Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Sevser Sahpaz.
The carob tree (C. siliqua L.) is an evergreen perennial tree from the Fabaceae (Leguminosae) family. Originating in the Mediterranean region, it now populates many parts of the world, including North and South America, Africa, and Australia. The tree grows up to 15 m tall and boasts long, dark green leathery leaves.
- carob
- Ceratonia siliqua
- phytochemistry
- pharmacology
- toxicity
1. Introduction
The carob tree (C. siliqua L.) is an evergreen perennial tree from the Fabaceae (Leguminosae) family. Originating in the Mediterranean region, it now populates many parts of the world, including North and South America, Africa, and Australia. The tree grows up to 15 m tall and boasts long, dark green leathery leaves [1,2][1][2]. Until 1980, the Ceratonia genus contained only one species, C. siliqua. However, another species, C. oreothauma, has since been discovered in East Africa and the Arabian Peninsula [3].
This plant has been utilized by humans since the ancient times. It is valued for its economic and culinary importance. Its seeds, also known as carob, are used as a food source for both humans and livestock [4,5][4][5]. High in carbohydrates and protein, they can be ground into a powder and used as a chocolate substitute [6]. The leaves, bark, and seeds have traditionally been used in medicine to treat various diseases, including diarrhea, diabetes, and hypertension [7,8][7][8]. In addition to its culinary uses, carob is believed to have several pharmacological activities, including antioxidant, antidiarrheal, antibacterial, antiulcer, and anti-inflammatory effects [9,10][9][10]. Rtibi et al., 2015, suggest using carob as a natural antioxidant in the form of a food supplement for the prevention of damage caused by oxidative stress [9]. In Morocco, the carob tree is particularly abundant in certain regions (Marrakech, El Ksiba, Khenifra, Beni Mellal, Meknes, Essaouira, Elhaouz, Kasba Tadla…); it provides farmers in these areas with a way to add value to land unsuitable for other crops. These regions could become the centers of carob production in Morocco. Nothing appears to hinder the success of the carob tree in these areas, with some specimens growing with minimal care and yielding interesting outputs [11].
2. Phytochemistry of C. siliqua L.
Phytochemical compounds in carob vary greatly, influenced by factors including environment, maturation stage, and tree parts [80][12]. In a study conducted by Fadel et al., 2011 [81][13], the phenolic compounds present in the aqueous acetone extracts of carob pulps and seeds were analyzed. These carob samples were harvested from two areas, Izouika and Reggada, in southwest Morocco. The High-Performance Liquid Chromatography (HPLC) analysis of the extracts showed their richness in phenolic compounds. In both areas, the pulp extracts were richer in phenolic compounds than the seeds, both qualitatively and quantitatively. The phenolic profile of the pulp was dominated by coumaric acid (20.52% in Izouika vs. 17.05% in Reggada) and gallic acid (17.8% in Izouika vs. 12.57% in Reggada). In seed extracts, coumaric acid and gallic acid were also the predominant phenolic acids, with coumaric acid representing 8.07% in Izouika and 8.18% in Reggada, while gallic acid represented 5.01% in Izouika and 3.95% in Reggada. Syringic acid, 4-hydroxybenzoic acid, and gentisic acid are all benzoic acids present in carob [82][14]. HPLC methods have been used to determine polyphenols in carob pods, revealing the presence of condensed tannins (proanthocyanidins), composed of flavan-3-ol groups and their galloyl esters, gallic acid, catechin, epicatechingallate (ECG), epigallocatechingallate (EGCG), and quercetin glycosides [83,84][15][16]. The presence of hydrolysable tannins (gallotanins and ellagitannins) has also been detected in carob pods [85][17]. Carob fiber was found to contain a rich variety of phenolic compounds, with a total of 24 polyphenol compounds identified, yielding 3.94 g/kg (dry weight). The profile was dominated by gallic acid in various forms: free gallic acid (42% of polyphenols by weight), gallotannins (29%), and methyl gallate (1%). Simple phenols, mainly cinnamic acid, comprised about 2% of the total, and major flavonoids were identified as the glycosides myricetin and quercetin-3-O-α-L-rhamnoside [80][12]. In another study, HPLC analysis showed the main compounds in mature carob pods to be pyrogallol (48.02 ± 3.55%), catechin (19.10 ± 2.11%), and tannic acid (9.01 ± 1.40%) [9]. In immature carob pods, pyrogallol (26.45 ± 3.03%), catechin (16.52 ± 2.34%), gallic acid (15.12 ± 2.31%), chlorogenic acid (15.01 ± 1.72%), and epicatechin (12.26 ± 1.04%) were detected [10]. The HPLC technique also identified several phenolic compounds in leaves such as kaempferol (77 ± 2.43%), tannic acid (13 ± 0.45%), catechin hydrate (4.30 ± 0.34%), and polydatin (0.85 ± 0.22%) [86][18]. Phytochemical screening of the crude ethyl acetate and methanolic extracts of three types of carob tree barks (spontaneous male, spontaneous female, and grafted female) indicated the presence of flavonoids and tannins. Alkaloids and saponins were not detected. The total phenolic contents from the ethyl acetate and methanolic extracts of the three varieties of C. siliqua L. barks varied from 0.46 to 0.76 (g/L gallic acid equivalents). In this study, the methanolic extract had a higher phenolic content than the ethyl acetate extract. These phenolic compounds and other reported bioactive compounds are generally more soluble in polar solvents [87][19]. Carobs are particularly rich in flavonols such as quercetin, myricetin, kaempferol, and their glycosidic derivatives. Quercetin and myricetin rhamnosides are usually the most abundant flavonoids in carob. The presence of flavones (apigenin, luteolin, and chrysoidium), flavanones (naringenin), or isoflavones (genistein) is low [80,83,88][12][15][20]. According to a semi-quantitative ultra-performance liquid chromatography (UPLC) analysis carried out on the leaves of C. siliqua from the southern region of Morocco (Tafraoute), it was found that the major compounds in the aqueous extract of C. siliqua were luteolin-7-glucoside followed by epicatechin, apigenin-7-glucoside, quercetin-3-O-glucoside, caffeic acid, gallic acid, and chlorogenic acid. This indicates that C. siliqua leaves represent a good source of natural bioactive compounds. No aglycons were identified in the sample [89][21] (Figure 21).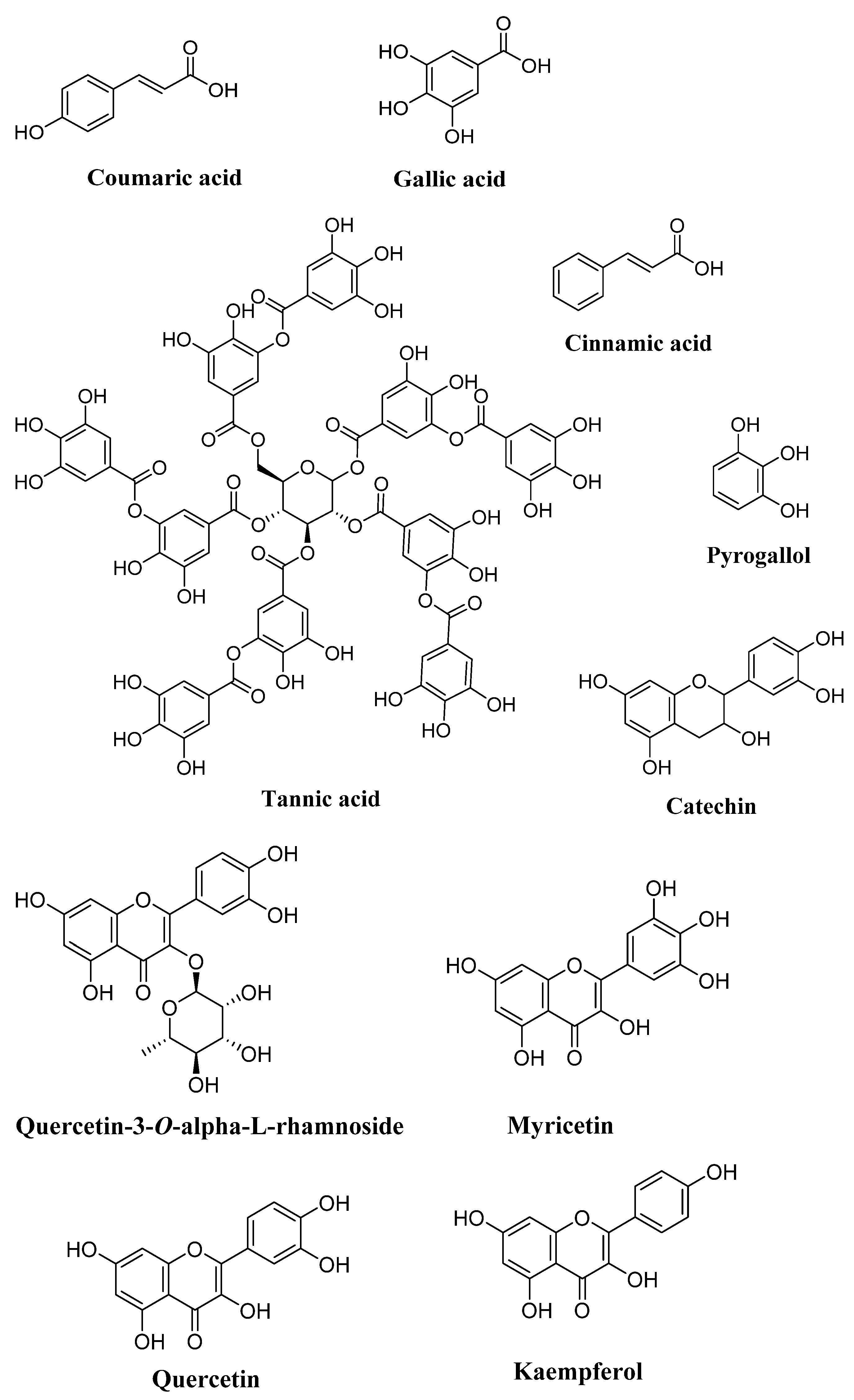
Figure 21.
Chemical compounds present in
C. siliqua
L. (drawn with ChemDraw 15.0).
- -
-
Total dietary fiber in carob pulp ranges from 30 to 40% [90][22].
- -
-
This fiber is primarily made up of lignin (50–65%), cellulose (15–25%), and hemicellulose (15–25%), with smaller amounts of pectin (0.5–2%), tannins (3–7%), and moisture (4–8%) [91][23].
- -
-
Carob fiber is primarily insoluble and is not easily fermented by gut bacteria [92][24].
- -
-
The soluble fiber content, which can be fermented in the colon, is significantly lower in carob and this portion contains simple carbohydrates [82][14].Table 31.Chemical composition of carob (C. siliquaL.).
Class Compounds Part of the Plant References Phenols Resorcinol Leaves, pods, pulp, seeds [102][34] Vanillin, fraxidin, 2,4-
bis(dimethylbenzyl)-6-butylphenolLeaves [102,109][34][42] Alizarin, hydroquinone, lignan
bis(trihydroxyphenyl)methanonePods [110,111][43][44] Phenolic acids Gallic acid, chlorogenic acid,
syringic acid, ferulic acid,
coumaric acid, cinnamic acidLeaves, pods, pulp, seeds [4,5,89,102,110,111,112,113,114,115,[116,117,118,119,120,121,122,123,124,125,126,127,5][128,21][129,130][4]34][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63] 4-Hydroxybenzoic acid, caffeic acid,
vanillic acid, gentisic acidLeaves, pods, pulp [5,89,102,112,113,114,119,121,122,125,126,127,128,129][5][21][34][45][46][47][52][54][55][58][59][60][61][62] Tannic acid Leaves, pods, seeds [4,122][4][55] Ellagic acid, rosmarinic acid Pods, pulp, seeds [111,119,125,126,127][44][52][58][59][60] Sinapic acid Pulp, seeds [111,126][44][59] Pyrogallol, methyl gallate, benzoic acid,
protocatechuic acidPods, pulp [4,5,110,111,119,120,122,128,129][4][5][43][44][52][53][55][61][62] Quinic acid Leaves, pods [109,120][42][53] Transferulic acid, O-feruloylrutinose, O-
feruloylrutinose isomer, p-coumaroyl-
galloylhexose, O-p-coumaroylrutinose,
siliquapyranonePods [110,120,125][43][53][58] 4-Hydroxy-coumaric acid Leaves [112,113][45][46] 5-Caffeoylquinic acid, myristic acid,
ascorbic acidPulp [111,126,131][44][59][64] Flavonoids Epicatechin, quercetin, kaempferol,
luteolin, catechin, apigeninLeaves, pods, pulp, seeds [4,5,589,102,]110,111,[21][34][43112,]113,115,[44]116,[45]118,[46]119,[120,121,48122,123,124,125,126,127,128,][49130,][51131,132][4][][52][53][54][55][56][57][58][59][60][61][63][64][65] Epigallocatechin gallate, rutin,
myricetin, naringeninLeaves, pods, pulp [4,5,5110,111,][43112,]113,114,[44]116,[117,118,45][46][47][49][50119,120,121,123,124,125,][51126,][52127,128][4][][53][54][56][57][58][59][60][61] Iso-rhamnetin Leaves, pods, seeds [102,110,111,119,125][34][43][44][52][58] Leucoanthocyanins Leaves, pulp, seeds [133][66] Genistein Leaves, pods [5,119][5][52] Quercitrin, catechin tannins Leaves, pulp [112,113,115,127,133][45][46][48][60][66] Anthocyanins Pods, pulp, seeds [5,134,135][5][67][68] Myricitrin, daidzein, flavonol, morin Leaves [102,112,113,114,116][34][45][46][47][49] Rhamnosides, chrysoeriol, tricetin
dimethyl ether, (iso)schaftoside-4′-O-
glucoside, gallocatechin, chrysoeriol-O-
deoxyheoxoside, dihydroxyflavanone
hexoside, tetrahydroxy flavanone, trihy-
droxy flavone (apigenin isomer), kamp-
feride, methoxykampferol, dihydroxy
flavanone, tricetin dimethyl ether, cirsi-
liol, flavone glycosides, hydroxytyrosolPods [5,110,119,120,122][5][43][52][53][55] Crismaritin, catechol, isoquercetrin,
flavonols 3′,4′,5,7-OH, 2-hexadecanol
scutellarin tetramethyl ether, silybin B,
hydroxytyrosol, catechin gallatePulp [4,126,][4][129,59][62]131[64] Apigenin flavone, chrysin aglycones Seeds [119][52]
References
- Hadi, M.Y.; Hameed, I.H.; Ibraheam, I.A. Ceratonia siliqua: Characterization, pharmaceutical products and analysis of bioactive compounds: A review. Res. J. Pharm. Technol. 2017, 10, 3585–3589.
- Battle, I.; Tous, J. Carob Tree: Ceratonia siliqua L.—Promoting the Conservation and Use of Underutilized and Neglected Crops. 17; Institute of Plant Genetics and Crops Plant Research, Gatersleben/International Plant Genetic Ressources Institute: Rome, Italy, 1997.
- Hillcoat, D.; Lewis, G.; Verdcourt, B. A new species of Ceratonia (Leguminosae-Caesalpinioideae) from Arabia and the Somali Republic. Kew Bull. 1980, 35, 261–271.
- Brassesco, M.E.; Brandão, T.R.S.; Silva, C.L.M.; Pintado, M. Carob bean (Ceratonia siliqua L.): A new perspective for functional food. Trends Food Sci. Technol. 2021, 114, 310–322.
- Basharat, Z.; Afzaal, M.; Saeed, F.; Islam, F.; Hussain, M.; Ikram, A.; Pervaiz, M.U.; Awuchi, C.G. Nutritional and functional profile of carob bean (Ceratonia siliqua): A comprehensive review. Int. J. Food. Prop. 2023, 26, 389–413.
- Loullis, A.; Pinakoulaki, E. Carob as cocoa substitute: A review on composition, health benefits and food applications. Eur. Food Res. Technol. 2018, 244, 959–977.
- Ali-Shtayeh, M.S.; Jamous, R.M.; Jamous, R.M.; Salameh, N.M. Complementary and alternative medicine (CAM) use among hypertensive patients in Palestine. Complement Ther. Clin. Pr. 2013, 19, 256–263.
- Baytop, T. Therapy with Medicinal Plants in Turkey (Past and Present); No: 3255; Istanbul University: Istanbul, Turkey, 1984; p. 359.
- Rtibi, K.; Jabri, M.A.; Selmi, S.; Souli, A.; Sebai, H.; El-Benna, J.; Amri, M.; Marzouki, L. Carob pods (Ceratonia siliqua L.) inhibit human neutrophils myeloperoxidase and in vitro ROS-scavenging activity. RSC Adv. 2015, 5, 84207–84215.
- Rtibi, K.; Selmi, S.; Jabri, M.-A.; Mamadou, G.; Limas-Nzouzi, N.; Sebai, H.; El-Benna, J.; Marzouki, L.; Eto, B.; Amri, M. Effects of aqueous extracts from Ceratonia siliqua L. pods on small intestinal motility in rats and jejunal permeability in mice. RSC Adv. 2016, 6, 44345–44353.
- Sbay, H. Le Caroubier au Maroc: Un Arbre D'avenir; Centre de Recherche Forestière du Haut Commissariat aux Eaux et Forêts et à la Lutte contre la Désertification: Rabat, Morocco, 2008.
- Owen, R.; Haubner, R.; Hull, W.; Erben, G.; Spiegelhalder, B.; Bartsch, H.; Haber, B. Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem. Toxicol. 2003, 41, 1727–1738.
- Fadel, F.; Fattouch, S.; Tahrouch, S.; Lahmar, R.; Benddou, A.; Hatimi, A. The phenolic compounds of Ceratonia siliqua pulps and seeds (Les composes phénoliques des pulpes et des graines de Ceratonia siliqua). J. Mater. Env. Sci. 2011, 2, 285–292.
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional components of carob fruit: Linking the chemical and biological space. Int. J. Mol. Sci. 2016, 17, 1875.
- Ortega, N.; Macia, A.; Romero, M.-P.; Trullols, E.; Morello, J.-R.; Angles, N.; Motilva, M.-J. Rapid determination of phenolic compounds and alkaloids of carob flour by improved liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2009, 57, 7239–7244.
- Corsi, L.; Avallone, R.; Cosenza, F.; Farina, F.; Baraldi, C.; Baraldi, M. Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia 2002, 73, 674–684.
- Avallone, R.; Plessi, M.; Baraldi, M.; Monzani, A. Determination of chemical composition of carob (Ceratonia siliqua): Protein, fat, carbohydrates, and tannins. J. Food Compos. Anal. 1997, 10, 166–172.
- Rtibi, K.; Jabri, M.-A.; Selmi, S.; Sebai, H.; Amri, M.; El-Benna, J.; Marzouki, L. Ceratonia siliqua leaves exert a strong ROS-scavenging effect in human neutrophils, inhibit myeloperoxydase in vitro and protect against intestinal fluid and electrolytes secretion in rats. RSC Adv. 2016, 6, 65483–65493.
- El Hajaji, H.; Lachkar, N.; Alaoui, K.; Cherrah, Y.; Farah, A.; Ennabili, A.; El Bali, B.; Lachkar, M. Antioxidant activity, phytochemical screening, and total phenolic content of extracts from three genders of carob tree barks growing in Morocco. Arab. J. Chem. 2011, 4, 321–324.
- Papagiannopoulos, M.; Wollseifen, H.R.; Mellenthin, A.; Haber, B.; Galensa, R. Identification and quantification of polyphenols in Carob Fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MS n. J. Agric. Food Chem. 2004, 52, 3784–3791.
- Abidar, S.; Boiangiu, R.S.; Dumitru, G.; Todirascu-Ciornea, E.; Amakran, A.; Cioanca, O.; Hritcu, L.; Nhiri, M. The aqueous extract from Ceratonia siliqua leaves protects against 6-hydroxydopamine in zebrafish: Understanding the underlying mechanism. Antioxidants 2020, 9, 304.
- Haber, B. Carob fiber benefits and applications. Cereal Foods World 2002, 47, 365.
- Marco, A.M.; De Mora, B.R.; Diaz, C.S. Method of Making Natural Carob Fiber. US Patent 5609905A, USA, 11 March 1997. Available online: https://patents.google.com/patent/US5609905A/en (accessed on 14 May 2023).
- Nasar-Abbas, S.M.; e-Huma, Z.; Vu, T.H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob kibble: A bioactive-rich food ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72.
- Khlifa, M.; Bahloul, A.; Kitane, S. Determination of chemical composition of carob pod (Ceratonia siliqua L.) and its morphological study. J. Mater. Env. Sci. 2013, 4, 348–353.
- Petit, M.; Pinilla, J. Production and purification of a sugar syrup from carob pods. LWT–Food Sci. Tech. 1995, 28, 145–152.
- Fidan, H.; Stankov, S.; Petkova, N.; Petkova, Z.; Iliev, A.; Stoyanova, M.; Ivanova, T.; Zhelyazkov, N.; Ibrahim, S.; Stoyanova, A. Evaluation of chemical composition, antioxidant potential and functional properties of carob (Ceratonia siliqua L.) seeds. J. Food Sci. Technol. 2020, 57, 2404–2413.
- Ayaz, F.A.; Torun, H.; Glew, R.H.; Bak, Z.D.; Chuang, L.T.; Presley, J.M.; Andrews, R. Nutrient content of carob pod (Ceratonia siliqua L.) flour prepared commercially and domestically. Plant. Foods Hum. Nutr. 2009, 64, 286.
- Sigge, G.; Iipumbu, L.; Britz, T. Proximate composition of carob cultivars growing in South Africa. S. Afr. J. Plant Soil 2011, 28, 17–22.
- Ayaz, F.A.; Torun, H.; Ayaz, S.; Correia, P.J.; Alaiz, M.; Sanz, C.; Grúz, J.; Strnad, M. Determination of chemical composition of anatolian carob pod (Ceratonia siliqua L.): Sugars, amino and organic acids, minerals and phenolic compounds. J. Food Qual. 2007, 30, 1040–1055.
- Smith, B.M.; Bean, S.R.; Schober, T.J.; Tilley, M.; Herald, T.J.; Aramouni, F. Composition and molecular weight distribution of carob germ protein fractions. J. Agric. Food Chem. 2010, 58, 7794–7800.
- Cebolla, Á.; Moreno, M.d.L.; Coto, L.; Sousa, C. Gluten immunogenic peptides as standard for the evaluation of potential harmful prolamin content in food and human specimen. Nutrients 2018, 10, 1927.
- Mahtout, R.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Gracia, I.; Hernández-Fernández, F.J.; Pérez de los Ríos, A.; Zaidia, F.; Sanchez-Segado, S.; Lozano-Blanco, L.J. Algerian carob tree products: A comprehensive valorization analysis and future prospects. Sustainability 2018, 10, 90.
- Ghanemi, F.Z.; Belarbi, M. Phytochemistry and Pharmacology of Ceratonia siliqua L. leaves. J. Nat. Prod. Res. App. 2021, 1, 69–82.
- Musa Özcan, M.; Arslan, D.; Gökçalik, H. Some compositional properties and mineral contents of carob (Ceratonia siliqua) fruit, flour and syrup. Int. J. Food Sci. Nutr. Eng. 2007, 58, 652–658.
- Dakia, P.A.; Wathelet, B.; Paquot, M. Isolation and chemical evaluation of carob (Ceratonia siliqua L.) seed germ. Food Chem. 2007, 102, 1368–1374.
- Iipumbu, L. Compositional Analysis of Locally Cultivated Carob (Ceratonia siliqua) Cultivars and Development of Nutritional Food Products for a Range of Market Sectors; Stellenbosch University: Stellenbosch, South Africa, 2008; Available online: https://api.semanticscholar.org/CorpusID:129160277 (accessed on 20 January 2023).
- El Hajaji, H.; Farah, A.; Ennabili, A.; Bousta, D.; Greche, H.; El Bali, B.; Lachkar, M. Etude comparative de la composition minérale des constituants de trois catégories de Ceratonia siliqua L. (Comparative study of the mineral composition of the constituents of three varieties of Ceratonia siliqua L.). J. Mater. Env. Sci. 2013, 4, 165–170.
- Oziyci, H.R.; Tetik, N.; Turhan, I.; Yatmaz, E.; Ucgun, K.; Akgul, H.; Gubbuk, H.; Karhan, M. Mineral composition of pods and seeds of wild and grafted carob (Ceratonia siliqua L.) fruits. Sci. Hortic. 2014, 167, 149–152.
- El Bouzdoudi, B.; Saïdi, R.; Khalid, E.; El Mzibri, M.; Nejjar, A.; El Kbiach, M.; Lamarti, A. Mineral composition of mature carob (Ceratonia siliqua L.) Pod: A Study. Int. J. Food Sci. Nutr. Eng. 2017, 7, 91–103.
- Dallali, S.; Aloui, F.; Selmi, H.; Sebei, H. Comparison of the chemical composition and the antioxidant activity of the leaves of Carob tree (Ceratonia siliqua L.) collected in three sites of Djebel Zaghouan (Tunisia). J. New Sci. Agric. Biotechnol. CIRS 2018, 21, 3429–3438.
- De Falco, B.; Grauso, L.; Fiore, A.; Bonanomi, G.; Lanzotti, V. Metabolomics and chemometrics of seven aromatic plants: Carob, eucalyptus, laurel, mint, myrtle, rosemary and strawberry tree. Phytochem. Anal. 2022, 33, 696–709.
- Farag, M.A.; El-Kersh, D.M.; Ehrlich, A.; Choucry, M.A.; El-Seedi, H.; Frolov, A.; Wessjohann, L.A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem. 2019, 283, 675–687.
- Samia, O.; Nesrine, M.; Feten, Z.K.; Riadh, K. Tunisian Ceratonia siliqua: Phytochemical Analysis, Antioxidant Activity, Preparation and Characterization of Carob Emulsion System. Eur. J. Nutr. Food Saf. 2022, 14, 41–52.
- Spizzirri, U.G.; Abduvakhidov, A.; Caputo, P.; Crupi, P.; Muraglia, M.; Oliviero Rossi, C.; Clodoveo, M.L.; Aiello, F.; Restuccia, D. Kefir enriched with carob (Ceratonia siliqua L.) leaves extract as a new ingredient during a gluten-free bread-making process. Fermentation 2022, 8, 305.
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Oqal, M.; Alqudah, M.; AbuDalo, R.; Nabil, A.-H. Ceratonia siliqua leaves ethanol extracts exert anti-nociceptive and anti-inflammatory effects. Heliyon 2022, 8, e10400.
- Abidar, S.; Yildiz, O.; Degirmenci, A.; Amakran, A.; El Maadoudi, M.; Nhiri, M. Glucose-mediated protein glycation: Contribution of methanolic extract of Ceratonia siliqua L. in protection and in vitro potential inhibition of acetylcholinesterase. J. Food Biochem. 2019, 43, e13009.
- Ghanemi, F.Z.; Belarbi, M.; Fluckiger, A.; Nani, A.; Dumont, A.; De Rosny, C.; Aboura, I.; Khan, A.S.; Murtaza, B.; Benammar, C. Carob leaf polyphenols trigger intrinsic apoptotic pathway and induce cell cycle arrest in colon cancer cells. J. Funct. Foods 2017, 33, 112–121.
- Aissani, N.; Coroneo, V.; Fattouch, S.; Caboni, P. Inhibitory effect of carob (Ceratonia siliqua) leaves methanolic extract on Listeria monocytogenes. J. Agric. Food Chem. 2012, 60, 9954–9958.
- Hsouna, A.B.; Trigui, M.; Jarraya, R.M.; Damak, M.; Jaoua, S. Identification of phenolic compounds by high performance liquid chromatography/mass spectrometry (HPLC/MS) and in vitro evaluation of the antioxidant and antimicrobial activities of Ceratonia siliqua leaves extracts. J. Med. Plant. Res. 2015, 9, 479–485.
- Eldahshan, O.A. Isolation and structure elucidation of phenolic compounds of carob leaves grown in Egypt. Curr. Res. J. Biol. Sci. 2011, 3, 52–55.
- Rasheed, D.M.; El-Kersh, D.M.; Farag, M.A. Ceratonia siliqua (carob-locust bean) outgoing and potential trends of phytochemical, economic and medicinal merits. In Wild Fruits: Composition, Nutritional Value and Products, 1st ed.; Mariod, A.A., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 481–498.
- Karmous, I.; Taheur, F.B.; Zuverza-Mena, N.; Jebahi, S.; Vaidya, S.; Tlahig, S.; Mhadhbi, M.; Gorai, M.; Raouafi, A.; Debara, M. Phytosynthesis of Zinc Oxide Nanoparticles Using Ceratonia siliqua L. and Evidence of Antimicrobial Activity. Plants 2022, 11, 3079.
- Soleimanzadeh, A.; Kian, M.; Moradi, S.; Mahmoudi, S. Carob (Ceratonia siliqua L.) fruit hydro-alcoholic extract alleviates reproductive toxicity of lead in male mice: Evidence on sperm parameters, sex hormones, oxidative stress biomarkers and expression of Nrf2 and iNOS. Avicenna J. Phytomed. 2020, 10, 35. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6941692/ (accessed on 30 March 2023).
- Rashed, K. Phytochemical and biological effects of Ceratonia siliqua L: A review. Int. J. Pharm. Sci. Res. Inter. 2021, 9, 1–8. Available online: https://www.ijipsr.com/index.php/IJIPSR/article/view/39/25 (accessed on 30 March 2023).
- Gregoriou, G.; Neophytou, C.M.; Vasincu, A.; Gregoriou, Y.; Hadjipakkou, H.; Pinakoulaki, E.; Christodoulou, M.C.; Ioannou, G.D.; Stavrou, I.J.; Christou, A. Anti-cancer activity and phenolic content of extracts derived from Cypriot carob (Ceratonia siliqua L.) pods using different solvents. Molecules 2021, 26, 5017.
- Goulas, V.; Georgiou, E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: Extraction optimization and application in food models. Foods 2020, 9, 20.
- Ghorbaninejad, Z.; Eghbali, A.; Ghorbaninejad, M.; Ayyari, M.; Zuchowski, J.; Kowalczyk, M.; Baharvand, H.; Shahverdi, A.; Eftekhari-Yazdi, P.; Esfandiari, F. Carob extract induces spermatogenesis in an infertile mouse model via upregulation of Prm1, Plzf, Bcl-6b, Dazl, Ngn3, Stra8, and Smc1b. J. Ethnopharmacol. 2023, 301, 115–760.
- Richane, A.; Rim, B.M.; Riadh, K.; Khaoula, A.; Nizar, M.; Hanen, B.I. Variability of phenolic compounds and antioxidant activities of ten Ceratonia siliqua L. provenances. Biochem. Syst. Ecol. 2022, 104, 104486.
- Martić, N.; Zahorec, J.; Stilinović, N.; Andrejić-Višnjić, B.; Pavlić, B.; Kladar, N.; Šoronja-Simović, D.; Šereš, Z.; Vujčić, M.; Horvat, O. Hepatoprotective effect of carob pulp flour (Ceratonia siliqua L.) extract obtained by optimized microwave-assisted extraction. Pharmaceutics 2022, 14, 657.
- Darwish, W.S.; Khadr, A.E.S.; Kamel, M.A.E.N.; Abd Eldaim, M.A.; El Sayed, I.E.T.; Abdel-Bary, H.M.; Ullah, S.; Ghareeb, D.A. Phytochemical characterization and evaluation of biological activities of egyptian carob pods (Ceratonia siliqua L.) aqueous extract: In vitro study. Plants 2021, 10, 2626.
- Mansouri, F.E.; Silva, J.C.E.; Cacciola, F.; Asraoui, F.; Tayeq, H.; Ben Amar, Y.M.; Lovillo, M.P.; Chouaibi, N.; Brigui, J. Evaluation of Different Extraction Methods on the Phenolic Profile and the Antioxidant Potential of Ceratonia siliqua L. Pods Extracts. Molecules 2022, 27, 6163.
- Amrani, A.; Bouakline, H.; Elkabous, M.; Brahmi, M.; Karzazi, Y.; El Bachiri, A.; Tahani, A. Ceratonia siliqua L seeds extract: Experimental analysis and simulation study. Mater. Today Proc. 2023, 72, 3705–3711.
- Atta, A.H.; Atta, S.A.; Khattab, M.; Abd El-Aziz, T.H.; Mouneir, S.M.; Ibrahim, M.; Nasr, S.M.; Ramadan, S. Ceratonia siliqua pods (Carob) methanol extract alleviates doxorubicin–induced nephrotoxicity via antioxidant, anti-inflammatory and anti-apoptotic pathways. Env. Sci. Pollut. Res. 2023, 30, 83421–83438.
- Ayache, S.B.; Reis, F.S.; Dias, M.I.; Pereira, C.; Glamočlija, J.; Soković, M.; Saafi, E.B.; Ferreira, I.C.; Barros, L.; Achour, L. Chemical characterization of carob seeds (Ceratonia siliqua L.) and use of different extraction techniques to promote its bioactivity. Food Chem. 2021, 351, 129263.
- Yahiaoui, K.; Bouchenak, O.; Boumaza, S.; Toubal, S.; Blizak, D.; Nouani, A.; Arab, K. Characterization and assessment of the antimicrobial function of total polyphenol extracts from pulps, leaves and seeds of two Ceratonia siliqua L. varieties. Alger. J. Envir. Sci. Tech. 2021, 7, 3429–3438.
- Ben Ayache, S.; Behija Saafi, E.; Emhemmed, F.; Flamini, G.; Achour, L.; Muller, C.D. Biological activities of aqueous extracts from carob plant (Ceratonia siliqua L.) by antioxidant, analgesic and proapoptotic properties evaluation. Molecules 2020, 25, 3120.
- Lakkab, I.; El Hajaji, H.; Lachkar, N.; Lefter, R.; Ciobica, A.; El Bali, B.; Lachkar, M. Ceratonia siliqua L. seed peels: Phytochemical profile, antioxidant activity, and effect on mood disorders. J. Funct. Foods 2019, 54, 457–465.
More
