There is a long and interesting history between honeybees and humans. From the beginning, honey has been utilized not only as a sweetener, but also as an ointment and a drug to treat several diseases. Until the discovery of antibiotics, honey was a very popular product used to protect and preserve skin and promote wound healing, to counteract gastrointestinal pains and disorders of the oral cavity, and for other diseases. After the development of antibiotic resistance, honey again gained interest for its use in wound management. Subsequently, more recently, in vitro and in vivo studies have displayed antimicrobial, antioxidant, and other effects of honey and honeybee products, as well as protection of cardiovascular, respiratory, nervous, and gastrointestinal systems.
- aquaporin-3
- honey
- honeydew honey
- propolis
- royal jelly
1. Introduction
2. Honey and Skin Regeneration
Honey played an important role in traditional medicine for centuries [17][15]. However, it has a limited application in modern medicine due to a lack of scientific support [18][16]. Since a few decades ago, thanks to the renewed interest in ethno-pharmacology and the use of principles of natural origin, several laboratories have begun to study the properties of honey not only in terms of antibacterial effects [27][17]. Honey has been evaluated for its effects on both keratinocytes and fibroblasts in the context of wound healing [28,29][18][19]. EMT is a biological process in which epithelial cells, which are typically organized in sheets and have a more stationary nature, undergo a transformation into mesenchymal cells, showing a more migratory phenotype and have a spindle-like shape [30][20]. Honey-driven wound closure is induced by keratinocyte re-epithelialization activation, but the EMT induction ability differs noticeably among honeys, according to their botanical origin [29][19]. Ca2+ signaling plays a crucial role in wound healing, including keratinocyte biology [31][21]. It regulates various cellular processes, such as migration, proliferation, differentiation, and cell–cell adhesion, all of which are critical for the successful closure and restoration of the wounded skin [32][22]. The understanding the complex interplay between keratinocyte and Ca2+ signaling [31][21] during wound healing is of crucial importance, providing insights into potential therapeutic targets for enhancing the healing process. This is the first observation demonstrating how honey exposure affects [Ca2+]i regulation in keratinocytes due to hydrogen peroxide production and redox regulation of ion channels. Honey has been shown to interact with the immune system, controlling cytokine production [7]. Honey is a natural ready-to-eat product rich in flavonoids. Some authors have proposed that honey flavonoids can mitigate inflammatory processes, and thus currently support studies of the anti-inflammatory potential of honeys [37][23]. Honey has been also reported to exhibit anti-inflammatory effects by modulating the production and release, in the monocytic cell line, MonoMac-6 (MM6), of pro-inflammatory cytokines, such as interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α), and consequently dampening inflammation in wound healing [38,39][24][25]. Likewise, honey seems to either reduce or activate the ROS production from neutrophils, also depending on the wound microenvironment [40][26]. Synthesis and deposition of collagen represents an important step of the extracellular matrix remodeling for wound repair [43][27]. Metalloproteinases (MMPs) and their inhibitors (TIMPs, tissue inhibitors of metalloproteinases) are essential in wound and tissue repair [46][28]. Majtan et al. [47][29] and Ranzato et al. [29][19] have reliably confirmed that honey exposure induces MMP-9 expression in a human keratinocyte cell line. In fibroblasts, TIMP or MMPs upregulation by honey was limited to MMP-3 induction with manuka, and TIMP-1 with buckwheat and manuka honey. The TIMP-1 increase upon buckwheat treatment has been correlated to the anti-inflammatory properties of this protein, considering that it has been boosted in fibroblasts by cytokine exposure [48][30].3. Honey and Endothelial Repair
In the framework of wound healing, endothelial repair is a crucial process that occurs to restore the integrity and functionality of blood vessels within the wound area [49][31]. In fact, optimal wound healing requires a coordinated response involving various cell types, including endothelial cells [50][32]. Endothelial cells play a central role in angiogenesis by proliferating, migrating, and organizing into functional blood vessels. Angiogenesis supplies oxygen and nutrients to the wound site, facilitating tissue repair [51][33]. Endothelial cells are activated in response to wound signals, such as growth factors and cytokines released by various cell types [52][34]. This activation triggers endothelial cell proliferation, migration, and the expression of adhesion molecules that facilitate their recruitment to the wound area. Disruption or dysfunction of endothelial repair can lead to impaired wound healing, such as delayed angiogenesis or impaired vascular integrity [53][35]. Exposure to honey produces an increase in extracellular H2O2, and this peroxide could pass in the cells through a specific aquaporin, i.e., aquaporin-3 (AQP-3). Such an aquaporin is also present in endothelial cells [57][36] and allows the entry of H2O2 that could start the signaling cascade. The increase in hydrogen peroxide, in the cytoplasm, induces the activation of Ca2+-channel TRPM2 [58][37], provoking an entry of Ca2+ from outside, the PLC-IP3 activation [59][38] and then the release of Ca2+ from the endoplasmic reticulum [60][39]. Taken together, these data suggest the pivotal role of honey-produced H2O2 as a mediator of endothelial cell physiology in response to buckwheat honey exposure, suggesting the central role played by Ca2+ signaling.4. Honeydew Honey
Honeydew honey (HH), also known as forest honey, is a type of honey produced by bees collecting honeydew secretions from aphids or other sap-sucking insects found in trees [63][40]. HH is increasingly valued by consumers and the food industry, due to its valuable nutritional and medicinal qualities, which are different from floral honey [64][41]. Moreover, HH showed equivalent or, in some cases, higher activities compared with medical-grade kanuka and manuka honey [65][42]. HH shows a darker color and a high polyphenol content, as well as more antioxidants and antibacterial activity compared to blossom honeys [66][43], highlighting this honey as a potential health-promoting food. However, in respect to blossom honey, there are few anecdotal data about the biological effects of HH. Martinotti et al. [64][41], due to wide ethnopharmacological use of HH, demonstrated that honeydew honey has low cytotoxicity on skin fibroblasts and keratinocytes, thereby allowing HH to be considered safe for external application on skin. Moreover, an in vitro scratch wound assay showed that HH produces an increase in wound-healing abilities in both skin cells. Analysis of cell signaling, through use of specific inhibitors, also demonstrated that HH acts in the same way in both cell types, and Ca2+ signaling seems to play a basic role.5. Propolis
Propolis is a resinous substance that bees collect from various sources, such as exudates, buds and plants in the north temperate zone, extending from the Tropic of Cancer to the Arctic Circle [69][44]. The main sources of propolis are willow, birch, alder, elm, beech, conifer, and horse-chestnut trees [70][45]. Bees utilized propolis to seal and protect their hives. Propolis has been traditionally used in folk medicine for its therapeutic effects, and scientific research in recent years has also considered its potential benefits for health. There are substantial data demonstrating that propolis possess antibacterial, antiviral, antifungal, and antiseptic effects, as well as anti-antioxidant and anti-inflammatory properties. Propolis exhibits broad-spectrum antimicrobial properties, helping to fight against various types of microorganisms, including bacteria, fungi, and viruses [73,74][46][47]. These effects are mainly due to the synergistic activity of the many compounds present in propolis [73][46]. It has generally been found that propolis’ antibacterial activity is greater against Gram-positive than Gram-negative bacteria [75][48]. This is explicated by the specific organization of the Gram-negative bacteria outer membrane and by the hydrolytic enzyme present in propolis [76][49]. In particular, artepillin C (3,5-diprenyl-p-coumaric acid) is one of the numerous phenolic compounds present in propolis [77][50]. Propolis also displays anti-inflammatory effects, principally due to its high content of polyphenols, thus inhibiting inflammatory mediator production, such as cytokines and prostaglandins, and supporting the healing process [79,80][51][52]. Propolis is generally considered an anti-oxidant; however, in some circumstances, it may also act as a pro-oxidant oxidative promoting environment [82][53]. For this pro-oxidant action, there is the need for transition metal ions and some phenolic compounds. These phenols are present in propolis (such as chrysin, pinocembrin, and galangin) and they act as temporary electron carriers in redox reactions, in which electrons from ferrous ions are relayed to oxygen molecules producing superoxide, after which H2O2 is made [81][54].6. Royal Jelly
Royal jelly (RJ) is an acid colloid (3.6–4.2 pH) composed mainly of sugar, proteins, lipids, water, vitamins, and some mineral salts [84[55][56],85], produced by worker bees and used to nourish and develop queen bees [86][57]. RJ, a traditional cure for various skin injuries [87][58], has not been extensively utilized in clinical practice or studied for wound management, principally due to the lack of knowledge on the RJ bioactive molecules and on the precise mechanisms boosting the wound repairing ability [88][59]. Therefore, there is limited scientific exploration focused on RJ’s effects on wound repair. However, some research has proposed that RJ expresses positive effects in promoting wound repair due to bioactive components. The topical application of RJ on diabetic foot ulcers suggests that RJ can positively boost wound repair [89[60][61],90], and RJ is able to promote tissue healing in animal models [91][62]. Bucekova and co-workers identified an RJ component able to induce the increase in matrix metalloproteinase-9 (MMP-9) [16][63]. They identified defensin-1 as the main factor responsible for inducing MMP-9 secretion and in vitro keratinocyte migration. Defensin-1 also improves wound closure and re-epithelialization in rats, promoting wound healing in vivo [16][63].7. Honey and “Green Chemistry”
Green chemistry focuses on designing and developing chemical procedures, processes, and products to make them environmentally friendly and more sustainable, minimizing their impact on human health and the planet. Green chemistry is devoted to decreasing or eliminating dangerous substance use, waste generation reduction, and endorsing energy proficiency. In this contest, honey utilization is a very promising way to realize this new “green” approach [97][64]. Honey is a renewable resource and it can be harvested without negatively impacting the environment. Moreover, honey is a non-toxic compound posing minimal risks to human health and to the environment. Honey has been successfully proposed as an agent in the green synthesis of silver nanoparticles (AgNPs) [97,98,99][64][65][66], widely used as standard antibacterial therapy for wounds [98,100,101][65][67][68]. Poly-sugars of honey can perform both reduction and stabilization of metallic ions required for AgNPs synthesis [102][69]. Moreover, Obot and collaborators [103][70] used honey and sunlight irradiation for silver nanoparticle production. Malaysian honey has also been used to realized silver nanoparticles in an easy, reproducible and cost-effective green approach [104][71]. Honey was utilized as a reducing and stabilizing agent in the place of dangerous chemicals, such as sodium borohydride and formamide. Taken together, information about honey usage for the synthesis of AgNPs shows that nanoparticles manufactured using honey with more antimicrobial efficacy showed better additive or synergistic properties than nanoparticles alone [105][72]. Some problems are still present, and the main limitation is the use of correct honey type or the use of standardized honey [106][73].8. Conclusions
Honey is included in the International Nomenclature of Cosmetic Ingredients (INCI) under the names of “Honey” or “Mel” (CAS no. 8028-66-8) and is classified as a humectant/emollient/moisturizing product. A huge amount of skin care formulations enclosing honey or other beehive products are available in the literature [114][74]. Honey has been shown to be much more than a simple food product, but rather a valuable medical product with multiple mechanisms and beneficial virtues, in particular as a wound-healing booster. Figure 32 summarizes the effects of honey on the classical phases of wound healing).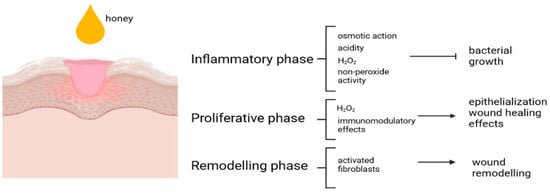
References
- Martinotti, S.; Ranzato, E. Honey, Wound Repair and Regenerative Medicine. J. Funct. Biomater. 2018, 9, 34.
- Ajibola, A.; Chamunorwa, J.P.; Erlwanger, K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. 2012, 9, 61.
- Martinotti, S.; Ranzato, E. Honey’s healing history. In Cellular and Molecular Mechanisms of Honey Wound Healing; Martinotti, R., Ed.; Nova Publishers Inc.: Hauppage, NY, USA, 2014.
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 2018, 25, 320–325.
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323.
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30.
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127.
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322.
- Ball, D. The Chemical Composition of Honey. J. Chem. Educ. 2007, 84, 1643–1646.
- Patrignani, M.; Fagúndez, G.A.; Tananaki, C.; Thrasyvoulou, A.; Lupano, C.E. Volatile compounds of Argentinean honeys: Correlation with floral and geographical origin. Food Chem. 2018, 246, 32–40.
- Escuredo, O.; Seijo, M.C. Honey: Chemical Composition, Stability and Authenticity. Foods 2019, 8, 577.
- Rossano, R.; Larocca, M.; Polito, T.; Perna, A.M.; Padula, M.C.; Martelli, G.; Riccio, P. What are the proteolytic enzymes of honey and what they do tell us? A fingerprint analysis by 2-D zymography of unifloral honeys. PLoS ONE 2012, 7, e49164.
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical Properties, Minerals, Trace Elements, and Heavy Metals in Honey of Different Origins: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233.
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011, 12, 9514–9532.
- Zumla, A.; Lulat, A. Honey—A remedy rediscovered. J. R. Soc. Med. 1989, 82, 384–385.
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160.
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013, 16, 731–742.
- Ranzato, E.; Martinotti, S.; Burlando, B. Honey exposure stimulates wound repair of human dermal fibroblasts. Burn. Trauma 2013, 1, 32–38.
- Ranzato, E.; Martinotti, S.; Burlando, B. Epithelial mesenchymal transition traits in honey-driven keratinocyte wound healing: Comparison among different honeys. Wound Repair Regen. 2012, 20, 778–785.
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587.
- Bikle, D.D.; Xie, Z.; Tu, C.L. Calcium regulation of keratinocyte differentiation. Expert Rev. Endocrinol. Metab. 2012, 7, 461–472.
- Ghilardi, S.J.; O’Reilly, B.M.; Sgro, A.E. Intracellular signaling dynamics and their role in coordinating tissue repair. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1479.
- Silva, B.; Biluca, F.C.; Gonzaga, L.V.; Fett, R.; Dalmarco, E.M.; Caon, T.; Costa, A.C.O. In vitro anti-inflammatory properties of honey flavonoids: A review. Food Res. Int. 2021, 141, 110086.
- Tonks, A.; Cooper, R.A.; Price, A.J.; Molan, P.C.; Jones, K.P. Stimulation of TNF-alpha release in monocytes by honey. Cytokine 2001, 14, 240–242.
- Tonks, A.J.; Cooper, R.A.; Jones, K.P.; Blair, S.; Parton, J.; Tonks, A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247.
- Majtan, J. Honey: An immunomodulator in wound healing. Wound Repair Regen. 2014, 22, 187–192.
- Ranzato, E.; Martinotti, S.; Volante, A.; Mazzucco, L.; Burlando, B. Platelet lysate modulates MMP-2 and MMP-9 expression, matrix deposition and cell-to-matrix adhesion in keratinocytes and fibroblasts. Exp. Dermatol. 2011, 20, 308–313.
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234.
- Majtan, J.; Kumar, P.; Majtan, T.; Walls, A.F.; Klaudiny, J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp. Dermatol. 2010, 19, e73–e79.
- Dasu, M.R.; Barrow, R.E.; Spies, M.; Herndon, D.N. Matrix metalloproteinase expression in cytokine stimulated human dermal fibroblasts. Burns 2003, 29, 527–531.
- Evans, C.E.; Iruela-Arispe, M.L.; Zhao, Y.Y. Mechanisms of Endothelial Regeneration and Vascular Repair and Their Application to Regenerative Medicine. Am. J. Pathol. 2021, 191, 52–65.
- Moccia, F.; Tanzi, F.; Munaron, L. Endothelial remodelling and intracellular calcium machinery. Curr. Mol. Med. 2014, 14, 457–480.
- Moccia, F.; Berra-Romani, R.; Tanzi, F. Update on vascular endothelial Ca(2+) signalling: A tale of ion channels, pumps and transporters. World J. Biol. Chem. 2012, 3, 127–158.
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295.
- Munaron, L.; Fiorio Pla, A. Endothelial calcium machinery and angiogenesis: Understanding physiology to interfere with pathology. Curr. Med. Chem. 2009, 16, 4691–4703.
- Da Silva, I.V.; Barroso, M.; Moura, T.; Castro, R.; Soveral, G. Endothelial Aquaporins and Hypomethylation: Potential Implications for Atherosclerosis and Cardiovascular Disease. Int. J. Mol. Sci. 2018, 19, 130.
- Shimizu, S.; Yonezawa, R.; Negoro, T.; Yamamoto, S.; Numata, T.; Ishii, M.; Mori, Y.; Toda, T. Sensitization of H2O2-induced TRPM2 activation and subsequent interleukin-8 (CXCL8) production by intracellular Fe2+ in human monocytic U937 cells. Int. J. Biochem. Cell Biol. 2015, 68, 119–127.
- Wang, L.; Negro, R.; Wu, H. TRPM2, linking oxidative stress and Ca. Curr. Opin. Immunol. 2020, 62, 131–135.
- Taylor, C.W.; Tovey, S.C. IP(3) receptors: Toward understanding their activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a004010.
- Pita-Calvo, C.; Vázquez, M. Honeydew Honeys: A Review on the Characterization and Authentication of Botanical and Geographical Origins. J. Agric. Food. Chem. 2018, 66, 2523–2537.
- Martinotti, S.; Calabrese, G.; Ranzato, E. Honeydew honey: Biological effects on skin cells. Mol. Cell Biochem. 2017, 435, 185–192.
- Bucekova, M.; Juricova, V.; Monton, E.; Martinotti, S.; Ranzato, E.; Majtan, J. Microwave processing of honey negatively affects honey antibacterial activity by inactivation of bee-derived glucose oxidase and defensin-1. Food Chem. 2018, 240, 1131–1136.
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Carmen Seijo, M. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856.
- Bonsignore, G.; Martinotti, S.; Ranzato, E. Propolis: A Multifaceted Approach for Wound Healing. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Murthy, H.N., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 689–697.
- Martinotti, S.; Ranzato, E. Propolis: A new frontier for wound healing? Burns Trauma 2015, 3, 9.
- Grange, J.M.; Davey, R.W. Antibacterial properties of propolis (bee glue). J. R. Soc. Med 1990, 83, 159–160.
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047.
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086.
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905.
- Beserra, F.P.; Gushiken, L.F.S.; Hussni, M.F.; Ribeiro, V.P.; Bonamin, F.; Jackson, C.J.; Pellizzon, C.H.; Bastos, J.K. Artepillin C as an outstanding phenolic compound of Brazilian green propolis for disease treatment: A review on pharmacological aspects. Phytother. Res. 2020, 35, 2274–2286.
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100.
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent Update on the Anti-Inflammatory Activities of Propolis. Molecules 2022, 27, 6473.
- Tsai, Y.C.; Wang, Y.H.; Liou, C.C.; Lin, Y.C.; Huang, H.; Liu, Y.C. Induction of oxidative DNA damage by flavonoids of propolis: Its mechanism and implication about antioxidant capacity. Chem. Res. Toxicol. 2012, 25, 191–196.
- Martinotti, S.; Pellavio, G.; Laforenza, U.; Ranzato, E. Propolis Induces AQP3 Expression: A Possible Way of Action in Wound Healing. Molecules 2019, 24, 1544.
- Melliou, E.; Chinou, I. Chemistry and bioactivity of royal jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992.
- Bagameri, L.; Baci, G.M.; Dezmirean, D.S. Royal Jelly as a Nutraceutical Natural Product with a Focus on Its Antibacterial Activity. Pharmaceutics 2022, 14, 1142.
- Fujita, T.; Kozuka-Hata, H.; Ao-Kondo, H.; Kunieda, T.; Oyama, M.; Kubo, T. Proteomic analysis of the royal jelly and characterization of the functions of its derivation glands in the honeybee. J. Proteome Res. 2013, 12, 404–411.
- Lin, Y.; Zhang, M.; Wang, L.; Lin, T.; Wang, G.; Peng, J.; Su, S. The in vitro and in vivo wound-healing effects of royal jelly derived from Apis mellifera L. during blossom seasons of Castanea mollissima Bl. and Brassica napus L. in South China exhibited distinct patterns. BMC Complement. Med. Ther. 2020, 20, 357.
- Yang, X.Y.; Yang, D.S.; Zhang, W.; Wang, J.M.; Li, C.Y.; Ye, H.; Lei, K.F.; Chen, X.F.; Shen, N.H.; Jin, L.Q.; et al. 10-Hydroxy-2-decenoic acid from Royal jelly: A potential medicine for RA. J. Ethnopharmacol. 2010, 128, 314–321.
- Abdelatif, M.; Yakoot, M.; Etmaan, M. Safety and efficacy of a new honey ointment on diabetic foot ulcers: A prospective pilot study. J. Wound Care 2008, 17, 108–110.
- Siavash, M.; Shokri, S.; Haghighi, S.; Mohammadi, M.; Shahtalebi, M.A.; Farajzadehgan, Z. The efficacy of topical Royal Jelly on diabetic foot ulcers healing: A case series. J. Res. Med. Sci. 2011, 16, 904–909.
- Siavash, M.; Shokri, S.; Haghighi, S.; Shahtalebi, M.A.; Farajzadehgan, Z. The efficacy of topical royal jelly on healing of diabetic foot ulcers: A double-blind placebo-controlled clinical trial. Int. Wound J. 2015, 12, 137–142.
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci. Rep. 2017, 7, 7340.
- Bonsignore, G.; Patrone, M.; Martinotti, S.; Ranzato, E. “Green” Biomaterials: The Promising Role of Honey. J. Funct. Biomater. 2021, 12, 72.
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int. J. Nanomed. 2014, 9, 2399–2407.
- Vijayaraghavan, K.; Nalini, S.P. Biotemplates in the green synthesis of silver nanoparticles. Biotechnol. J. 2010, 5, 1098–1110.
- Wang, L.; Zhang, T.; Li, P.; Huang, W.; Tang, J.; Wang, P.; Liu, J.; Yuan, Q.; Bai, R.; Li, B.; et al. Use of Synchrotron Radiation-Analytical Techniques To Reveal Chemical Origin of Silver-Nanoparticle Cytotoxicity. ACS Nano 2015, 9, 6532–6547.
- Sudmann, E.; Vik, H.; Rait, M.; Todnem, K.; Andersen, K.J.; Julsham, K.; Flesland, O.; Rungby, J. Systemic and local silver accumulation after total hip replacement using silver-impregnated bone cement. Med. Prog. Technol. 1994, 20, 179–184.
- Philip, D. Honey mediated green synthesis of gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 650–653.
- Obot, I.B.; Umorena, S.A.; Johnsona, A.S. Sunlight-mediated synthesis of silver nanoparticles using honey and its promising anticorrosion potentials for mild steel in acidic environments. J. Mater. Environ. Sci. 2013, 4, 1013–1018.
- Haiza, H.; Azizan, A.; Mohidin, A.H.; Halin, D.S.C. Green Synthesis of Silver Nanoparticles Using Local Honey. Nano Hydrids 2013, 4, 87–98.
- Al-Brahim, J.S.; Mohammed, A.E. Antioxidant, cytotoxic and antibacterial potential of biosynthesized nanoparticles using bee honey from two different floral sources in Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 363–373.
- González Fáa, A.J.; Juanb, A.; Di Nezio, M.S. Synthesis and Characterization of Silver Nanoparticles Prepared with Honey: The Role of Carbohydrates. Anal. Lett. 2017, 50, 877–888.
- McLoone, P.; Oluwadun, A.; Warnock, M.; Fyfe, L. Honey: A Therapeutic Agent for Disorders of the Skin. Cent. Asian J. Glob. Health 2016, 5, 241.
