Impressively increasing availability of mechanical circulatory/cardiac support systems (MCSs) worldwide, together with the deepening of the knowledge of critical care medical practitioners, has inevitably led to the discussion about further improvements of intensive care associated to MCS. An appealing topic of the left ventricle (LV) overload related to VA ECMO support endangering myocardial recovery is being widely discussed within the scientific community. Unloading of LV leads to the reduction in LV end-diastolic pressure, reduction in pressure in the left atrium, and decrease in the LV thrombus formation risk. Consequently, better conditions for myocardial recovery, with comfortable filling pressures and a better oxygen delivery/demand ratio, are achieved. The combination of VA ECMO and Impella device, also called ECPELLA, seems to be a promising strategy that may bring the improvement of CS mortality rates.
1. VA ECMO-Generated Circulation and Harlequin Syndrome
Blood flow generated by VA ECMO is non-physiological. The blood stream is directed against the physiological flow (if any is preserved) in the aorta. Thus, two extreme conditions can emerge. If the
left ventricle (LV
) function is maintained to some extent, then there is a meeting (mixing) point of the physiological and ECMO blood stream somewhere in the aortic arch. Thus, if the LV function is preserved enough to shift this mixing point distal from the origin of the
left common carotid artery (or
left subclavian artery), a condition called Harlequin syndrome may ensue. Harlequin, or north–south syndrome is reported in 8.8% of cases
[1]. Pathophysiologically, the main issue is that the blood from the native output of the LV may be poorly oxygenated due to concomitant pulmonary dysfunction. On the other hand, ECMO-generated blood stream contains highly oxygenated blood. As a result of the phenomenon, hypoxia of areas supplied by the branches originating from the aortic arch and perfused by the native left ventricle output may be observed. This is particularly dangerous considering a possibility of cerebral hypoxic damage
[2][3]. Not less importantly, the myocardium itself is compromised in this situation, as coronary arteries originate from the most proximal part of the aorta
[4]. Owing to these anatomical considerations, the myocardium may still suffer from inadequate oxygen delivery. Based on explained hemodynamic principles, monitoring via an arterial line for the sampling of blood gases should be placed in the artery of the right upper extremity (typically
radial artery). Another possibility to detect hypoxic cerebral perfusion is the use of near-infrared-spectroscopy (NIRS) monitoring, attached to patient’s forehead
[3]. If pulmonary dysfunction is advanced and the LV function is better than expected, the appropriate solution seems to be adding another returning venous cannula into the
internal jugular vein and thus upgrading to V-AV ECMO to assure adequate oxygenation of the blood leaving the LV.
2. Left Ventricle Overload
Thanks to large bore cannulas and modern pumps, VA ECMO can provide substantial flow support; typical flows usually range around 3 to 4.5 L per minute. VA ECMO achieves this by directly drawing blood from the systemic venous system, which reduces the right ventricular preload and alleviates peripheral venous congestion. The flow rate (Q) is determined by the pressure gradient created by the pump and is significantly influenced by the cannula’s radius (directly proportional to r^4). Additionally, it is inversely proportional to fluid viscosity (η) and cannula length (l), as per Poiseuille’s law Q = πPr^4/8ηl
[5].
While it may seem that this redirection of blood away from the heart would also reduce the LV preload and alleviate pulmonary congestion, this is not the right understanding of the situation. This is partly due to the fact that ECMO, despite providing higher flows, elevates blood pressure. Consequently, even with the increased ECMO-induced arterial flow, some blood continues to flow through the pulmonary circuit (as not all of it is diverted into the drainage cannula and instead passing through the right atrium and right ventricle). Factors such as the Thebesian drainage of coronary blood flow, the presence of aortic regurgitation, and the return of bronchial blood flow to the LA contribute to blood returning to the LV. To exit the LV, blood must overcome the ECMO-induced increase in arterial pressure, necessitating the LV to generate sufficient pressure
[5].
It is obvious that in patients with peripheral VA ECMO, it is not possible to completely drain the blood entering the heart; thus, residual basic circulation through the natural pathway still exists. This phenomenon may cause serious clinical issues in another extreme hemodynamic situation, which will be emphasized in this research. In case of severe LV dysfunction without any effective stroke volume, the impaired function of the LV is furthermore deteriorated by the increase in the afterload caused by the blood stream originating from the ECMO. As a consequence of this, typical hemodynamic pattern may
[4][6] be seen:
- (1)
-
increased end-diastolic LV pressure (LVEDP);
-
- (2)
-
increased LV wall tension;
-
- (3)
-
worsening of mitral valve regurgitation;
-
- (4)
-
increased LA pressure;
-
- (5)
-
elevation of pulmonary venous pressure;
-
- (6)
-
increased risk of LV thrombosis
-
Even if there would not be clinically significant deterioration in LV pulsatility, the pathophysiology connected to the adaptation of the LV to the increased afterload is working behind the scenes. Thus, firstly, LVEDP is increased, which is followed with increased calcium sensitivity of cardiomyocytes resulting in higher contractile power. These adaptation mechanisms, important for the preservation of the LV stroke volume despite the increased afterload, are tightly tied to a higher oxygen demand of the myocardium
[1].
Adjusting VA ECMO flow to the lowest sufficient value accompanied with thorough restrictive fluid management using diuretics, hemodialysis (CVVHD), or continuous veno-venous hemofiltration (CVVH) and inotrope agents might help with left heart decompression. However, this conservative approach might not be possible in most severe cardiogenic shock patients presented with a completely akinetic LV.
Patients with aortic valve regurgitation and a competent mitral valve are logically more endangered with the LV overload. Considering another valve pathology, the presence of mitral valve regurgitation enables the transmission of pressure from an overloaded LV into the LA and pulmonary circulation (increase in PCWP (pulmonary capillary wedge pressure) and PAP (pulmonary artery pressure) is measured) resulting in the congestion and deterioration of oxygenation.
In extremis, when the leaflets of the aortic valve are not being opened, clinically presented as a non-pulsatile flow, the thrombosis of the aortic valve, aortic root, and LV may occur (with following fatal systemic thromboembolic complications). Described alterations in the physiology of the heart may lead not only to the development of pulmonary edema (by the increase in pulmonary venous pressure) but even to the worsening of myocardial ischemia (due to the elevation of LV wall tension and LVEDP) and the frequency of malignant ventricular arrhythmias. These changes can be summarized in terms of increased mechanical stress and strain.
3. How to Recognize Left Ventricle Overload?
Detecting LV distention and pulmonary edema during VA ECMO support plays a crucial role in patient care. Various clinical indicators can be utilized to monitor and identify patients who may be at risk. Firstly, one straightforward approach involves assessing the presence and extent of aortic valve opening through arterial pulse pressure tracing. As ECMO flow increases, the mean arterial pressure rises, but the pulse pressure and stroke volume decrease, indicating a reduction in aortic valve opening
[5].
Secondly, echocardiography can provide direct visualization of the degree and duration of aortic valve opening. The use of an M-Mode through the aortic valve can help determine if and to what degree the aortic valve is opening. However, it is worth noting that when it comes to assessing LV distention, echocardiography may have limitations. This is because changes in LV dimensions may not accurately reflect changes in LVEDP due to the nonlinear nature of the LVEDP relationship and pericardial constraints
[5]. Additionally, variations in the LV chamber size during ECMO support can be misleading as indicators of ventricular distention may be influenced by previous pathology (present before VA ECMO).
Thirdly, progressive hypoxia in blood exiting the LV, which can be measured from the right radial artery or by cerebral oximetry, may indicate the perfusion of the upper circulation with deoxygenated blood due to worsening pulmonary edema
[5] Lastly, deteriorating pulmonary edema observed in chest X-rays can signal increasing PCWP. However, this finding may occur late and lacks specificity, as radiographic changes can also result from other conditions such as acute respiratory distress syndrome or infection. While each of these four measures can help detect aortic valve opening and LV loading, they only offer indirect indicators of monitoring for increases in PCWP. The most reliable index for assessing LV filling pressures is to have a pulmonary artery catheter (PAC) in place and directly measure either the pulmonary artery diastolic pressure or PCWP
[5]Unloading of the LV seems to be a reasonable solution to preserve the LV function by decreasing the energy demand and possibly achieve better outcomes. Several modalities were described (
Table 1) for this purpose
[4][6][7]:
- (1)
-
pharmacological management (maintaining of the appropriate stroke volume by the administration of inotropic agents, i.e., dobutamine, PDE III inhibitors, levosimendan);
-
- (2)
-
surgical (vent in the LV/left atrium/pulmonary artery);
-
- (3)
-
percutaneous devices (such as an intra-aortic balloon pump (IABP) and percutaneous axial flow devices like the Impella family by Abiomed).
-
Table 1. Different modalities of LV unloading (based on Belohlavek et al.
[2] and Meani et al.
[8]; LA = left atrium, PA = pulmonary artery, LV = left ventricle).
Considering the surgical approach sternotomy or thoracotomy is usually used to place a venting cannula into the pulmonary vein, directly to the LV, left atrium, or even pulmonary artery. An obvious disadvantage of these methods is the degree of invasiveness and high risk of bleeding complications.
Therefore, percutaneous methods with limited invasiveness have become most widely used. Nowadays, in 84% of cases, LV unloading was provided by percutaneous devices, and 16% of cases underwent surgical procedure in order to unload the overdistended LV
[7].
4. Impella Device—The New Hope for Effective Left Ventricle Unloading
The Impella device is a catheter-based, small measured percutaneous axial flow LVAD, which is able to create a blood flow of up to 2.5/3.5/5.5 L/min depending on a particular type of the machine. The Impella family currently contains Impella CP, 5.5, and RP (for the right heart support)
[9][10]. Impella CP is implanted via the
femoral artery; Impella 5.5 requires arteriotomy and a vascular graft.
5. History of Impella
The origin of the idea of how blood flow is created in Impella leads to the ancient Greece, where Archimedes’s screw was invented (approx. 200–300 BC). The American physician Richard Wampler, a father of the reborn idea, developed a predecessor of the Impella in 1985. After years of improvements, in approx. 2000, finally the Impella family of devices was revealed
[9][10].
6. Why to Unload?
LV overload after VA ECMO implantation puts myocardial recovery in danger. Unloading of the LV leads to the reduction in the LV end-diastolic pressure, reduction in the pressure in the left atrium, and the decrease in the LV thrombus formation risk. To conclude, better conditions for myocardial recovery, with comfortable filling pressures and a better oxygen delivery/demand ratio, are achieved
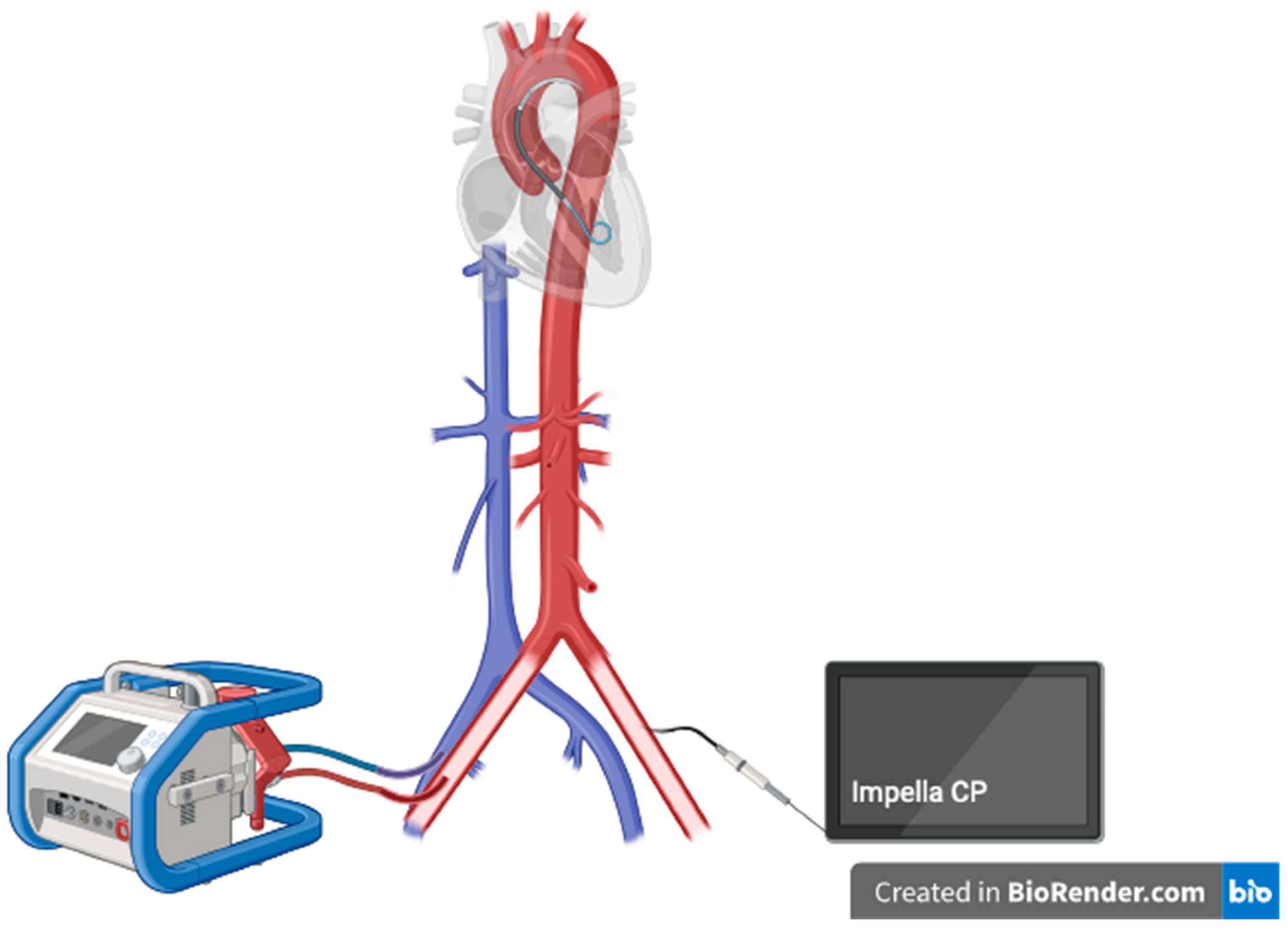
[11]. Currently, a growing evidence on possible lower mortality associated with the use of Impella device as a modality for the LV unloading in ECMO patients evolves. The combination of VA ECMO and Impella is usually labeled as ECPELLA or ECMELLA, which is shown in
Scheme 1.
Scheme 1. ECPELLA configuration for LV unloading.
Drawbacks of using Impella are increased risk of bleeding complications, hemolysis, and abdominal compartment syndrome
[11]. Notably, the use of ECPELLA brings a higher risk of need for renal replacement therapy (RRT) in comparison with VA ECMO alone
[11]. The rational consequence of the ECPELLA configuration is the higher risk of Harlequin syndrome, whereas adding another venous cannula via the
internal jugular vein (for the return of oxygenated blood) and transformation to V-AV ECMO + Impella should be a solution to the issue
[12].
Impella family devices are contraindicated for patients with LV thrombosis, mechanical aortic valve prosthesis, moderate-to-severe aortic valve disease, and severe peripheral artery disease (due to the risk of limb ischemia and/or technical issues with implantation). The above-mentioned list of limitations is even more bounding in critically ill patients due to the higher frequency of these pathological conditions.