extensive use, variations in color shades and intensity, expensive production, and issues related to stability and solubility [2]. Nowadays, microorganisms, including bacteria, fungi, and algae, have been shown to be an excellent alternative source of natural pigments. For the large-scale production of pigments, microorganisms are more suitable, due to a clear understanding of their cultural techniques, processing, and ease of handling. Natural pigments from microbes, especially from bacteria and fungi, have been reported worldwide by many researchers [1,10,12,13,14,15,16,17,18,19,20][1][10][12][13][14][15][16][17][18][19][20]. Many bacterial species have been reported to possess potential for pigment production [10[10][21][22][23],21,22,23], but their pathogenic nature as well as associated toxicity have blocked production and commercialization. This eventually opened a new avenue for producing pigments from fungi and for their various applications.3. Fungal Pigments
Fungi have been shown to be a good and readily available alternative source of natural pigments [1,20,24,25,26][1][20][24][25][26]. Fungi have immense advantages over plants such as season-independent pigment production, easy and fast growth in a cheap culture medium, production of pigments with different color shades and of more stable, soluble pigments, and easy processing [10,27][10][27]. Fungi belonging to the Monascaceae, Trichocomaceae, Nectriaceae, Hypocreaceae, Pleosporaceae, Cordycipitaceae, Xylariaceae, Chaetomiaceae, Sordariaceae, Chlorociboriaceae, Hyaloscyphaceae, Hymenochaetaceae, Polyporaceae, Ophiostomataceae, Tremellaceae, Herpotrichiellaceae, and Tuberaceae families have been described as potent pigment producers [8,12,20,25,26,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45][8][12][20][25][26][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45]. These fungi are known to synthesize a variety of pigments as secondary metabolites. They are prolific producers of pigments belonging to several chemical classes, such as carotenoids, melanins, azaphilones, flavins, phenazines, quinones, monascin, violacein, indigo, etc. [16,25,26,46,47,48,49][16][25][26][46][47][48][49]. The use of Monascus pigments for the production of red mold rice (ang-kak) is the oldest recorded use of fungal pigments by humans. Certain species of Monascus, viz., Monascus ruber and Monascus purpureus, have been reported to be good potential producers of pigments worldwide. Studies have shown the potential of the red pigment produced by M. ruber as an important food colorant as well as food additive [50,51][50][51]. Many new pigments produced by M. ruber, such as N-glucosylrubropunctamine, N-glucosylmonascorubramine, monarubrin, rubropunctin, etc., have been discovered (Figure 1) [52,53,54][52][53][54]. Recently, researchers revealed the first detailed biosynthetic pathway of Monascus azophilone pigments (MonAzPs) in M. ruber M7, based on targeted gene knockouts, heterologous gene expression, as well as in vitro enzymatic and chemical reactions [55]. Along with M. ruber, M. purpureus was also reported to produce a variety of novel pigments, such as monapurone A–C, monasphilone A–B, monapilol A–D, and 9-(1-hydroxyhexyl)-3-(2-hydroxypropyl)-6a-methyl-9,9a-dihydrofuro[2,3-h] isoquinoline-6,8 (2H,6aH)-dione (Figure 1) [56,57,58,59][56][57][58][59]. Another study reports on the physicochemical (pH, light, and heat stability) properties of the red pigment of M. purpureus [60].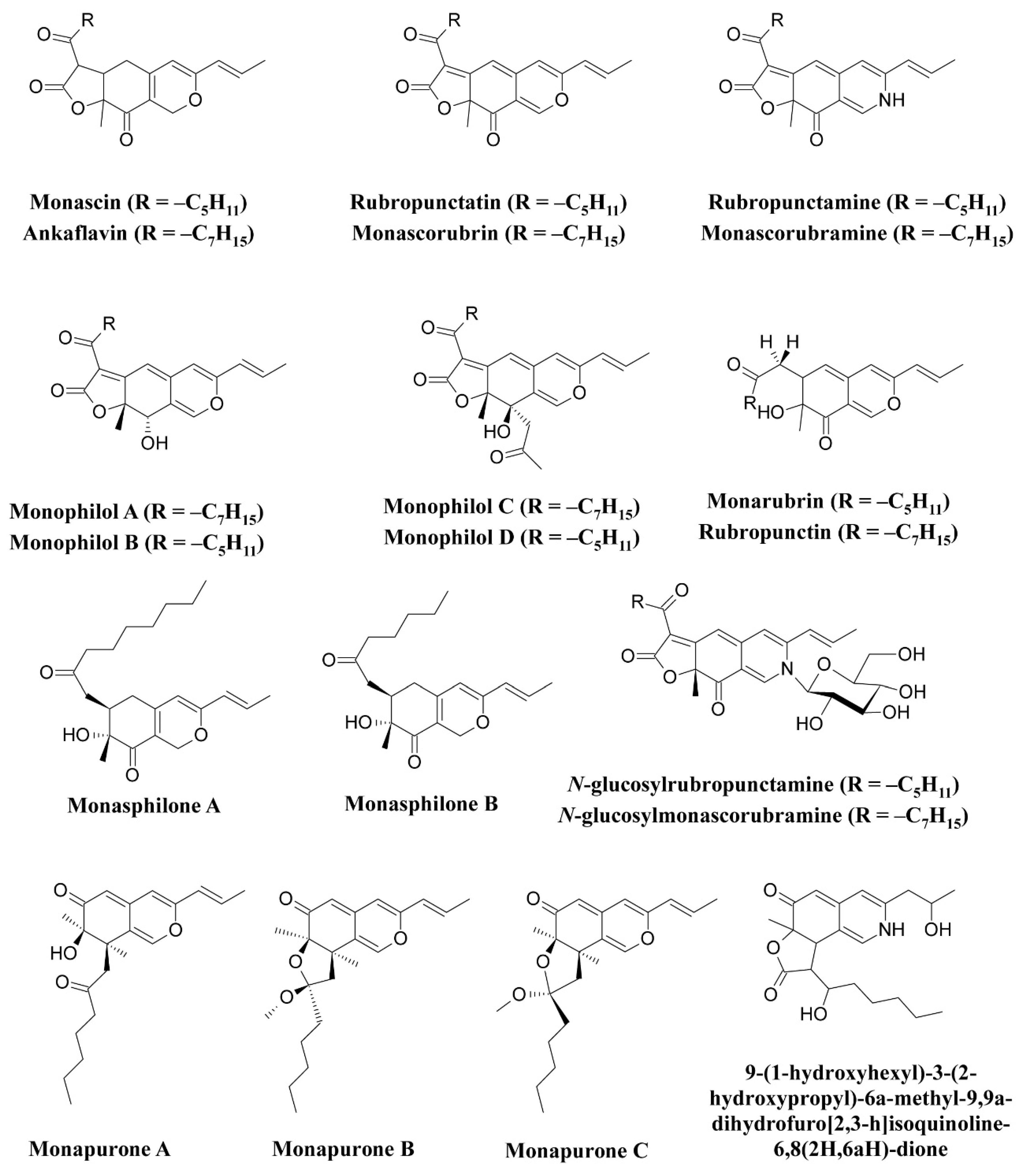
Figure 1.
Pigments reported from
Monascus
species (
M. ruber
and
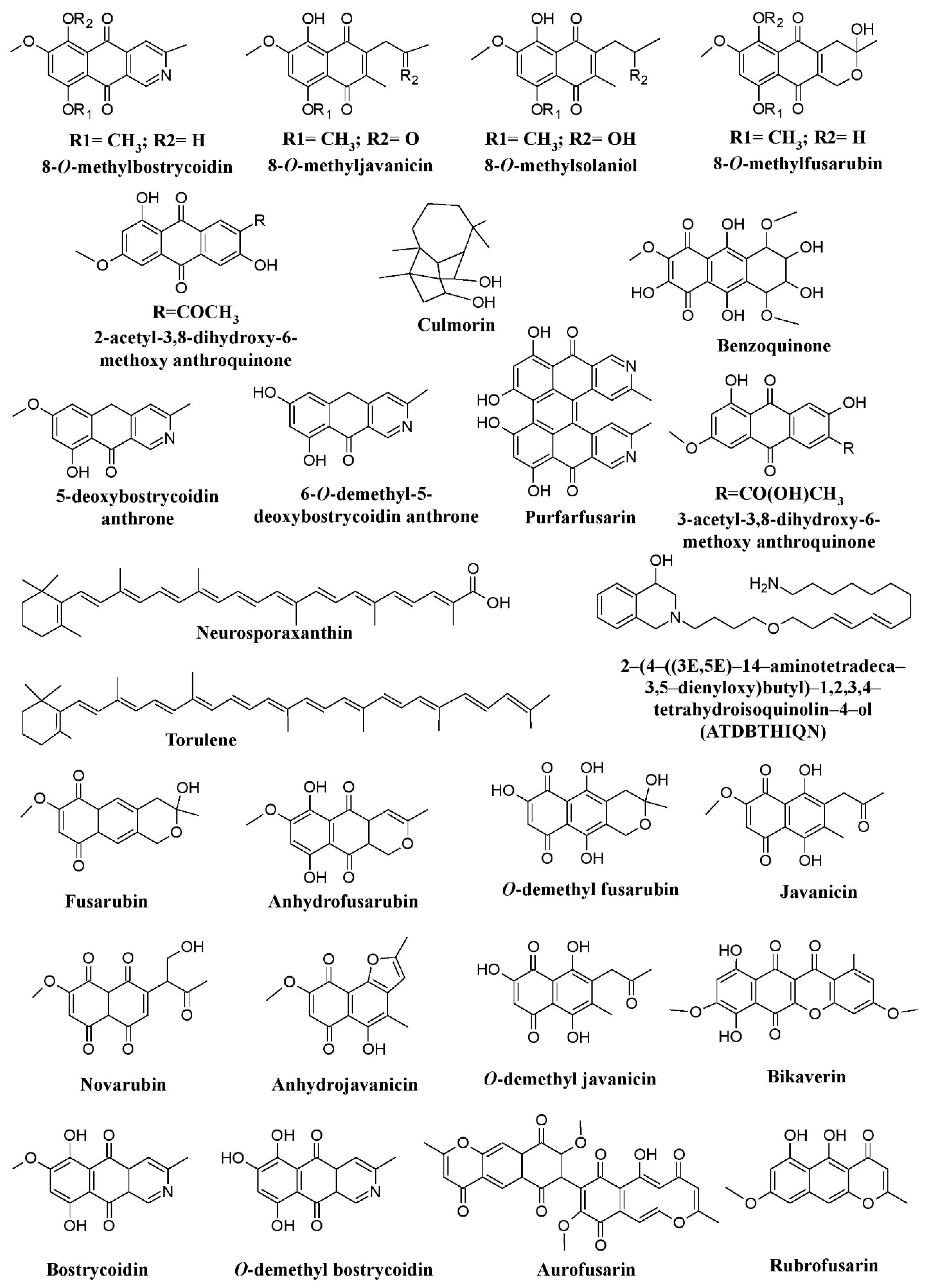
Figure 2.
Pigments from fungal genera of Nectriaceae (
Fusarium
,
Fusicolla
, and
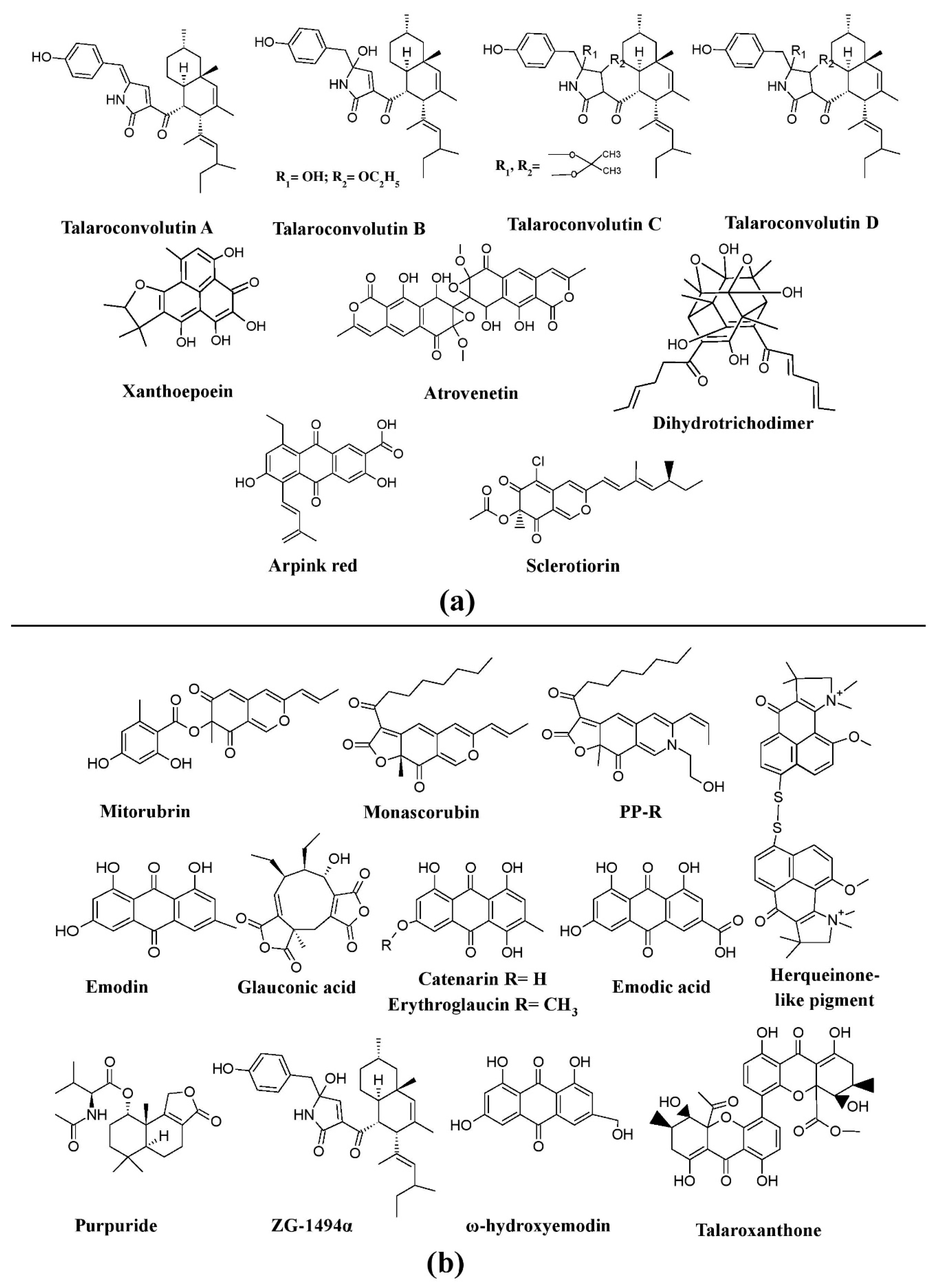
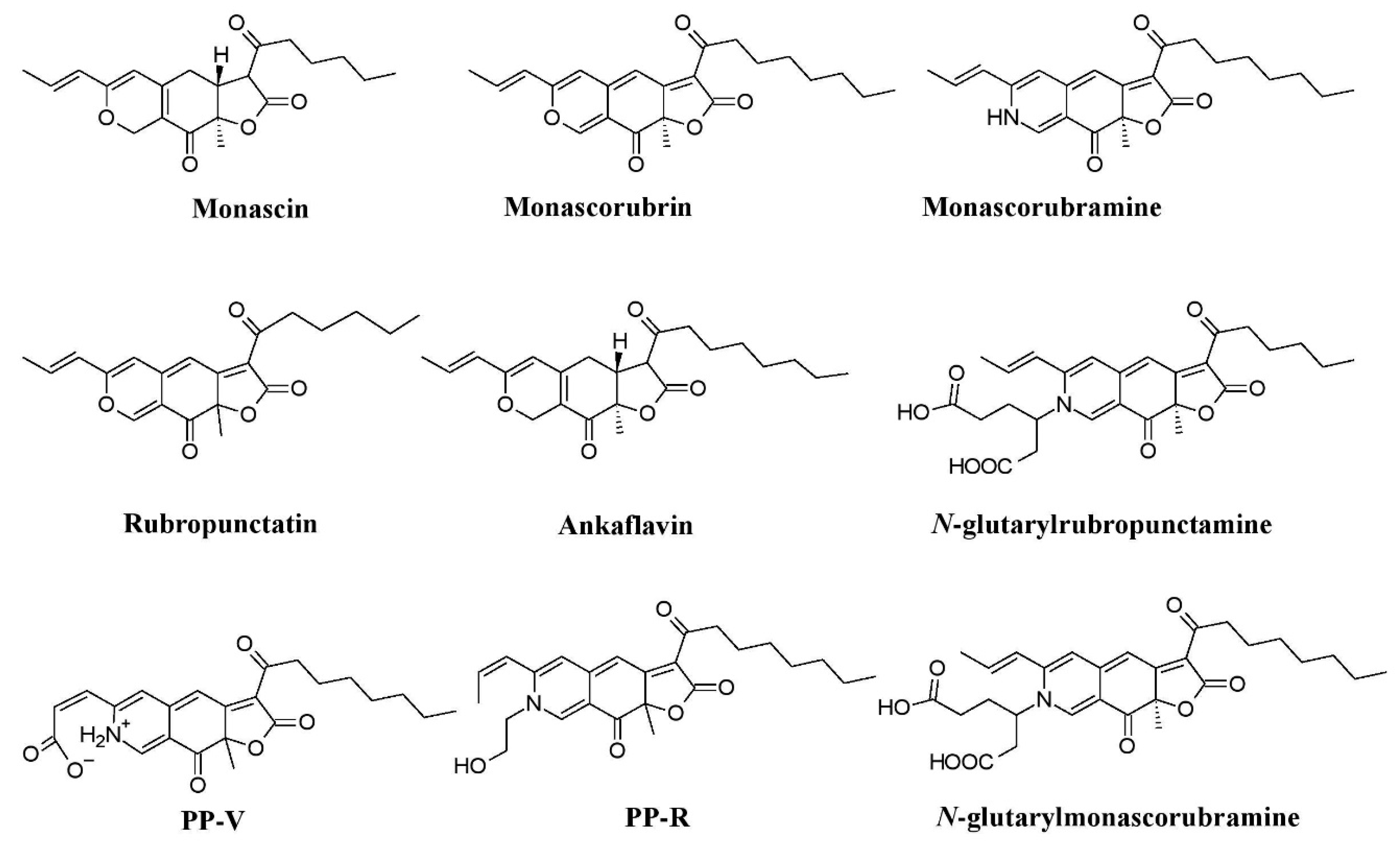
Figure 4.
Monascus
–like azaphilone pigments of
Penicillium
and
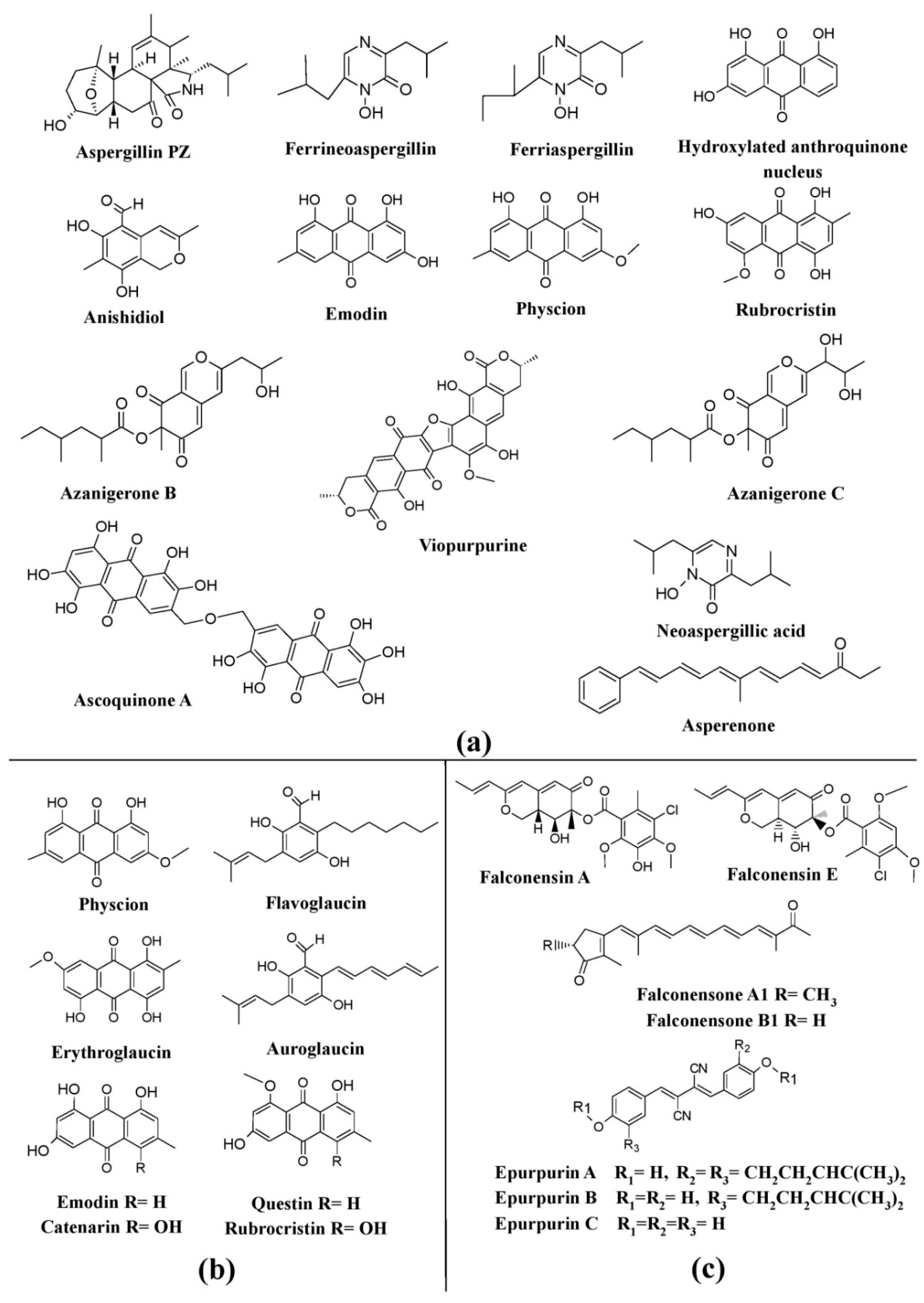
Figure 5. Pigments from the genus Aspergillus and its teleomorphic genera. (a) Structures of pigments produced by Aspergillus species. (b) Pigments produced by species of Eurotium (teleomorph of Aspergillus). (c) Pigments produced by species of Emericella (teleomorph of Aspergillus), re-drawn from [25].
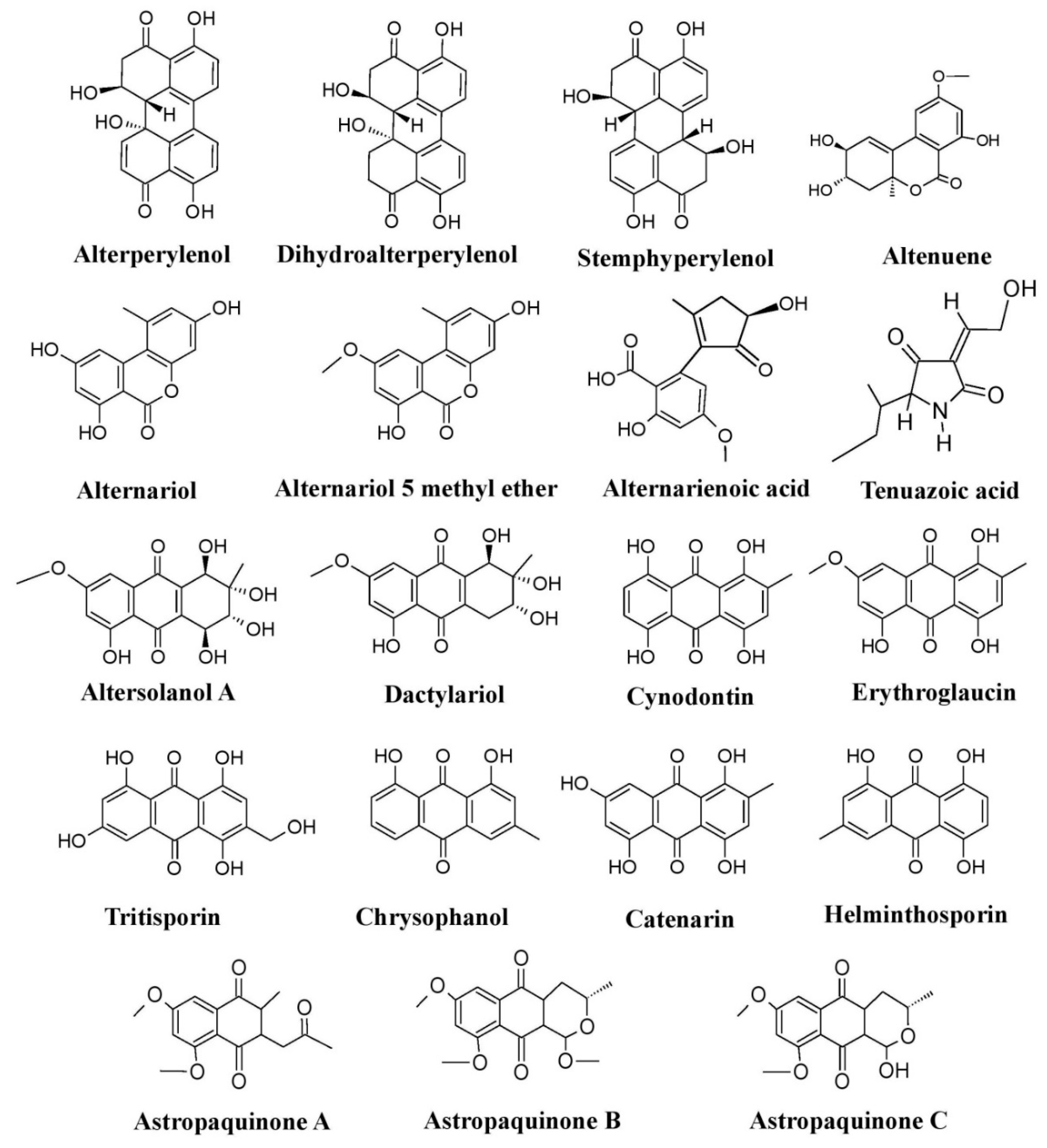
Figure 6.
Pigments produced by members of the fungal family Pleosporaceae (species of
Alternaria
,
Curvularia
,
Astrosphaeriella
, and
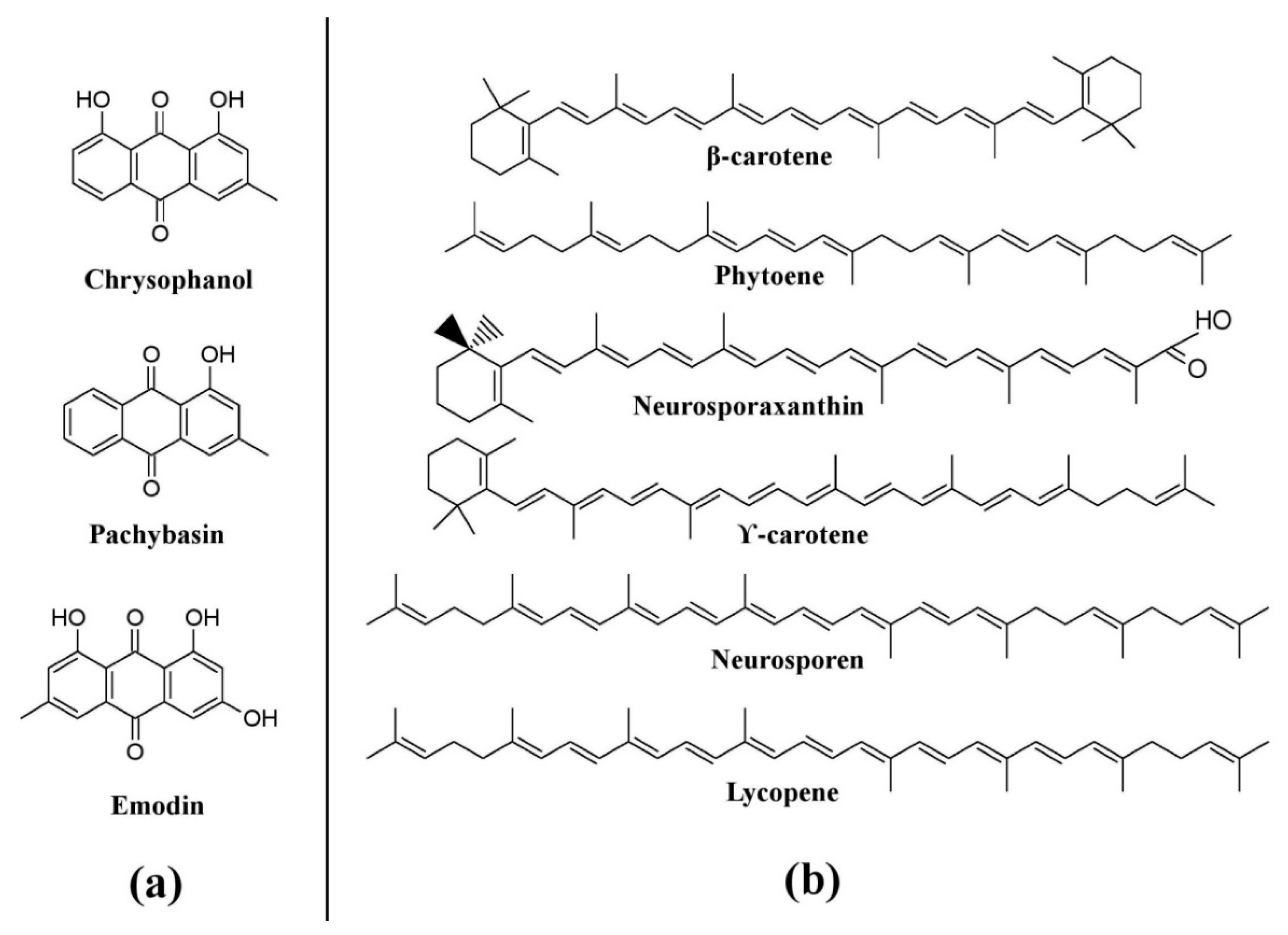
Figure 7.
Pigments from other fungi. (
a
) Pigments from
Trichoderma
b
) Pigments from
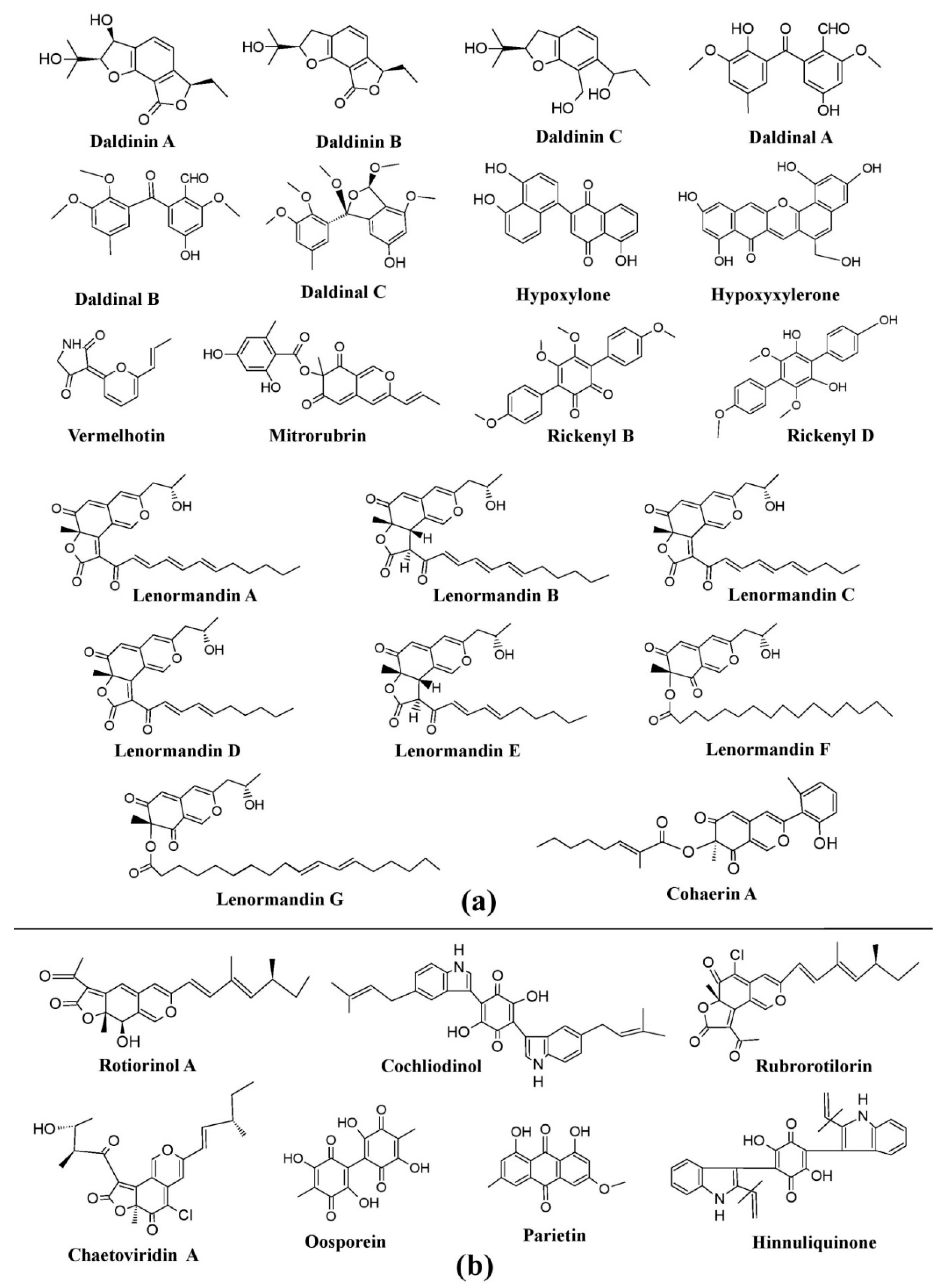
Figure 8. Pigments from the fungi of Xylariaceae and Chaetomiaceae families. (a) Pigments from members of the Xylariaceae family (species of Daldinia, Hypoxylon, and Jackrogersella), re-drawn from [25]. (b) Pigments from members of the Chaetomiaceae family (species of Chaetomium and Achaetomium) and Hypoxylaceae, re-drawn from [25,84][25][104].
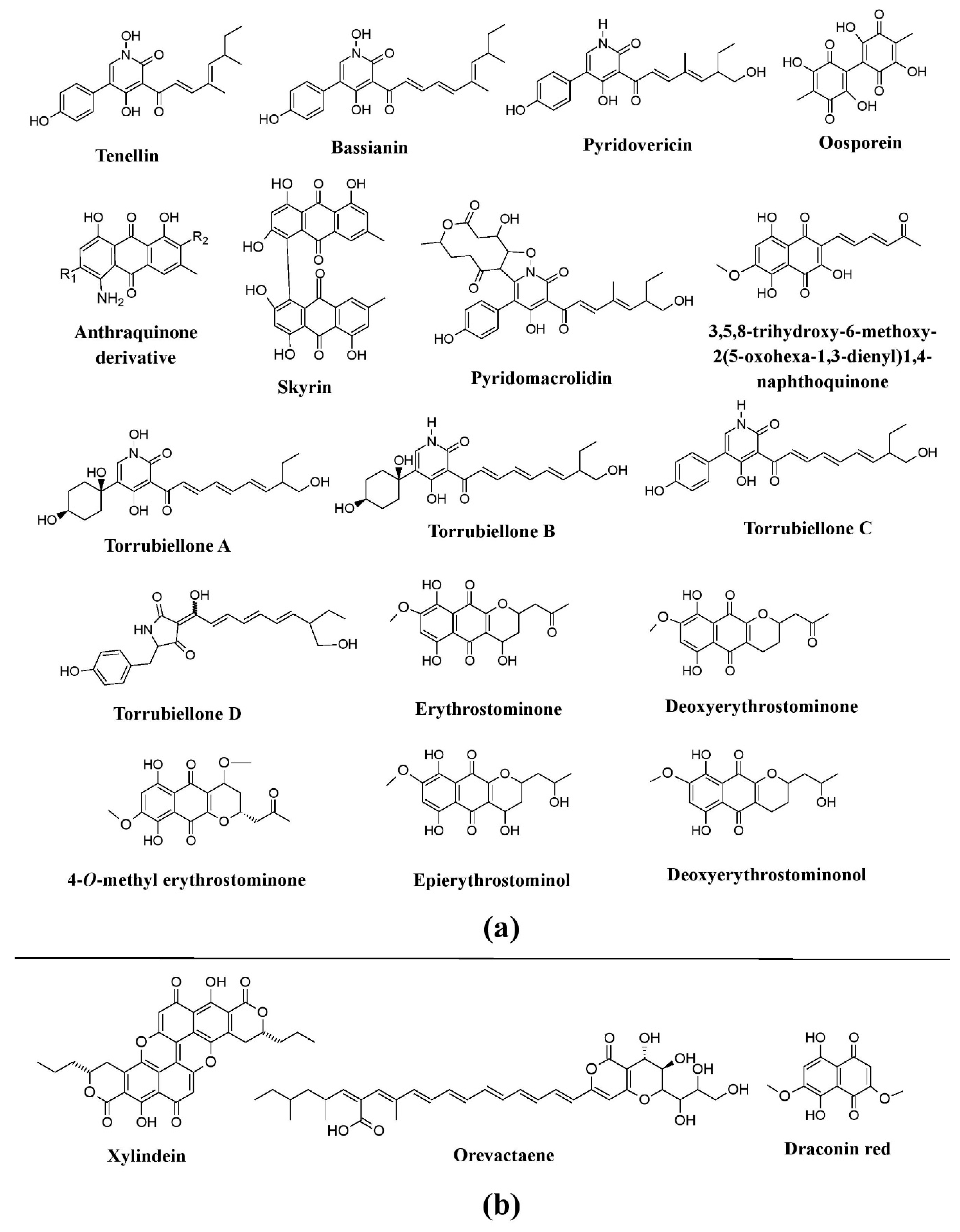
Figure 9. Pigments from the fungi of the Cordycipitaceae family and some other group. (a) Pigments from members of the families Cordycipitaceae (species of Beauveria, Torrubiella, Cordyceps, Hyperdermium, and Lecanicillium) and Ophiocordycipitaceae (Ophiocordyceps sp.), re-drawn from [25,41,73,74,75,125][25][41][105][106][107][108]. (b) Pigments known from other groups of fungi (species of Chlorociboria, Scytalidium, and Epicoccum), re-drawn from [37,41][37][41].
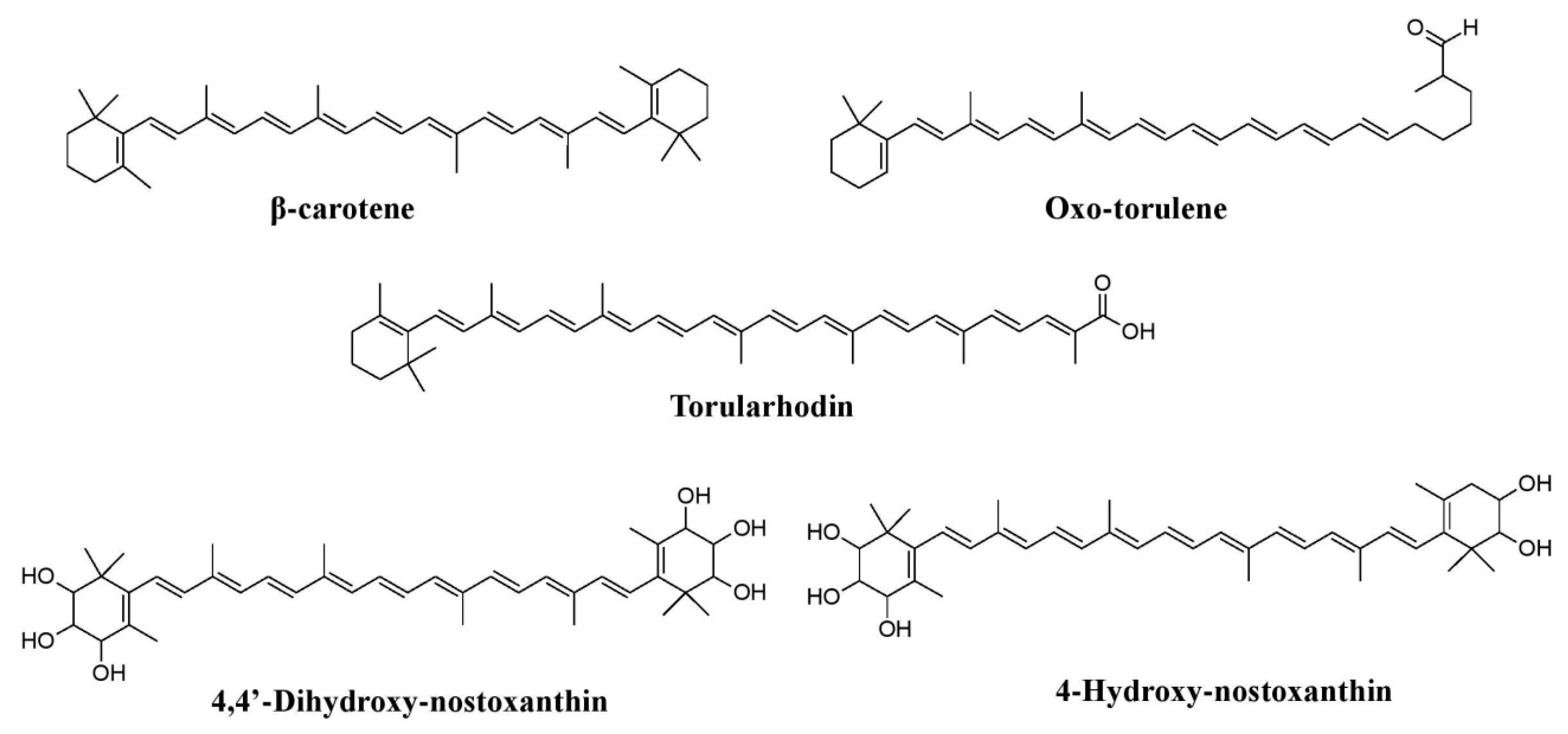
Figure 10.
Pigments reported from yeasts such as
Rhodotorula glutini
and
References
- Rao, M.P.N.; Xiao, M.; Li, W.J. Fungal and bacterial pigments: Secondary metabolites with wide application. Front. Microbiol. 2017, 8, 1113.
- Downham, A.; Collins, P. Coloring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22.
- Osman, M.Y.; Sharaf, I.A.; Osman, H.M.Y.; El-Khouly, Z.A.; Ahmed, E.I. Synthetic organic food coloring agents and their degraded products: Effects on human and rat cholinesterases. Br. J. Biomed. Sci. 2004, 61, 128–132.
- Babitha, S. Microbial pigments. In Biotechnology for Agro-Industrial Residues Utilization; Nigam, P.S., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 147–162.
- Samanta, A.K.; Agarwal, P. Application of natural dyes on textiles. Indian J. Fibre Text. Res. 2009, 34, 384–399.
- Ratna, P.B.S. Pollution due to synthetic dyes toxicity and carcinogenicity studies and remediation. Int. J. Environ. Sci. 2012, 3, 940–955.
- Arora, S. Textile dyes: Its impact on the environment and its treatment. J. Bioremediat. Biodegrad. 2014, 5, 1.
- Akilandeswari, P.; Pradeep, B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1631–1643.
- Chattopadhyay, P.; Chatterjee, S.; Sen, S.K. Biotechnological potential of natural food grade biocolorants. Afr. J. Biotechnol. 2008, 7, 2972–2985.
- Joshi, V.K.; Attri, D.; Bala, A.; Bhushan, S. Microbial pigments. Indian J. Biotechnol. 2003, 2, 362–369.
- Aberoumand, A. A review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. World J. Dairy Food Sci. 2011, 6, 71–78.
- Dufossé, L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006, 44, 313–321.
- Latha, B.V.; Jeevaratnam, K. Purification and characterization of the pigments from Rhodotorula glutinis DFR-PDY isolated from a natural source. Glob. J. Biotechnol. Biochem. 2010, 5, 166–174.
- Nagpal, N.; Munjal, N.; Chatterjee, S. Microbial pigments with health benefits—A mini review. Trends Biosci. 2011, 4, 157–160.
- Ahmad, W.A.; Ahmad, W.Y.W.; Zakaria, Z.A.; Yusof, N.Z. Isolation of pigment-producing bacteria and characterization of the extracted pigments. In Application of Bacterial Pigments as a Colorant; SpringerBriefs in Molecular Science; Springer: Berlin/Heidelberg, Germany, 2012; pp. 25–44.
- Kirti, K.; Amita, S.; Priti, S.; Kumar, A.M.; Jyoti, S. Colorful world of microbes: Carotenoids and their applications. Adv. Biol. 2014, 1, 1–13.
- Mata-Gomez, L.C.; Montanez, J.C.; Mendez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12.
- Kumar, A.; Vishwakarma, H.S.; Singh, J.; Dwivedi, S.; Kumar, M. Microbial pigments: Production and their applications in various industries. Int. J. Phram. Chem. Biol. Sci. 2015, 5, 203–212.
- Sarkar, S.L.; Saha, P.; Sultana, N.; Akter, S. Exploring textile dye from microorganisms, an eco-friendly alternative. Microbiol. Res. J. Int. 2017, 18, 1–9.
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Multifaceted applications of microbial pigments: Current knowledge, challenges and future directions for public health implications. Microorganisms 2019, 7, 186.
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial pigments and their applications. Process. Biochem. 2013, 48, 1065–1079.
- Gupta, C.; Prakash, D.; Gupta, S. Natural useful therapeutic products from microbes. J. Microbiol. Exp. 2014, 1, 30–37.
- Numan, M.; Bashir, S.; Mumtaz, R.; Tayyab, S.; Rehman, N.U.; Khan, A.L.; Shinwari, Z.K.; Al-Harrasi, A. Therapeutic applications of bacterial pigments: A review of current status and future opportunities. 3 Biotech 2018, 8, 207.
- Indra Arulselvi, P.; Umamaheswari, S.; Ranandkumar Sharma, G.; Karthik, C.; Jayakrishna, C. Screening of yellow pigment producing bacterial isolates from various eco-climatic areas and analysis of the carotenoid produced by the isolate. J. Food Process. Technol. 2014, 5, 292.
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and colorants from filamentous fungi. In Fungal Metabolites; Merillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 499–568.
- Gmoser, R.; Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Filamentous ascomycetes fungi as a source of natural pigments. Fungal Biol. Biotechnol. 2017, 4, 4.
- Manikprabhu, D.; Lingappa, K. γ Actinorhodin a natural and attorney source for the synthetic dye to detect acid production of fungi. Saudi J. Biol. Sci. 2013, 20, 163–168.
- Blanchette, R.A.; Wilmering, A.M.; Baumeister, M. The use of green-stained wood caused by the fungus Chlorociboria in intarsia masterpieces from the 15th century. Holzforsch Int. J. Biol. Chem. Phys. Technol. Wood 1992, 46, 225–232.
- Butler, M.J.; Day, A.W. Fungal melanins: A review. Can. J. Microbiol. 1998, 44, 1115–1136.
- Sakaki, T.; Shibata, M.; Mukai, K.; Sakai, M.; Wakamatsu, K.; Miyauchi, S. Chlorociboria aeruginosa pigment as algicide. Jpn. Kokai Tokkyo Koho JP 2002, 2002291493.
- Carvalho, J.C.; Pandey, A.; Babitha, S.; Soccol, C.R. Production of Monascus biopigments: An overview. Agro Food Ind. Hi-Tech 2003, 14, 37–42.
- Feng, Y.; Shao, Y.; Chen, F. Monascus pigments. J. Appl. Microbiol. Biotechnol. 2012, 96, 1421–1440.
- Robinson, S.C.; Tudor, D.; Snider, H.; Cooper, P.A. Stimulating growth and xylindein production of Chlorociboria aeruginascens in agar-based systems. AMB Express 2012, 2, 15.
- Tudor, D. Fungal Pigment Formation in Wood Substrate. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2013.
- Tudor, D.; Robinson, S.C.; Cooper, P.A. The influence of pH on pigment formation by lignicolous fungi. Int. Biodeterior. Biodegrad. 2013, 80, 22–28.
- Robinson, S.C.; Tudor, D.; Zhang, W.R.; Ng, S.; Cooper, P.A. Ability of three yellow pigment producing fungi to colour wood under controlled conditions. Int. Wood Prod. J. 2014, 5, 103–107.
- Hinsch, E.M.; Chen, H.L.; Weber, G.; Robinson, S.C. Colorfastness of extracted wood-staining fungal pigments on fabrics: A new potential for textile dyes. J. Text. Appar. Technol. Manag. 2015, 9, 1–11.
- Tam, W.T.E.; Tsang, C.C.; Lau, K.P.S.; Woo, C.Y.P. Polyketides, toxins, and pigments in Penicillium marneffei. Toxins 2015, 7, 4421–4436.
- Hernandez, V.A.; Galleguillos, F.; Robinson, S. Fungal pigments from spalting fungi attenuating blue stain in Pinus spp. Int. Biodeterior. Biodegrad. 2016, 107, 154–157.
- Robinson, S.C.; Michaelsen, H.; Robinson, J.C. Spalted Wood, History, Science and Art of an Unique Material; Schiffer Publishing: Atglen, PA, USA, 2016; pp. 1–288.
- Souza, P.N.C.; Grigoletto, T.L.B.; Moraes, L.A.B.; Abreu, L.M.; Souza, L.H.; Santos, C.; Galvao, L.R.; Cardoso, P.G. Production and chemical characterization of pigments in filamentous fungi. Microbiology 2016, 162, 12–22.
- Agurto, M.E.P.; Gutierrez, S.M.V.; Chen, H.-L.; Robinson, S.C. Wood-rotting fungal pigments as colorant coatings on oil-based textile dyes. Coatings 2017, 7, 152.
- Avalos, J.; Pardo-Medina, J.; Parra-Rivero, O.; Ruger-Herreros, M.; Rodriguez-Ortiz, R.; Hornero-Mendez, D.; Limon, M.C. Carotenoid biosynthesis in Fusarium. J. Fungi 2017, 3, 39.
- Pombeiro-Sponchiado, S.R.; Sousa, G.S.; Andrade, J.C.; Lisboa, H.F.; Gonçalves, R.C. Production of melanin pigment by fungi and its biotechnological applications. In Melanin; Blumenberg, M., Ed.; InTechOpen: London, UK, 2017; pp. 45–75.
- Vega Gutierrez, P.; Robinson, S. Determining the presence of spalted wood in spanish marquetry woodworks of the 1500s through the 1800s. Coatings 2017, 7, 188.
- Mortensen, A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006, 78, 1477–1491.
- Nagia, F.A.; El-Mohamedy, R.S.R. Dyeing of wool with natural anthraquinone dyes from Fusarium oxysporum. Dyes Pigm. 2007, 75, 550–555.
- Mapari, S.A.S.; Thrane, A.U.; Meyer, A.S. Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol. 2010, 28, 300–307.
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.S.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61.
- Went, F.A.F.C. Monascus purpureus le champignon de l’ang-quac une nouvelle thelebolee. Ann. Des. Sci. Nat. Bot. Biol. Veg. 1895, 8, 1–18.
- Fabre, C.E.; Santerre, A.L.; Loret, M.O.; Baberian, R.; Pareilleux, A.; Goma, G.; Blanc, P.J. Production and food applications of the red pigments of Monascus ruber. J. Food Sci. 1993, 58, 1099–1102.
- Hajjaj, H.; Klaebe, A.; Loret, M.O.; Tzedakis, T.; Goma, G.; Blanc, P.J. Production and identification of N-glucosylrubropunctamine and N-glucosylmonascorubramine from Monascus ruber and occurrence of electron donor-acceptor complexes in these red pigments. Appl. Environ. Microbiol. 1997, 63, 2671–2678.
- Lian, X.; Wang, C.; Guo, K. Identification of new red pigments produced by Monascus ruber. Dyes Pigm. 2007, 73, 121–125.
- Loret, M.O.; Morel, S. Isolation and structural characterization of two new metabolites from Monascus. J. Agric. Food Chem. 2010, 58, 1800–1803.
- Chen, W.; Chen, R.; Liu, O.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 4917–4925.
- Hsu, Y.-W.; Hsu, L.-C.; Liang, Y.-H.; Kuo, Y.-H.; Pan, T.-M. Monaphilones A–C, three new antiproliferative azaphilone derivatives from Monascus purpureus NTU 568. J. Agric. Food Chem. 2010, 58, 8211–8216.
- Li, J.-J.; Shang, X.-Y.; Li, L.-L.; Liu, M.-T.; Zheng, J.-Q.; Jin, Z.L. New cytotoxic azaphilones from Monascus purpureus-fermented rice (red yeast rice). Molecules 2010, 15, 1958–1966.
- Hsu, Y.-W.; Hsu, L.-C.; Liang, Y.-H.; Kuo, Y.-H.; Pan, T.-M. New bioactive orange pigments with yellow fluorescence from Monascus-fermented Dioscorea. J. Agric. Food Chem. 2011, 59, 4512–4518.
- Mukherjee, G.; Singh, S.K. Purification and characterization of a new red pigment from Monascus purpureus in submerged fermentation. Process Biochem. 2011, 46, 188–192.
- Kaur, B.; Chakraborty, D.; Kaur, H. Production and evaluation of physicochemical properties of red pigment from Monascus purpureus MTCC 410. Internet J. Microbiol. 2008, 7, 1–6.
- Steyn, P.S.; Wessels, P.L.; Marasas, W.F.O. Pigments from Fusarium moniliforme Shledon: Structure and 13C nuclear magnetic resonance assignments of an azaanthraquinone and three naphthoquinones. Tetrahedron 1979, 35, 1551–1555.
- Pradeep, F.S.; Palaniswamya, M.; Ravib, S.; Thangamanib, A.; Pradeep, B.V. Larvicidal activity of a novel isoquinoline type pigment from Fusarium moniliforme KUMBF1201 against Aedes aegypti and Anopheles stephensi. Process Biochem. 2015, 50, 1479–1486.
- Medenstev, A.G.; Arinbasarova, A.; Akimenko, V.K. Biosynthesis of naphthoquinone pigments by fungi of the genus. Fusarium. Prikl. Biokhim. Mikrobiol. 2005, 41, 573–577.
- Lebeau, J.; Petit, T.; Clerc, P.; Dufossé, L.; Caro, Y. Isolation of two novel purple naphthoquinone pigments concomitant with the bioactive red bikaverin and derivates thereof produced by Fusarium oxysporum. Biotechnol. Prog. 2019, 35, e2738.
- Frandsen, R.J.N.; Rasmussen, S.A.; Knudsen, P.B.; Uhlig, S.; Petersen, D.; Lysoe, E.; Gotfredsen, C.H.; Giese, H.; Larsen, T.O. Black perithecial pigmentation in Fusarium species is due to the accumulation of 5-deoxybostrycoidin-based melanin. Sci. Rep. 2016, 6, 26206.
- Cambaza, E. Comprehensive description of Fusarium graminearum pigments and related compounds. Foods 2018, 7, 165.
- Zheng, L.; Cai, Y.; Zhou, L.; Huang, P.; Ren, X.; Zuo, A.; Meng, X.; Xu, M.; Liao, X. Benzoquinone from Fusarium pigment inhibits the proliferation of estrogen receptor-positive MCF-7 cells through the NF–κB pathway via estrogen receptor signalling. Int. J. Mol. Med. 2017, 39, 39–46.
- Wollenberg, R.D.; Saei, W.; Westphal, K.R.; Klitgaard, C.S.; Nielsen, K.L.; Lysøe, E.; Gardiner, D.M.; Wimmer, R.; Sondergaard, T.S.; Sørensen, J.L. Chrysogine biosynthesis is mediated by a two-module nonribosomal peptide synthetase. J. Nat. Prod. 2017, 80, 2131–2135.
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U.; Frisvad, J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Fact. 2009, 8, 24.
- Pandey, N.; Jain, R.; Pandey, A.; Tamta, S. Optimisation and characterization of the orange pigment produced by a cold-adapted strain of Penicillium sp. (GBPI_P155) isolated from mountain ecosystem. Mycology 2018, 9, 81–92.
- Dhale, M.A.; Vijay-Raj, A.S. Pigment and amylase production in Penicillium sp. NIOM-02 and its radical scavenging activity. Int. J. Food Sci. Technol. 2009, 44, 2424–2430.
- Jiang, Y.; Li, H.B.; Chen, F.; Hyde, K.D. Production potential of water-soluble Monascus red pigment by a newly isolated Penicillium sp. J. Agric. Technol. 2005, 1, 113–126.
- Teixeira, M.F.S.; Martins, M.S.; Da Silva, J.C.; Kirsch, L.S.; Fernandes, O.C.C.; Carneiro, A.L.B.; De Conti, R.; Durrn, N. Amazonian biodiversity: Pigments from Aspergillus and Penicillium-characterizations, antibacterial activities and their toxicities. Curr. Trends Biotechnol. Pharm. 2012, 6, 300–311.
- Suzuki, S.; Hosoe, T.; Nozawa, K.; Kawai, K.; Yaguchi, T.; Udagawa, S. Antifungal substances against pathogenic fungi, Talaroconvolutins, from Talaromyces convolutes. J. Nat. Prod. 2000, 63, 768–772.
- Santos, P.O.; Ferraz, C.G.; Soares, A.C.F.; Miranda, F.M.; da Silva, F.; de Abreu Roque, M.R. Sclerotiorin, a novel pigment from Penicillium mallochii. In Proceedings of the 6th Brazilian Conference on Natural Products, Federal University of Espirito Santo Victoria, Vitoria, Brazil, 5–8 November 2017.
- Sardaryan, E. Strain of the Microorganism Penicillium oxalicum var. Armeniaca and Its. Application. Patent EP1070136B1, 4 August 2004.
- Heo, Y.M.; Kim, K.; Kwon, S.L.; Na, J.; Lee, H.; Jang, S.; Kim, C.H.; Jung, J.; Kim, J.J. Investigation of filamentous fungi producing safe, functional water-soluble pigments. Mycobiology 2018, 46, 269–277.
- Ogihara, J.; Kato, J.; Oishi, K.; Fujimoto, Y. PP-R, 7-(2-Hydroxyethyl)-Monascorubramine, a red pigment produced in the mycelia of Penicillium sp. AZ. J. Biosci. Bioeng. 2001, 91, 44–47.
- Viggiano, A.; Salo, O.; Ali, H.; Szymanski, W.; Lankhorst, P.P.; Nygard, Y.; Bovenberg, R.A.L.; Driessena, A.J.M. Pathway for the biosynthesis of the pigment Chrysogine by Penicillium chrysogenum. Appl. Environ. Microbiol. 2018, 84, 1–11.
- Frisvad, J.C.; Yilmaz, N.; Thrane, U.; Rasmussen, K.B.; Houbraken, J.; Samson, R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 2013, 8, e84102.
- Bhardwaj, S.; Shukla, A.; Mukherjee, S.; Sharma, S.; Guptasarma, P.; Chakraborti, A.K.; Chakrabarti, A. Putative structure and characteristics of red water-soluble pigment secreted by Penicillium marneffei. Med. Mycol. 2007, 45, 419–427.
- Koolen, H.H.F.; Menezes, L.S.; Souza, M.P.; Silva, F.M.A.; Almeida, F.G.O.; de Souza, A.Q.L.; Nepel, A.; Barison, A.; da Silva, F.H.; Evangelistae, D.E.; et al. Talaroxanthone, a novel xanthone dimer from the endophytic fungus Talaromyces sp. associated with Duguetia stelechantha (Diels) R. E. Fries. J. Braz. Chem. Soc. 2013, 24, 880–883.
- Zhai, M.-M.; Li, J.; Jiang, C.-X.; Shi, Y.P.; Di, D.L.; Crews, P.; Wu, Q.-X. The bioactive secondary metabolites from Talaromyces species. Nat. Prod. Bioprospect. 2016, 6, 1–24.
- Chadni, Z.; Rahaman, M.H.; Jerin, I.; Hoque, K.M.F.; Reza, M.A. Extraction and optimization of red pigment production as secondary metabolites from Talaromyces verruculosus and its potential use in textile industries. Mycology 2017, 8, 48–57.
- Mendez-Zavala, A.; Contreras-Esquivel, J.C.; Lara-Victoriano, F.; Rodriguez-Herrera, R.; Aguilar, C.N. Fungal production of the red pigment using a xerophilic strain Penicillium purpurogenum GH-2. Rev. Mex. Ing. Quim. 2007, 6, 267–273.
- Sethi, B.K.; Parida, P.; Sahoo, S.L.; Dikshit, B.; Pradhan, C.; Sena, S.; Behera, B.C. Extracellular production and characterization of red pigment from Penicillium purpurogenum BKS9. Alger. J. Nat. Prod. 2016, 4, 379–392.
- Ogbonna, C.N.; Aoyagi, H.; Ogbonna, J.C. Isolation and identification of Talaromyces purpurogenus and preliminary studies on its pigment production potentials in solid-state cultures. Afr. J. Biotechnol. 2017, 16, 672–682.
- Arai, T.; Koganei, K.; Umemura, S.; Kojima, R.; Kato, J.; Kasumi, T.; Ogihara, J. Importance of the ammonia assimilation by Penicillium purpurogenum in amino derivative Monascus pigment, PP-V production. AMB Express 2013, 3, 19.
- Pattenden, G. Synthesis of Asperenone, a new pigment from Aspergillus niger and Aspergillus awamori. Tetrahedron Lett. 1969, 10, 4049–4052.
- Ray, A.C.; Eakin, R.E. Studies on the biosynthesis of Aspergillin by Aspergillus niger. J. Appl. Microbiol. 1975, 30, 909–915.
- Zabala, A.O.; Xu, W.; Chooi, Y.-H.; Tang, Y. Discovery and characterization of a silent gene cluster that produces azaphilones from Aspergillus niger ATCC 1015 reveal a hydroxylation-mediated pyran-ring formation. Chem. Biol. 2012, 19, 1049–1059.
- Brown, D.W.; Solvo, J.J. Isolation and characterization of sexual spore pigments from Aspergillus nidulans. Appl. Environ. Microbiol. 1994, 60, 979–983.
- Wang, C.C.; Chiang, Y.M.; Kuo, P.L.; Chang, J.K.; Hsu, Y.L. Norsolorinic acid from Aspergillus nidulans inhibits the proliferation of human breast adenocarcinoma MCF-7 cells via Fas-mediated pathway. Basic Clin. Pharmacol. Toxicol. 2008, 102, 491–497.
- Youngchim, S.; Morris-Jones, R.; Hay, R.J.; Hamilton, A.J. Production of melanin by Aspergillus fumigatus. J. Med. Microbiol. 2004, 53, 175–181.
- Hosoe, T.; Mori, N.; Kamano, K.; Itabashi, T.; Yaguchi, T.; Kawai, K. A new antifungal yellow pigment from Aspergillus nishimurae. J. Antibiot. 2011, 64, 211–212.
- Akilandeswari, P.; Pradeep, B.V. Aspergillus terreus KMBF1501 a potential pigment producer under submerged fermentation. Int. J. Pharm. Pharm. Sci. 2017, 9, 38–43.
- Assante, G.; Camarda, L.; Locci, R.; Merlini, L. Isolation and structure of red pigments from Aspergillus flavus and related species, grown on a differential medium. J. Agric. Food Chem. 1981, 29, 785–787.
- Narendrababu, B.N.; Shishupala, S. Spectrophotometric detection of pigments from Aspergillus and Penicillium isolates. J. Appl. Biol. Biotechnol. 2017, 5, 53–58.
- Devi, S.; Kumar, H.A.K.; Ramachandran, G.; Subramanian, C.; Karuppan, P. Growth and mass spectrometry profile of Alternaria alternata pigment grown in maize grain extract. J. Microbiol. Biotechnol. Food Sci. 2014, 4, 179–184.
- Suemitsu, R.; Nakamura, A.; Isono, F.; Sano, T. Isolation and identification of Dactylariol from the culture liquid of Alternaria porri (Ellis) Ciferri. Agric. Biol. Chem. 1982, 46, 1693–1694.
- Okuno, T.; Natsume, I.; Sawai, K.; Sawamura, K.; Furusaki, A.; Matsumoto, T. Structure of antifungal and phytotoxic pigments produced by Alternaria sps. Tetrahedron Lett. 1983, 24, 5653–5656.
- Gupta, C.; Sharma, D.; Aggarwal, S.; Nagpal, N. Pigment production from Trichoderma spp. for dyeing of silk and wool. Int. J. Sci. Nat. 2013, 4, 351–355.
- Avalos, J.; Prado-Cabrero, A.; Estrada, A.F. Neurosporaxanthin production by Neurospora and Fusarium. In Microbial Carotenoids from Fungi: Methods and Protocols; Barredo, J.-L., Ed.; Springer Protocols: Totowa, NJ, USA, 2012; pp. 263–274.
- O’Leary, M.A.; Hanson, J.R.; Yeoh, B.L. The structure and biosynthesis of hinnuliquinone, a pigment from Nodulisporium hinnuleum. J. Chem. Soc. Perkin Trans. 1 1984, 1, 567–569.
- Velmurugan, P.; Lee, Y.H.; Nanthakumar, K.; Kamala-Kannan, S.; Dufossé, L.; Mapari, S.A.S.; Oh, B.T. Water-soluble red pigments from Isaria farinosa and structural characterization of the main colored component. J. Basic Microbiol. 2010, 50, 581–590.
- Wat, C.-K.; Mcinnes, A.G.; Smith, D.G.; Wright, J.L.C.; Vining, L.C. The yellow pigments of Beauveria species. Structures of tenellin and bassianin. Can. J. Chem. 1977, 55, 4090–4098.
- Isaka, M.; Chinthanom, P.; Supothina, S.; Tobwor, P. Pyridone and tetramic acid alkaloids from the spider pathogenic fungus Torrubiella sp. BCC 2165. J. Nat. Prod. 2010, 73, 2057–2060.
- Takahashi, S.; Uchida, K.; Kakinuma, N.; Hashimoto, R.; Yanagisawa, T.; Nakagawa, A. The structures of Pyridovericin and Pyridomacrolidin, new metabolites from the entomopathogenic fungus, Beauveria bassiana. J. Antibiot. 1998, 51, 1051–1054.
- Williamson, P.R.; Wakamatsu, K.; Ito, S. Melanin biosynthesis in Cryptococcus neoformans. J. Bacteriol. 1998, 180, 1570–1572.
- Harki, E.; Talou, T.; Dargent, R. Purification, characterization and analysis of melanin extracted from Tuber melanosporum vitt. Food Chem. 1997, 58, 69–73.
- Suryanarayanan, T.S.; Ravishankar, J.P.; Venkatesan, G.; Murali, T.S. Characterization of the melanin pigment of a cosmopolitan fungal endophyte. Mycol. Res. 2004, 108, 974–978.
- Cho, Y.J.; Park, J.P.; Hwang, H.J.; Kim, S.W.; Choi, J.W.; Yun, J.W. Production of red pigment by submerged culture of Paecilomyces sinclairii. Lett. Appl. Microbiol. 2002, 35, 195–202.
- Kot, A.M.; Błażejak, S.; Gientka, I.; Kieliszek, M.; Bryś, J. Torulene and torularhodin: “new” fungal carotenoids for industry? Microb. Cell Fact. 2018, 17, 49.
- Pollmann, H.; Breitenbach, J.; Wolff, H.; Bode, H.B.; Sandmann, G. Combinatorial biosynthesis of novel multi-hydroxy carotenoids in the red yeast Xanthophyllomyces dendrorhous. J. Fungi 2017, 3, 9.
- Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufossé, L. Anthraquinones and derivatives from marine-derived fungi: Structural diversity and selected biological activities. Mar. Drugs 2016, 14, 64.
- Fouillaud, M.; Venkatachalam, M.; Llorente, M.; Magalon, H.; Cuet, P.; Dufossé, L. Biodiversity of pigmented fungi isolated from the marine environment in La Reunion Island, Indian Ocean: New resources for colored metabolites. J. Fungi 2017, 3, 36.
- Trisuwan, K.; Khamthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Anthraquinone, cyclopentanone, and naphthoquinone derivatives from the sea fan-derived fungi Fusarium spp. PSU–F14 and PSU–F135. J. Nat. Prod. 2010, 73, 1507–1511.
- Huang, C.H.; Pan, J.H.; Chen, B.; Yu, M.; Huang, H.B.; Zhu, X.; Lu, Y.J.; She, Z.G.; Lin, Y.C. Three bianthraquinone derivatives from the mangrove endophytic fungus Alternaria sp. ZJ9–6B from the South China Sea. Mar. Drugs 2011, 9, 832–843.
- Li, D.-L.; Li, X.-M.; Wang, B.-G. Natural anthraquinone derivatives from a marine mangrove plant-derived endophytic fungus Eurotium rubrum: Structural elucidation and DPPH radical scavenging activity. J. Biotechnol. 2009, 19, 675–680.
- Sibero, M.T.; Triningsih, D.W.; Radjasa, O.K.; Sabdono, A.; Trianto, A. Evaluation of antimicrobial activity and identification of yellow pigmented marine sponge-associated fungi from Teluk Awur, Jepara, Central Java. Indones. J. Biotechnol. 2016, 21, 1–11.
- Chintapenta, L.K.; Rath, C.C.; Maringinti, B.; Ozbay, G. Pigment production from a mangrove Penicillium. Afr. J. Biotechnol. 2014, 13, 2668–2674.
- Lebeau, J.; Venkatachalam, M.; Fouillaud, M.; Petit, T.; Vinale, F.; Dufossé, L.; Caro, Y. Production and new extraction method of polyketide red pigments produced by ascomycetous fungi from terrestrial and marine habitats. J. Fungi 2017, 3, 34.
- Wang, W.; Liao, Y.; Chen, R.; Hou, Y.; Ke, W.; Zhang, B.; Gao, M.; Shao, Z.; Chen, J.; Li, F. Chlorinated azaphilone pigments with antimicrobial and cytotoxic activities isolated from the deep sea-derived fungus Chaetomium sp. NA–S01–R1. Mar. Drugs 2018, 16, 61.
- Venkatachalam, M.; Zelena, M.; Cacciola, F.; Ceslova, L.; Girard-Valenciennes, E.; Clerc, P.; Dugo, P.; Mondello, L.; Fouillaud, M.; Rotondo, A.; et al. Partial characterization of the pigments produced by the marine-derived fungus Talaromyces albobiverticillius 30548. Towards a new fungal red colorant for the food industry. J. Food Compost. Anal. 2018, 67, 38–47.
- Duarte, A.W.F.; de Menezes, G.C.A.; e Silva, T.R.; Bicas, J.L.; Oliveira, V.M.; Rosa, L.H. Antarctic fungi as producers of pigments. In Fungi of Antarctica; Rosa, L., Ed.; Springer: Cham, Switzerland, 2019; pp. 305–318.
More
