Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Laurent Dufossé and Version 2 by Peter Tang.
Microbial oddities such as versatile pigments are gaining more attention in research due to their widely perceived applications as natural food colorants, textiles, antimicrobial activities, and cytotoxic activities.
- microbial pigments
- pigment compounds
- food colorants
- bioactive pigment molecules
- pigment applications
1. Introduction
Microbial communities have an enormous potentiality to produce diverse and mesmerizing aesthetic traits, such as knack emission of bioluminescence and fluorescence, formation of magnetosomes, production of bioactive metabolites, and different pigments for scientific succulence. Colloquially, directly or indirectly, microbial communities play an important integrated role in the biosphere by regulating biogeochemical and ecological processes [1]. Regardless of their role in the environment, they offer several benefits to humanity; one such benefit is pigment production by several microbes, of which deserved importance is being highlighted in recent times, and there are still more untapped sources to explore many unknown pigmented compounds [2]. The importance of microbial pigments has been emphasized in different applications, such as cosmetics, food, pharmaceuticals, and textiles, and these compounds are also well-known to exhibit cytotoxic, antioxidant, antimicrobial, antimalarial, anticancer, antitumor, and antifouling activities [3][4][5][6][7][3,4,5,6,7].
Pigments are molecules that absorb a specific wavelength of light and reflect the rest of the pulchritude visible spectrum (380–750 nm). Pigment production is one of the charismatic traits of microbes. Apparently, microbial pigments are not merely colors, but they possess a mixture of diverse chemical components with multifaceted potential biological activities [8]. In the last two decades, studies on pigmented microorganisms from terrestrial and marine ecosystems have tremendously expanded, resulting in the use of pigments in cancer-related research.
Microbial pigmented molecules such as bacteriochlorophylls, carotenoids, flavins, indigoids, melanins, pheomelanin, monascins, phenazines, phenazostatin D, prodigiosin, quinone precursors, violacein, glaukothalin, pycocyanin, xanthomonadin, phenazine, canthaxanthin, astaxanthin, β-carotene, etc. are produced as biproducts by several microorganisms [3][9][10][11][12][3,9,10,11,12]. Many of these compounds and their derivatives are reported to show wide range of cell specific biological activities which are expressed in effective/inhibitory/lethal/subleathal concentrations such as effective dose (ED), growth inhibitory concentration (GIC), minimum inhibitory concentration (MIC), Half maximal effective concentration (EC50), half maximal growth inhibition (GI50), half maximal inhibitory concentration (IC50), half maximal lethal concentration (LC50), half maximal lethal dose (LD50), and tumour growth inhibition of 50% (TGI50) [13].
There are several synthetic colorants that are being developed as immunosuppressive and anticancer drugs [14]. Historical notes on several synthetic dyes and pigments are of industrial applications (textile, cosmetics, and food), and their disadvantages have recently been well-detailed [15][16][15,16]. Since a number of synthetic pigments and their biproducts are found to display toxic, teratogenic, and carcinogenic properties, the exploration of natural pigments from microbes has emerged in recent years due to their biodegradability than synthetic counterparts [17]. Several natural pigment compounds originated from different sources are being used as food colorants [18]. Irrespective of plant, animal, and synthetic pigments, microbial pigments are much mellower and highly preferred due to their higher productivity and optimizable culture conditions. Pigment productions by Monascus, Rhodotorula, marine actinomycetes, marine Pseudoalteromonas, and marine cyanobacterial species have been widely studied.
2. Microbial Pigments and Chemical Structures
Microbial pigments are usually seen in two forms as pigments diffused out into the media and pigments retained within the cells. To produce any kind of pigmented compound with potential biological properties from any organism for industrial applications, the following requirements need to be fulfilled: Organisms should be amenable to culture, have a fast growth rate, be optimizable, have a high productivity in limited space and in short time, be available and able to produce throughout the year, be nontoxigenic, be nonpathogenic, be able to grow in a wide range of nutrients such as carbon and nitrogen sources, and be tolerant to a broad spectrum of physical (light and temperature) and chemical (pH and osmolarity) parameters used in production. Mostly, these requirements are met with microbes; hence, many research studies have been prioritized and focused on microbes (particularly marine bacteria are highly preferred due to their higher productivity and optimizable culture conditions) as potential sources over plants, animals, and synthetic compounds. Further inclination towards optimization and improvement of mass production of pigments are encouraged with genetic engineering as well. There are four main sources of pigments for various applications as mentioned above: (1) Plant-derived pigments, (2) animal-derived pigments, (3) microbial pigments, and (4) synthetic pigments. Here, merely microbial pigments are reviewed due to their insatiable demand over the rest of the sources and for better understanding on the same aspect. Some of the carotenoid pigments, such as acetylenic carotenoids appear to be restricted to certain environments, e.g., some marine bacteria Planococcus maritimus and Rubritalea squalenifaciens biosynthesize acyclic C30-type carotenoic acids [19]. In nature, C50 Carotenoids such as sarcinaxanthin and decaprenoxanthin are exclusively biosynthesized by Halobacteria, Halococcus, Actinomycetales, Flavobacterium dehydrogenans, Arthrobacter sp., Micrococcus luteus, Dietzia sp., Corynebacterium poinsettiae, C. glutamicum, and a strain of Pseudomonas, and these carotenoids appear to be not produced by plants [20][21][20,21]. Similarly, aryl carotenoids such as isorenieratene, 3-hydroxy-isorenieratene and 3,3′-di-hydroxy-isorenieratene are found in very few microorganisms, such as Brevibacterium linens, Streptomyces mediolani, and Mycobacterium aurum [21]. Identifying metabolic pathways and genes responsible for such rare phenomena are of great importance for genetic engineering studies to develop rare carotenoids with therapeutic application. The chemical structures of some of the major pigment molecules are given below: carotenoids: Acyl glyco-carotenoic acid (diapolycopenedioic acid) (1), adonixanthin (2), alloxanthin (3), anhydrorhodovibrin (4), antheraxanthin (5), astaxanthin (6), aleuriaxanthin (7), aphanicin (8), aphanizophyll (9), auroxanthin (10), β-carotene (11), bacterioruberin (12), caloxanthin (13), canthaxanthin (14), chlorobactene (15), chloroxanthin (16), crocoxanthin (17), cryptoxanthin (18), deinoxanthin (19), decaprenoxanthin (20), demethylspheroidene (21), demethylspheroidenone (22), diadinoxanthin (23), diatoxanthin (24), diapolycopene (25), dinoxanthin (26), echinenone (27), ergoxanthin (28), escholtzxanthin (29), eutreptiellanone (30), flavacin (31), flexirubin (32), flexixanthin (33), fucoxanthin (34), gyroxanthin diester (35), heteroxanthin (36), isorenieratene (37), lutein (38), loroxanthin (39), lycopene (40), monadoxanthin (41), mutachrome (42), mutatoxanthin (43), mycosporine (44), myxobactin (45), myxobactone (46), keto-myxocoxanthin (47), myxoxanthophyll (48), Nanocystis exedens pigments (49), nostoxanthin (50), neoxanthin (51), neurosporene (52), okenone (53), oscilloxanthin (54), phoenicoxanthin (55), phytoene (56), prasinoxanthin (57), pyrroxanthin (58), rhodopin (59), rhodovibrin (60), salinixanthin (61), saproxanthin (62), sarcinaxanthin (63), siphonein (64), siphonaxanthin (65), spheroidene (66), spheroidenone (67), spirilloxanthin (68), staphyloxanthin (69), torulene (70), torularhodin (71), vaucherixanthin (72), violaxanthin (73), vioxanthin (74), xanthomonadines (75), xanthophyll (76), and zeaxanthin (77); phycobiliproteins: cyanophycin (78), phycocyanin (79), phycocyanobilin (80), phycoerythrin (81), phycoerythrobilin (82), and phycourobilin (83); flavins: ankaflavin (84), monascoflavin (85), riboflavin (86), roseoflavin (87), and toxoflavin (88); melanins: melanin precursors such as catechol (89), 1,8-dihydroxynaphthalene (DHN) (90), Dopa (91), eumelanin (92), l-glutaminyl-4-hydroxybenzene (GHB) (93), homogentisic acid (HGA) (94), lincomycin (95), phaeomelanin (96), phenazostatin D (97), and all trans-retinal (98); heterocyclic pigments: cycloprodigiosin (99), indigotine (indigo) (100), indigoidine (101), prodigiosin (102), undecylprodigiosin (103), and violacein (104); phenazine compounds: actinomycin D (105), chlororaphine (106), Dihydrophencomycin (107), griseolutein (108), iodinin (109), myxin (cuprimycin) (110), oxychlororaphine (111), phenazine-1-carboxylic acid (112), phenozostatin D (113), pyocyanin (114), pyorubin (aeruginosinA+B) (115), and pyoverdin (116); quinones: arpink red (117), averythrin (118), austrocortinin (119), bostrycoidin (120), catenarin (121), cercosporin (122), chlorobiumquinone (123), 7-chloroemodin (124), chrysophanol (125), citreorosein (126), cynodontin (127), dermocybin (128), dermoglaucin (129), dermorubin (130), draconin (131), elsinochromes (132), emodin (133), erythroglaucin (134), fallacinal (135), flaviolin (136), flavomannin (137), fusarubin (138), helminthosporin (139), javanicin (140), juglone (141), Karuquinone A (142), menaquinone-7 (143), naphthoquinone (144), nectriachrysone (145), pachybasin (146), parietinic acid (147), phomaligin A (148), phomarin (149), physcion (150), piloquinone (151), questin (152), rubellin D (153), rubrocristin (154), skyrin (155), spinulosin (156), teloschistin (157), tritisporin (158), and Xylindein (159); monascus pigments: ankaflavin (84), lovastatin (160), monascins (161), monascoflavin (85), monascorubramine (162), monascorubrin (163), rubropunctamine (164), and rubropunctatine (165); other compounds: acetylazulene (166), actinorhodin (167), akashin (168), albidin (169), alterperylenol (170), amitenone (171), ammosamide A (172), ammosamide B (173), atranorin (174), atromentin (175), aulosirazole (176), aurantricholide B (177), aurasperone A (178), azaphilone (179), azulene (180), boviquinone 3 (181), bromoalterochromides (182), calycin (183), candidin (184), chloronatronochrome (185), chrysogenin (186), citrinin (187), cochliodinol (188), cordycepin (189), cordycepoid A (190), diastaphenazine (191), dihydroalterperylenol (192), dihydroxyazulene (193), dolastatin (194), epicocconone (195), floccosin (196), fluorescein (197), fonsecin (198), glauconic acid (199), glaukothalin (200), gomphidic acid (201), granadaene (202), grevilline A (203), gyrophoric acid (204), haematopodin (205), hyaluromycin (206), hypericin (207), iridosporin (208), lactaroviolin (209), laetiporic acid A (210), lilacinone (211), luteosporin (212), magnesidin (213), marineosin A (214), marinone (215), melanocrocin (216), mitorubrin (217), mycenaaurin A (218), N-carboxamidostaurosporine (219), natronochrome (220), nicotine (221), nostocine A (222), panosialin (223), peridinin (224), pestalone (225), Phlegmacin A (226), phenoxazine (227), porphyrin (228), pulvinic acid (229), purpuride (230), pyrandione (231), pyrrocidine A (232), rhizocarpic acid (233), roseophilin (234), rubrolone (235), rubrosporin (236), rubrosulphin (237), rumbrin (238), sanguinone A (239), scytonemin (240), siroheme (241), sorbicillin (242), stearoyldeterrol (243), sterigmatocystin (244), streptochlorin (245), tambjamine (246), tetrabromopyrrole (247), thermorubin (248), tryptanthrin (249), variegatorubin (250), viomellein (251), viopurpurin (252), vulpinic acid (253), xanthomegnin (254), bacterial luciferin (255), dinoflagellate luciferin (256), blue fluorescent protein-lumazine (BFP) (257) and yellow fluorescent protein (YFP) chromophore (258); and chlorophylls: bacteriochlorophylls (259), chlorophylls (260) and divinyl-chlorophylls (261) occurring in different microorganisms are illustrated (Figure 1; Table S1). Here, thwe researchers aalso categorize the microbial pigments into two categories: (1) Fluorescent pigments (phycoerythrins, fluorescein, epicocconone, BFP, and YFP) and (2) nonfluorescent pigments (rest of the pigmented compounds as detailed above).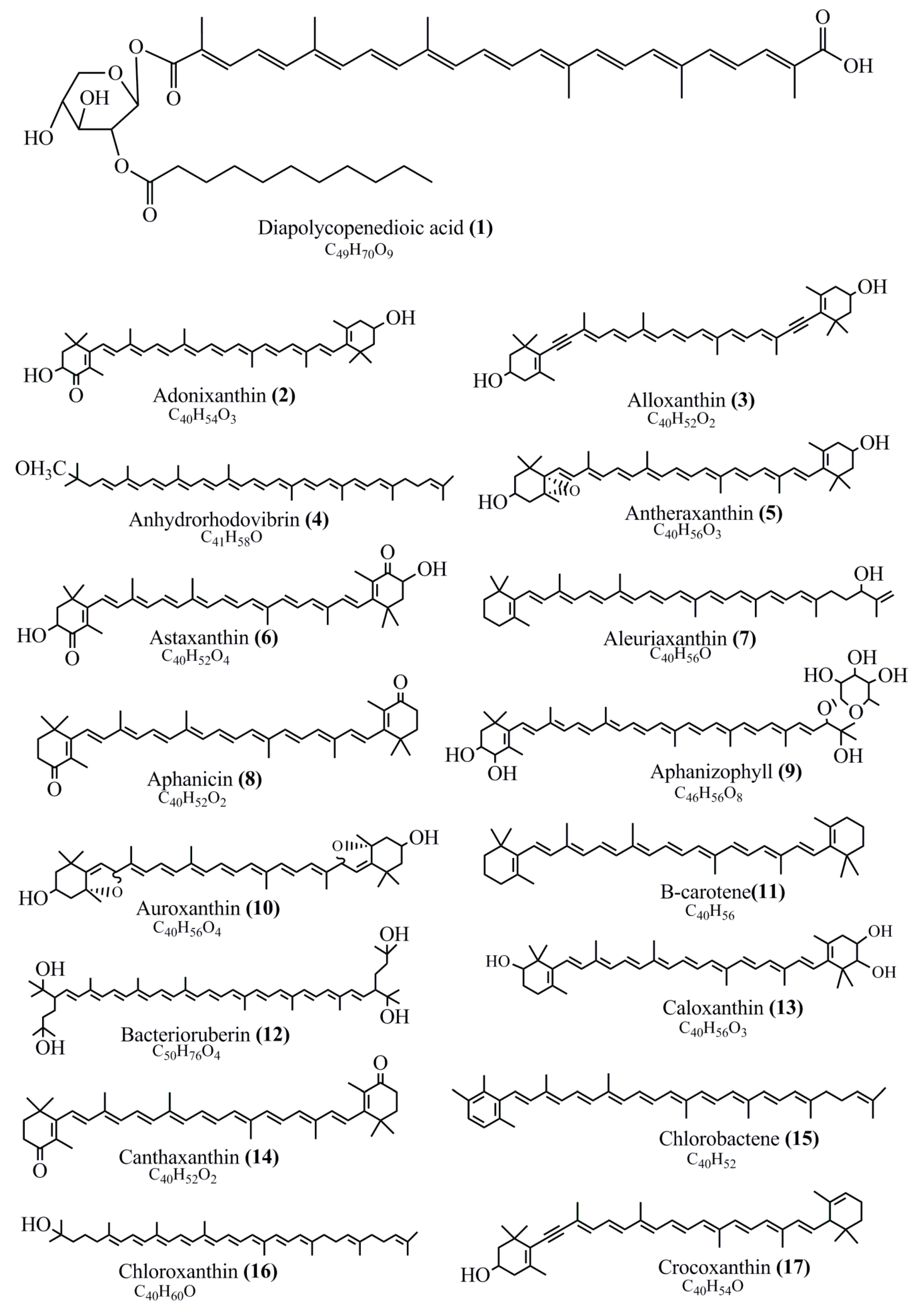
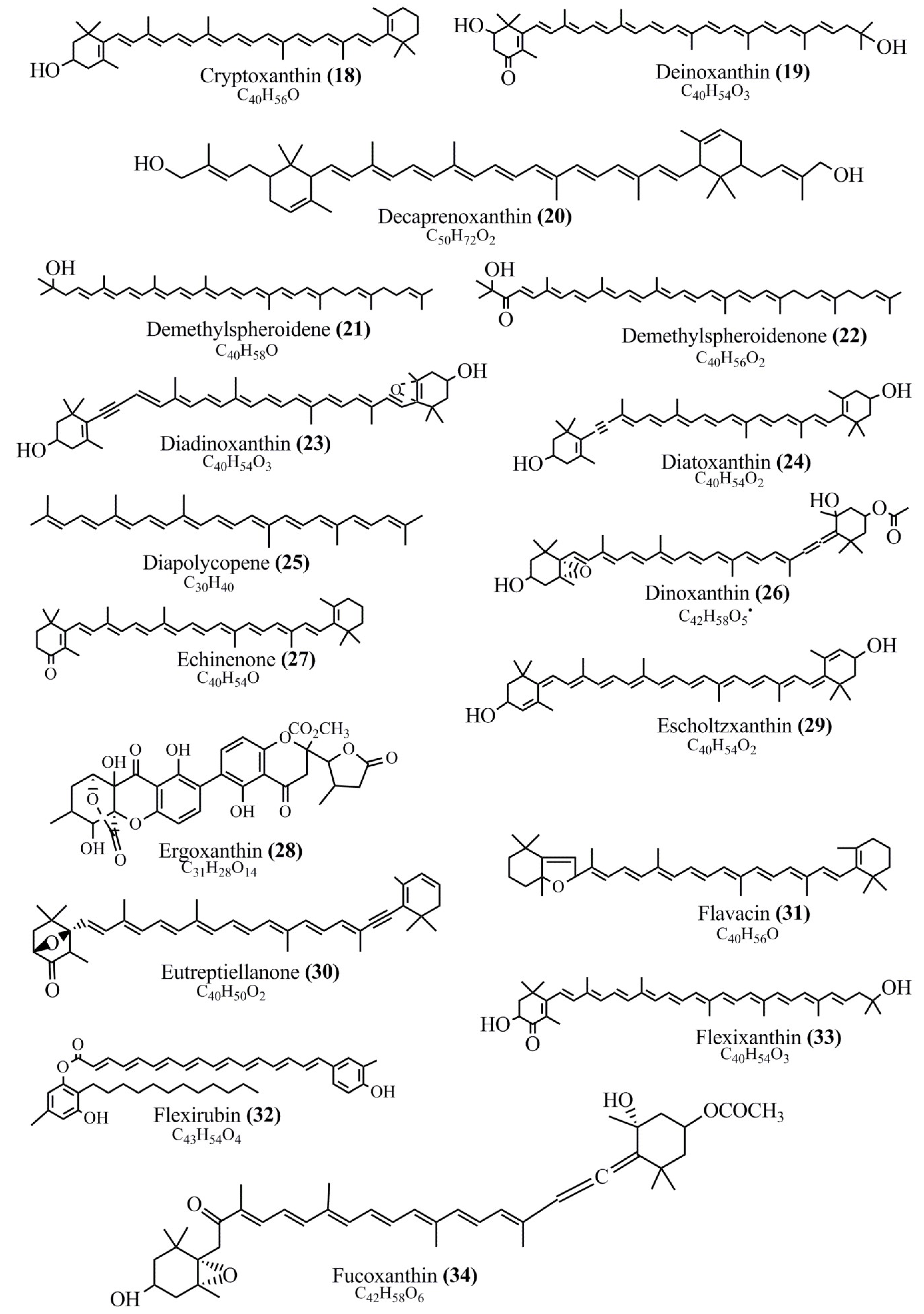
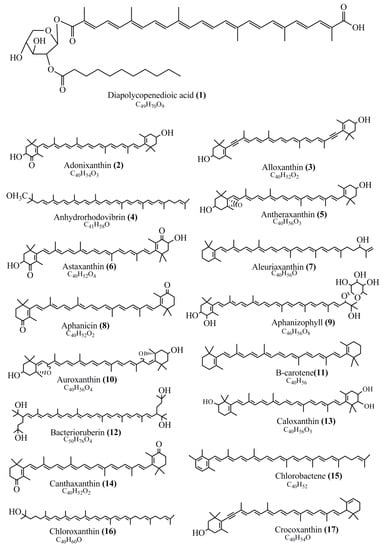
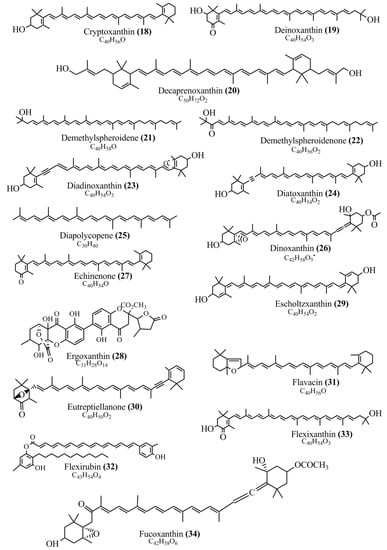
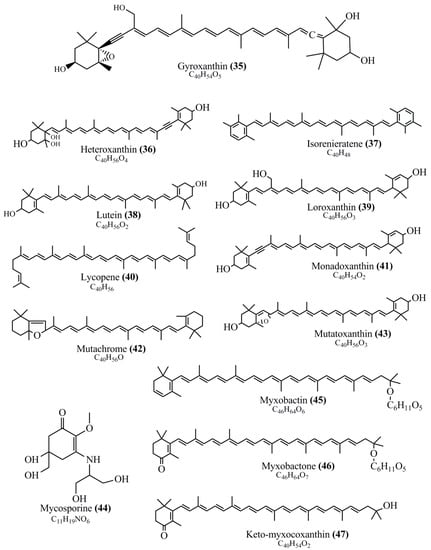
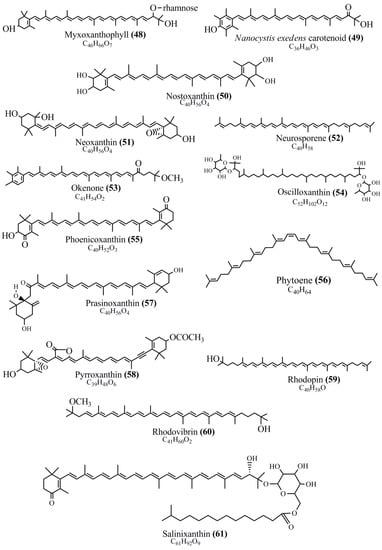
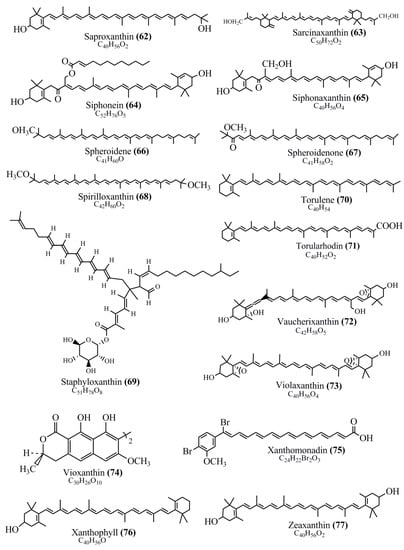
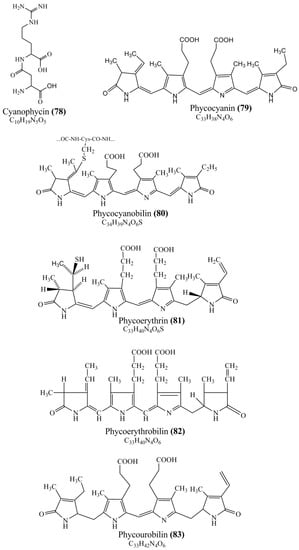
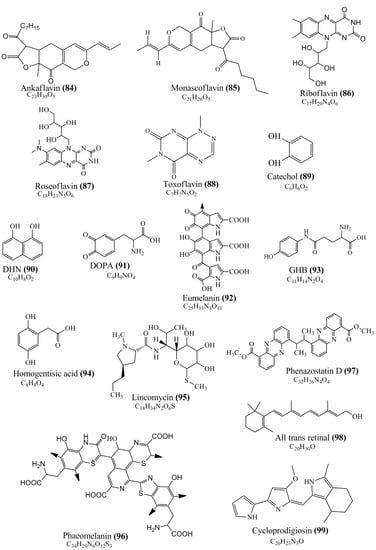
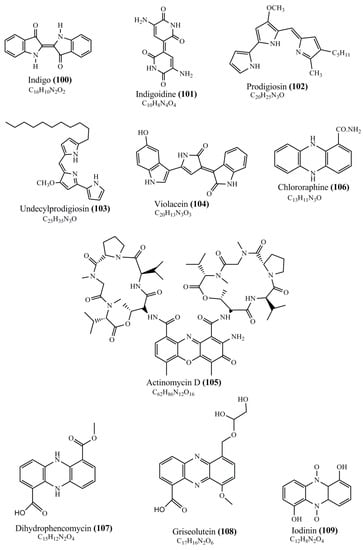
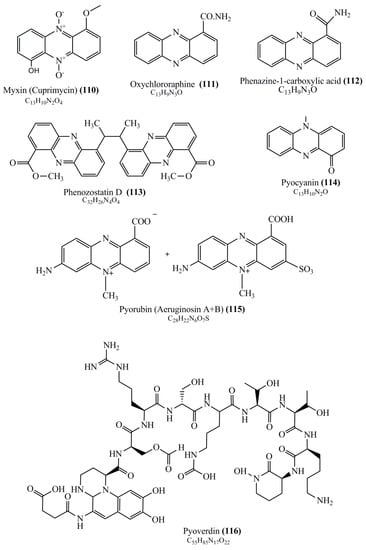
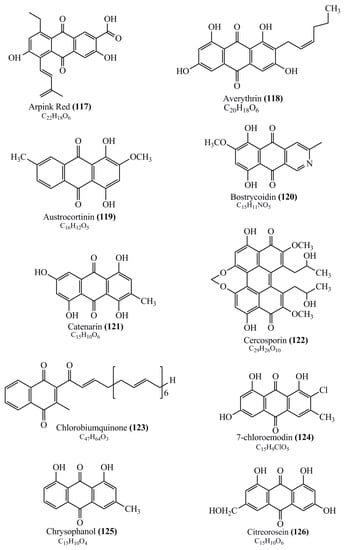
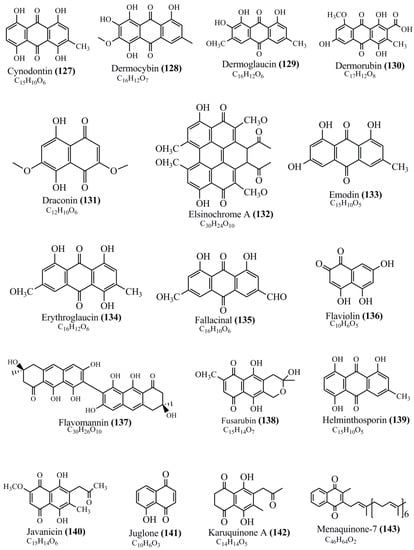
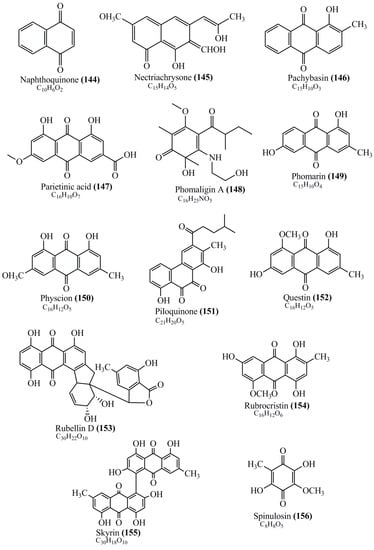
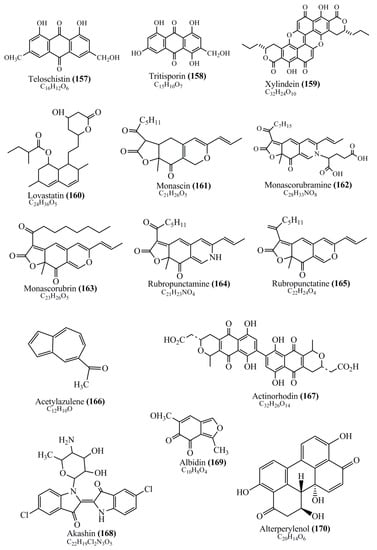
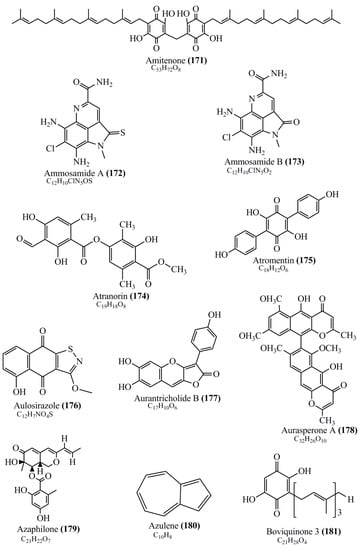
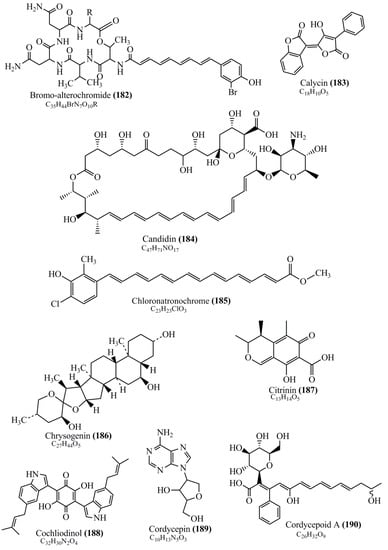
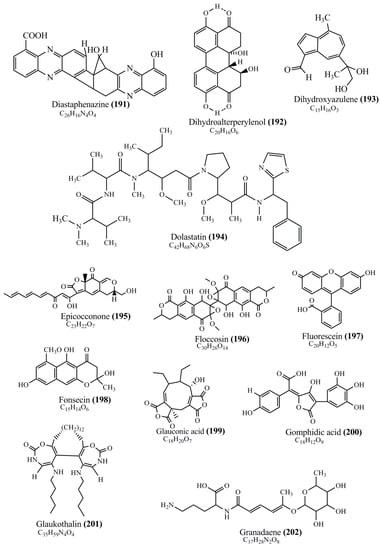
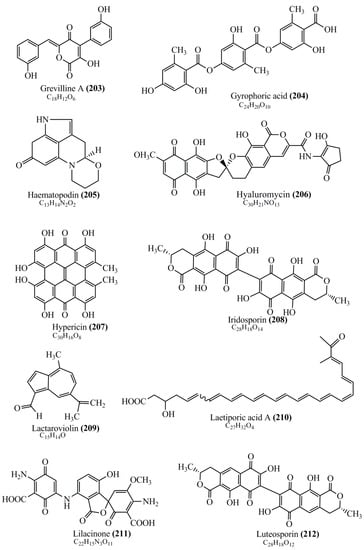
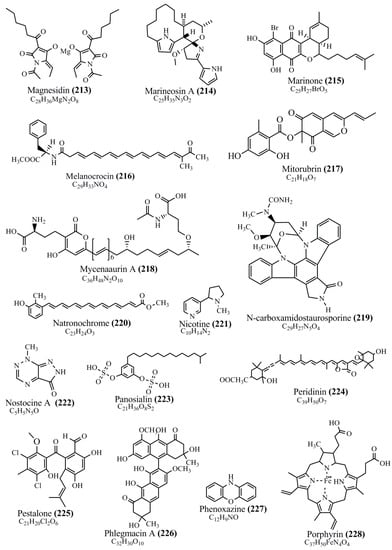
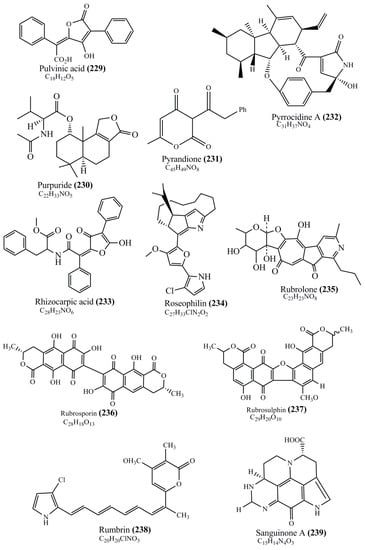
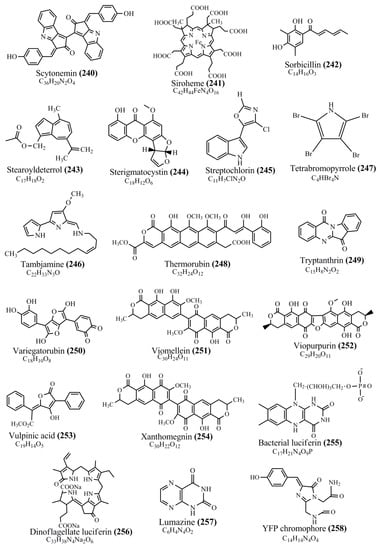
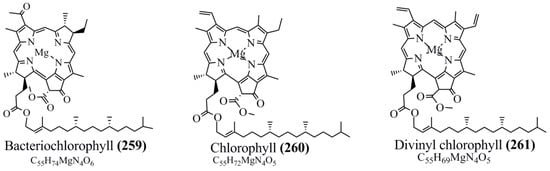
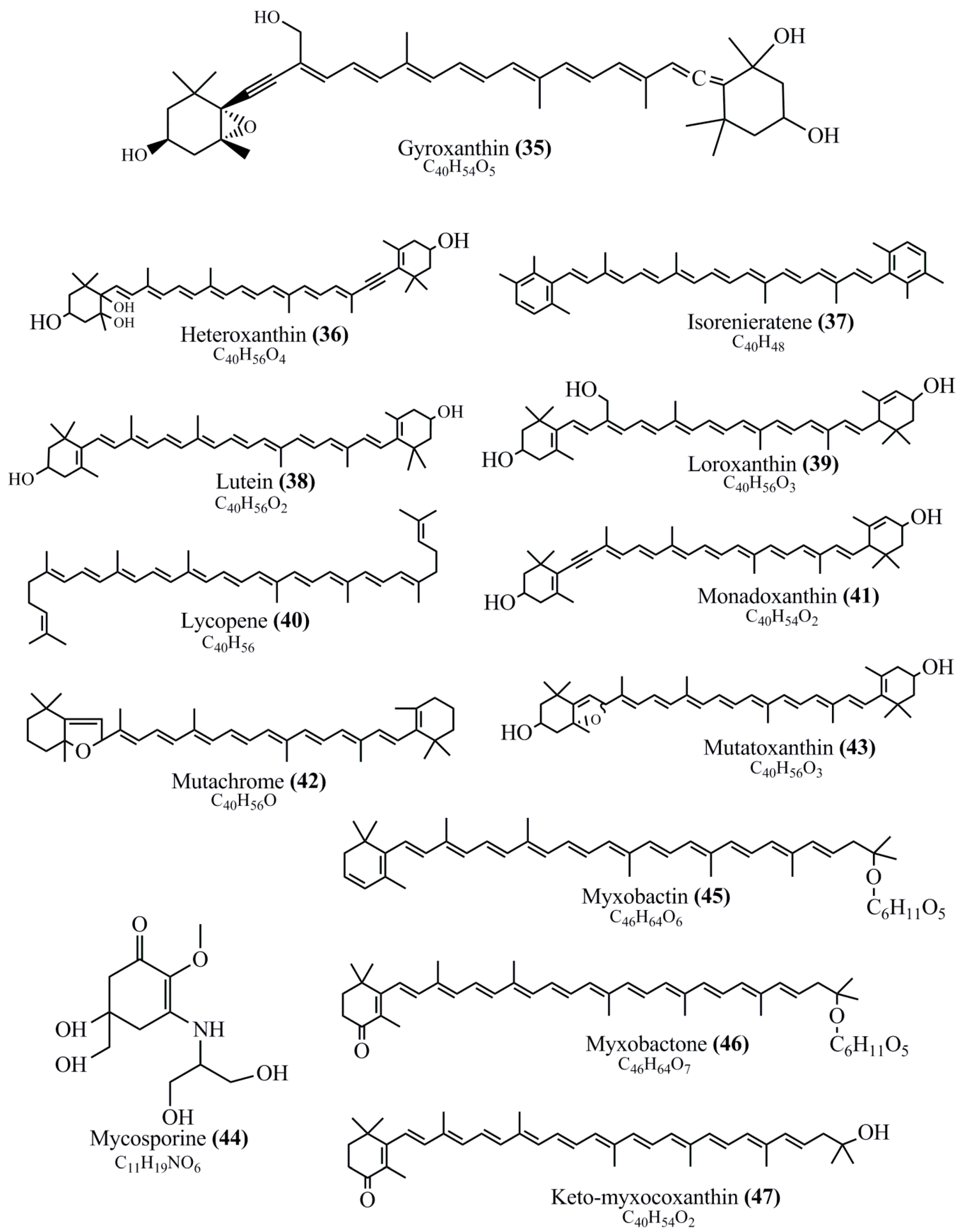
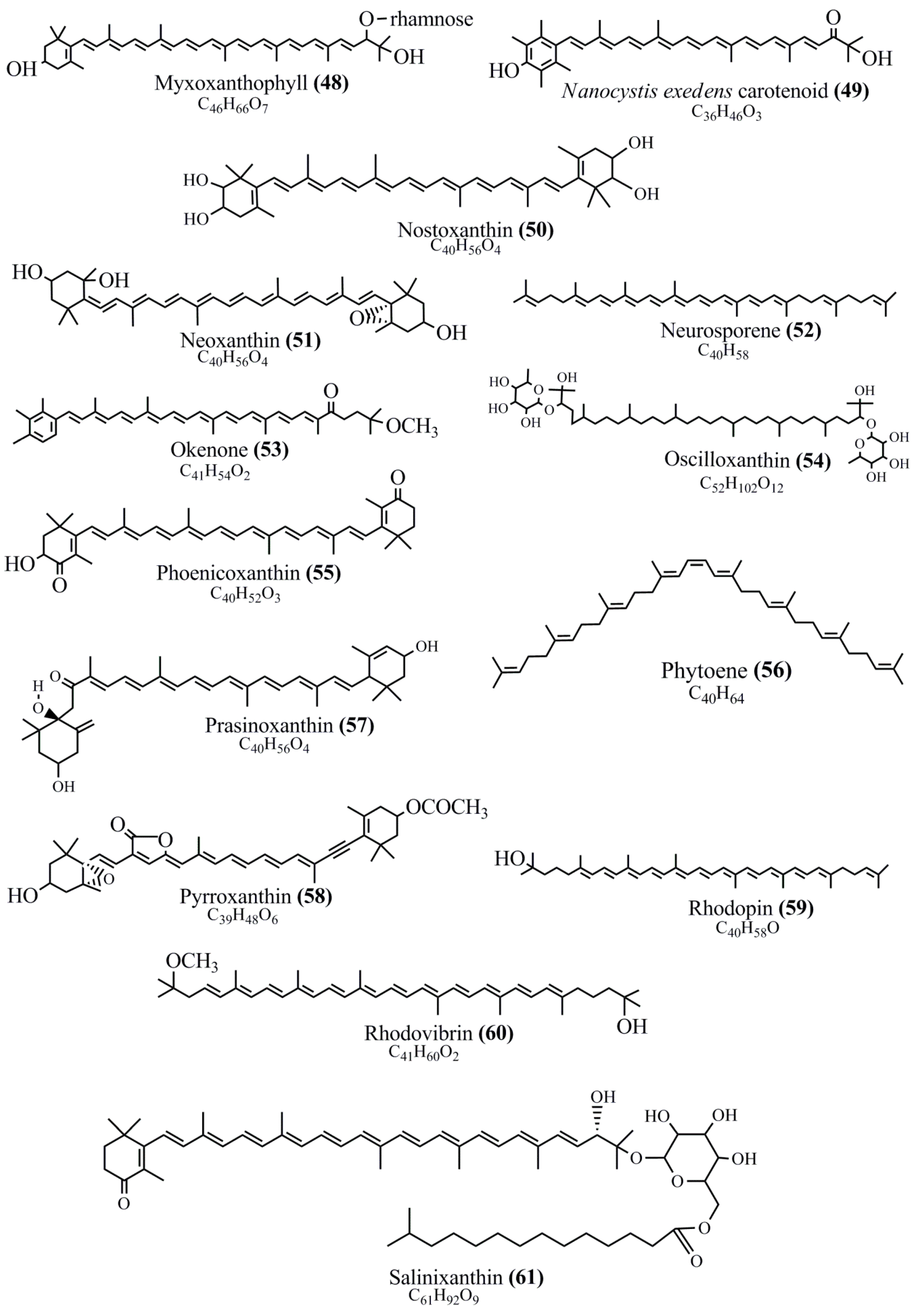
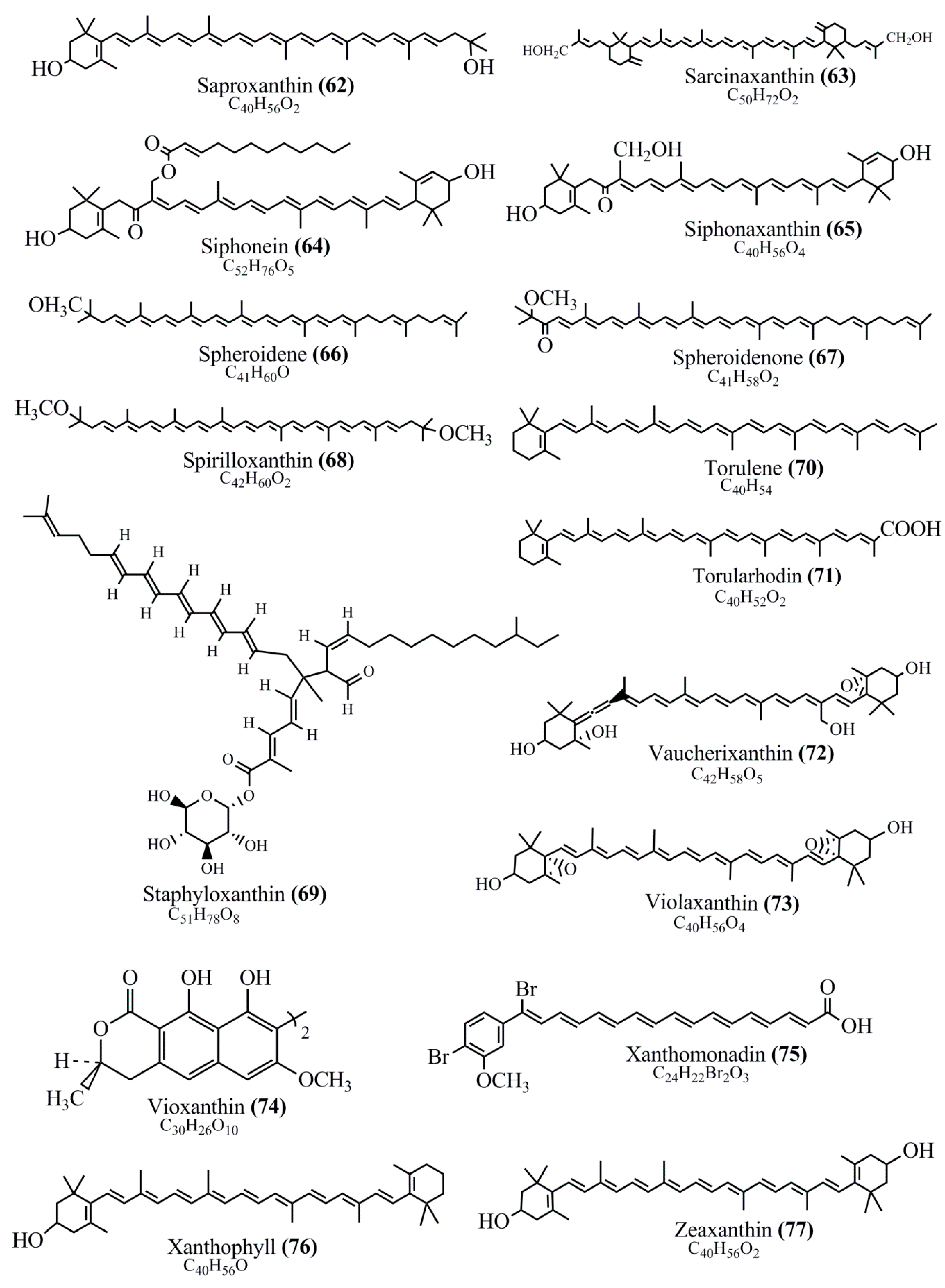
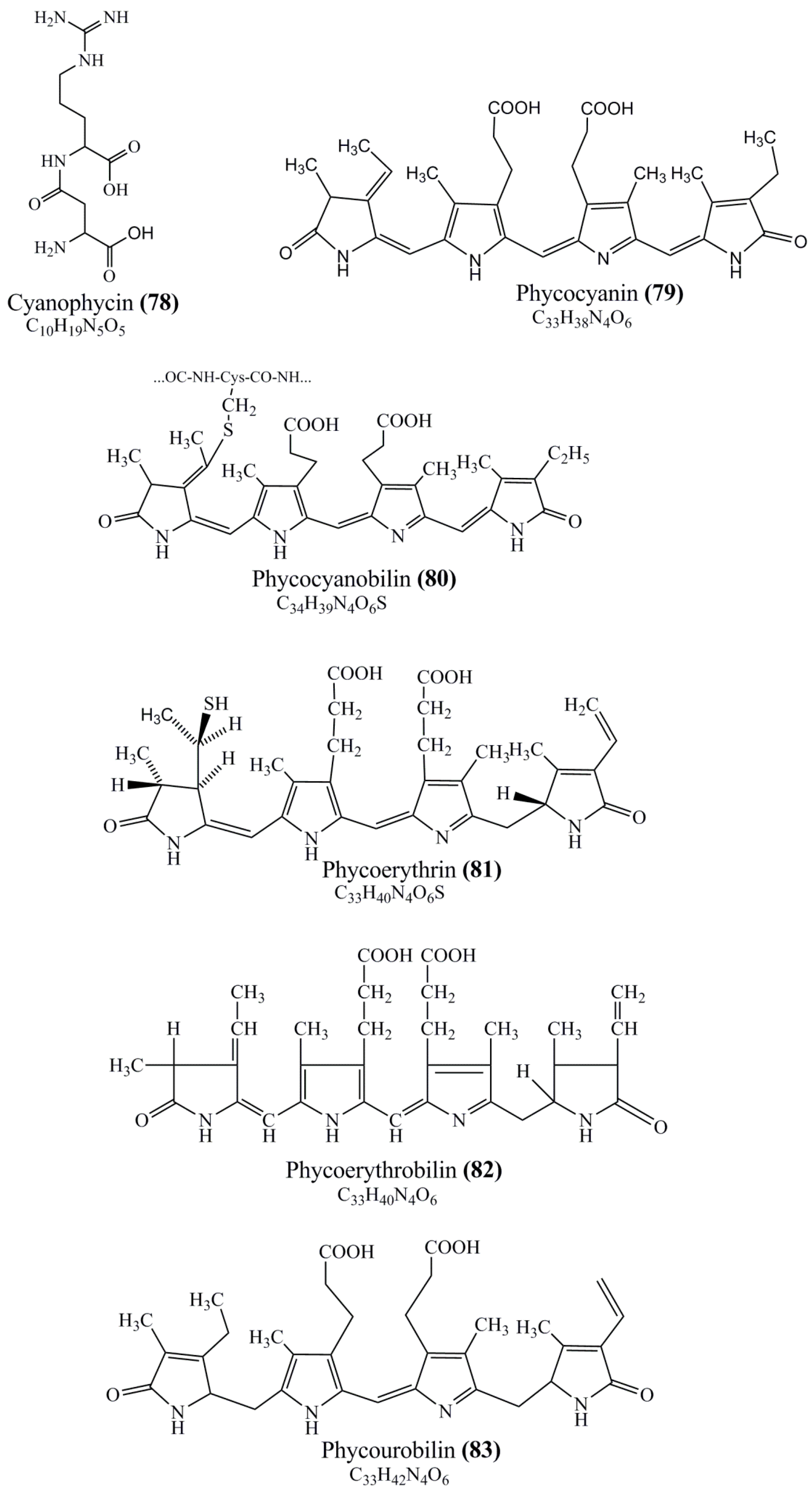
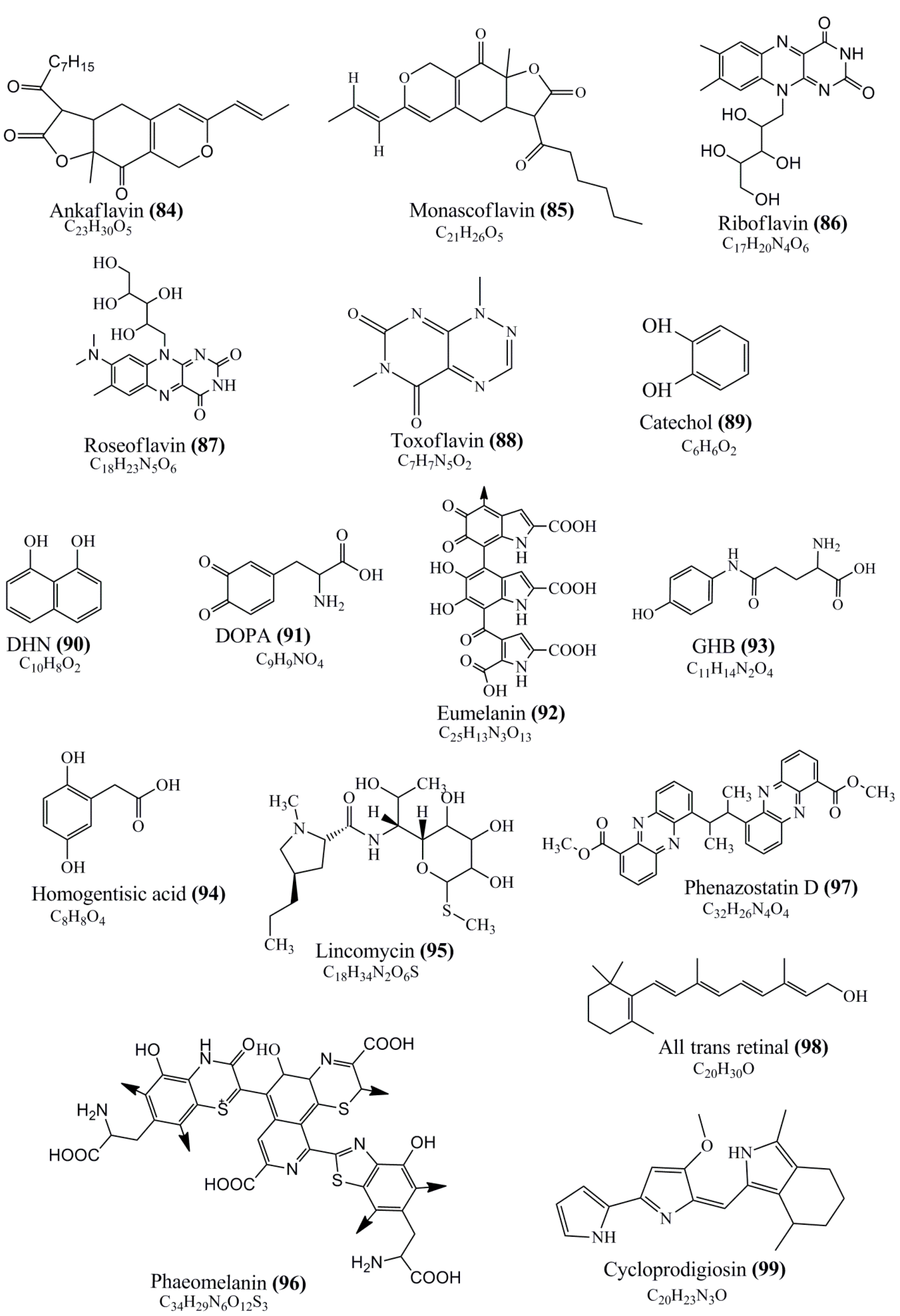
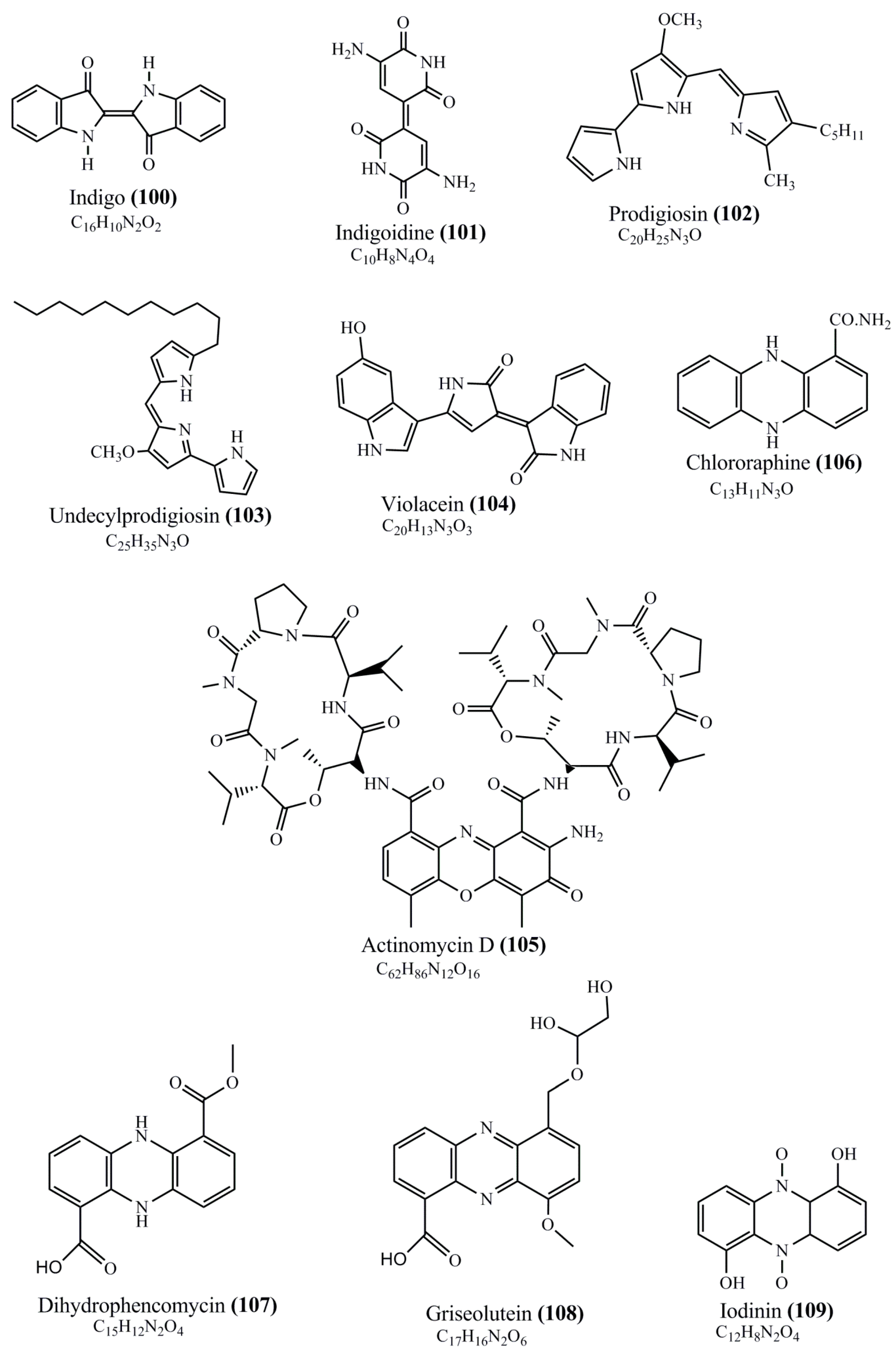


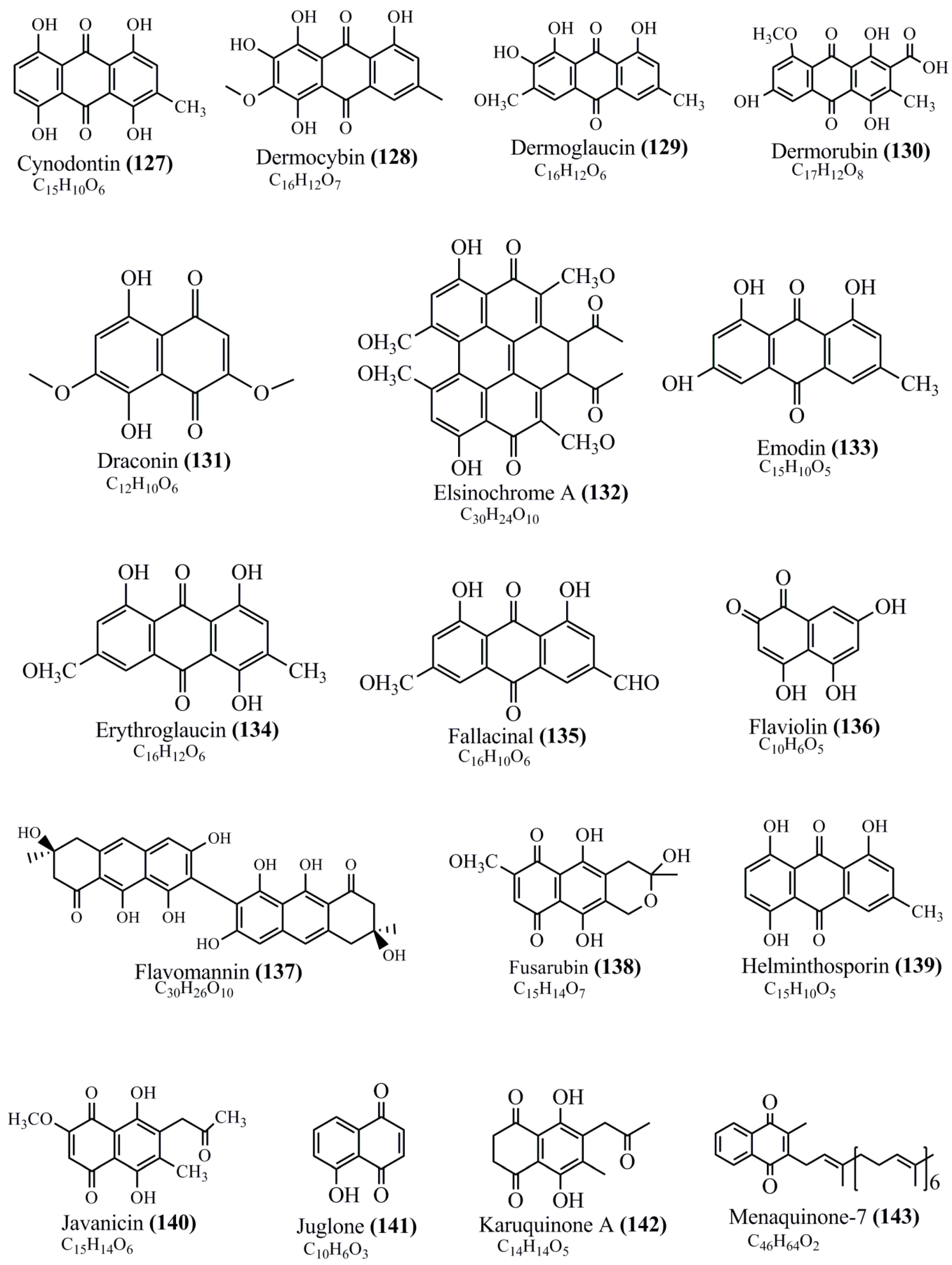
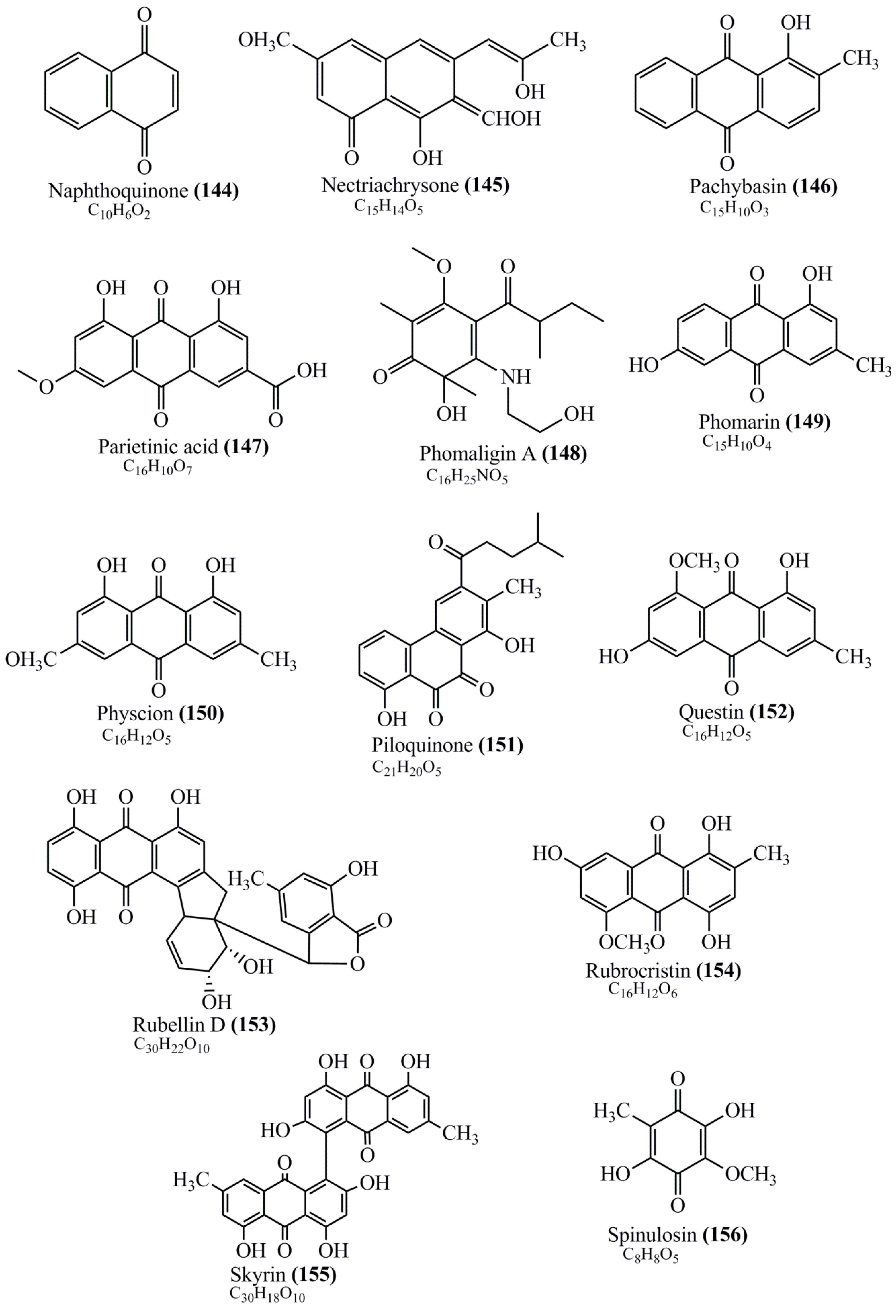

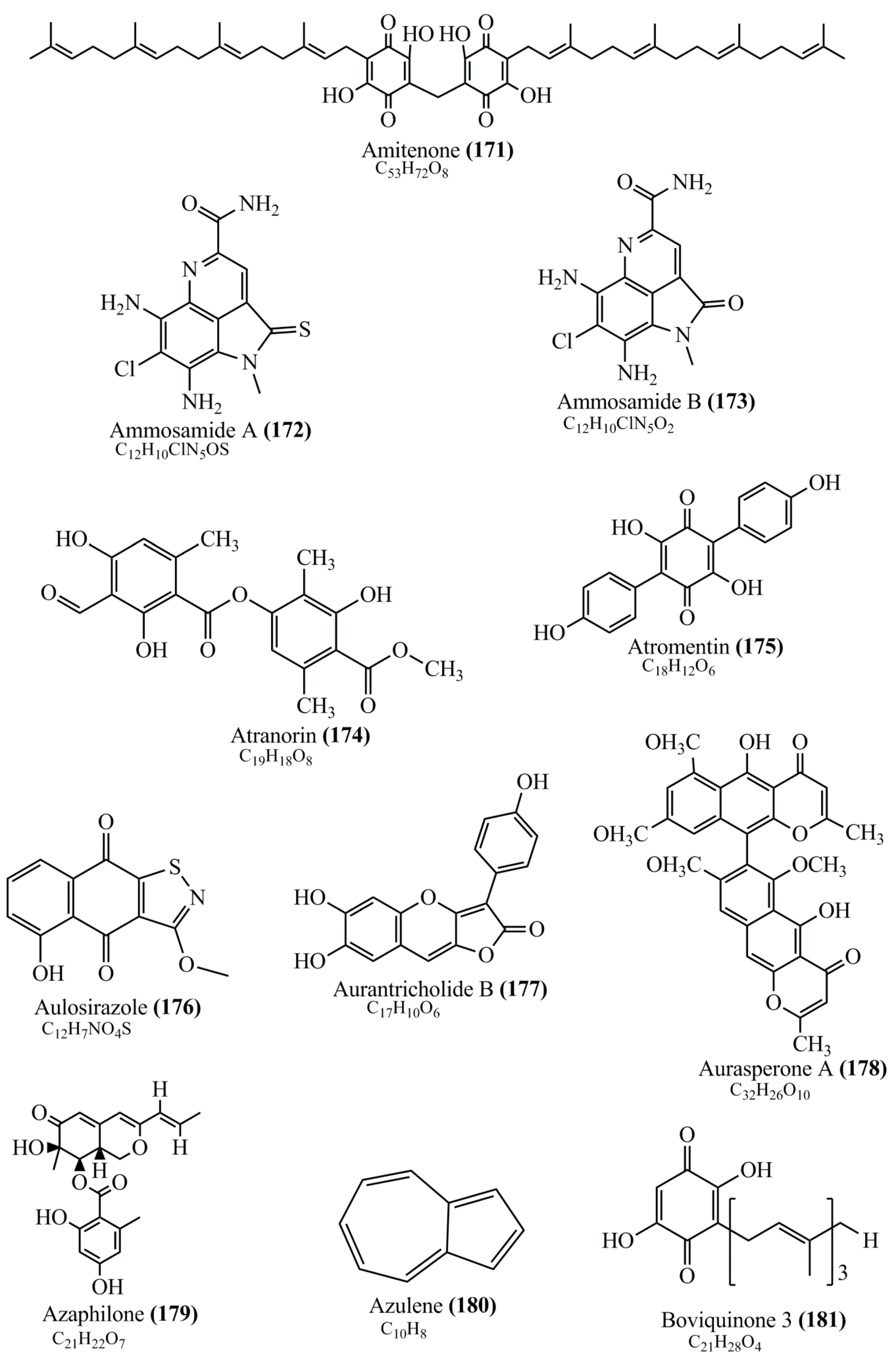
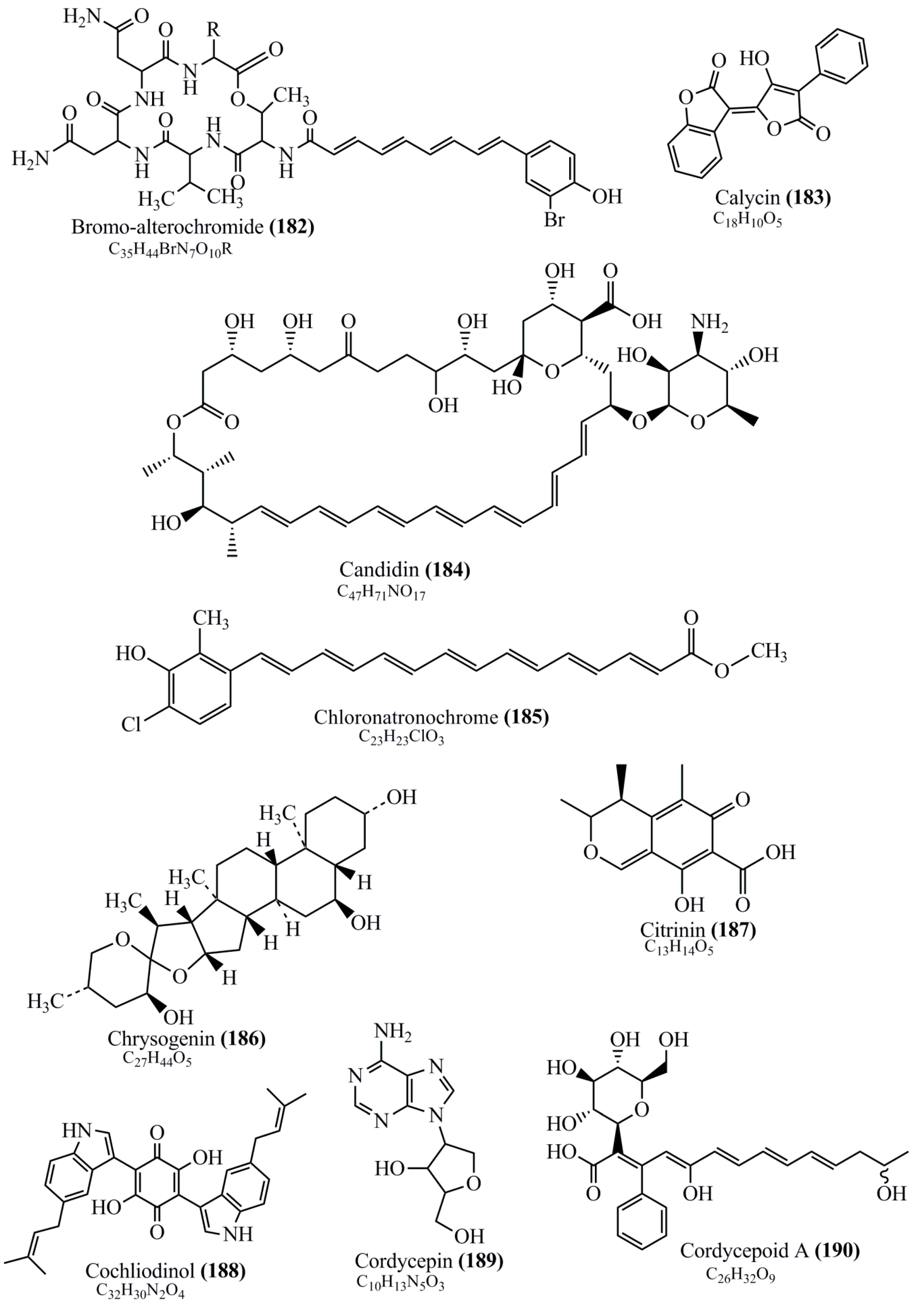
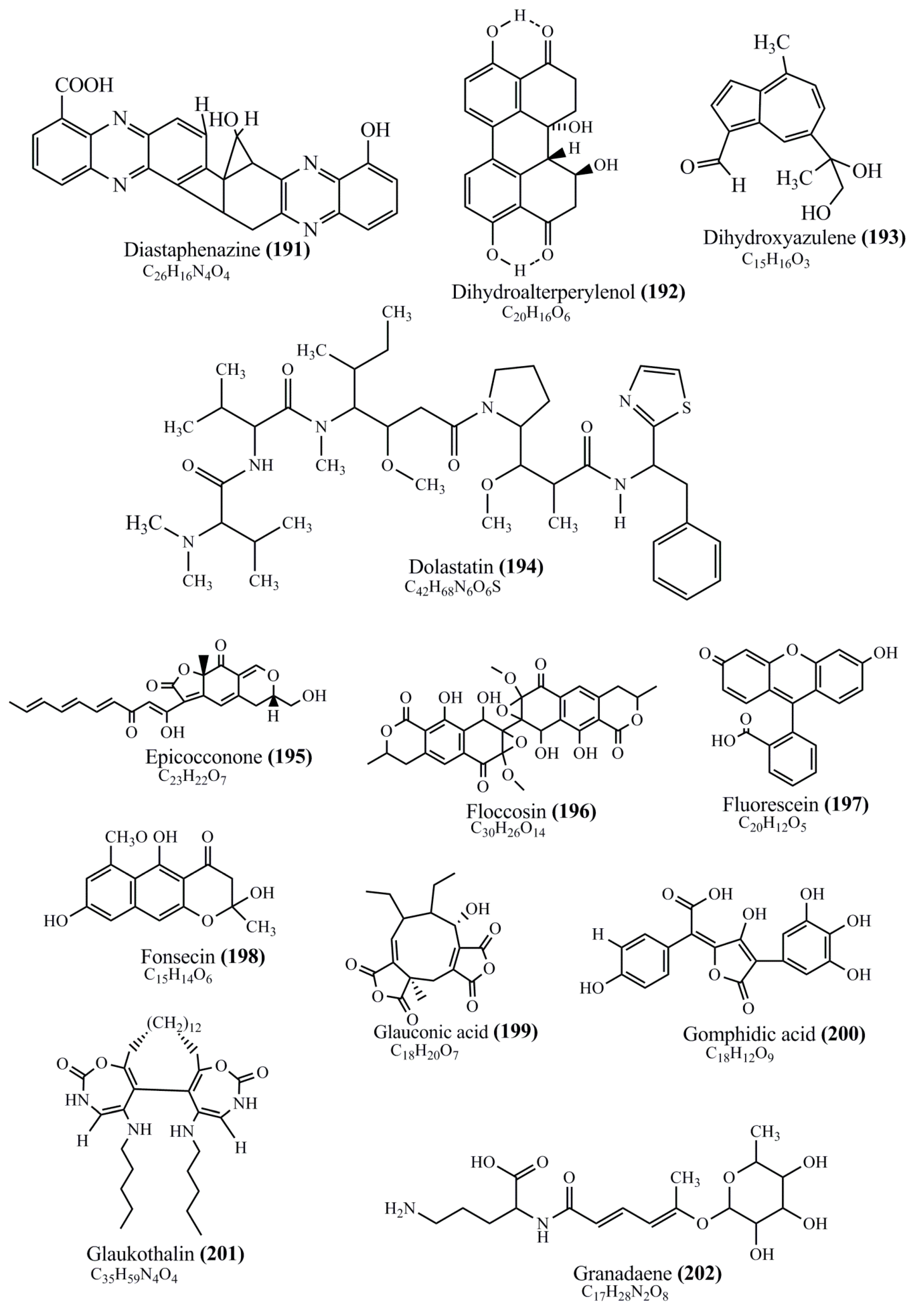
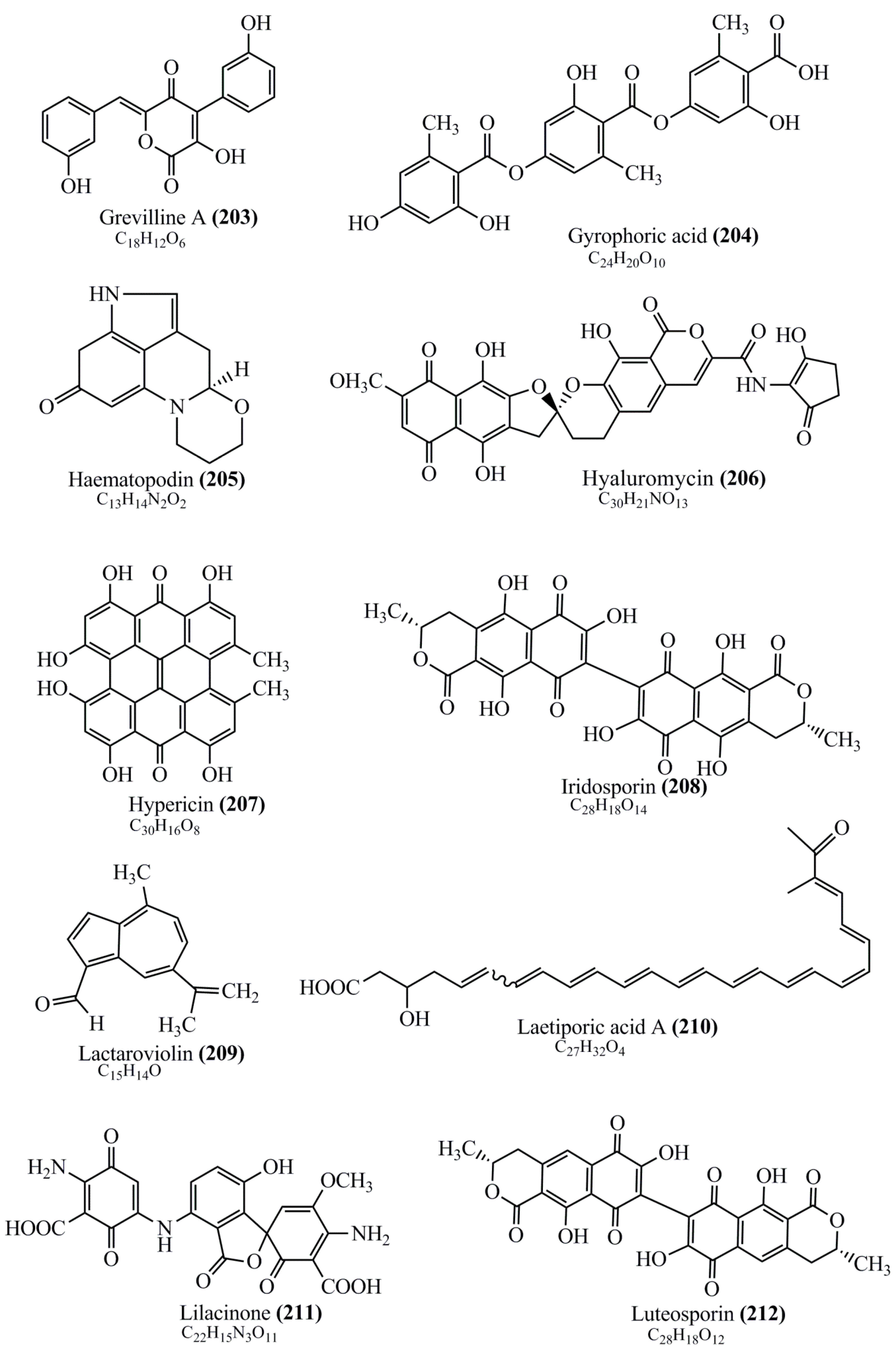
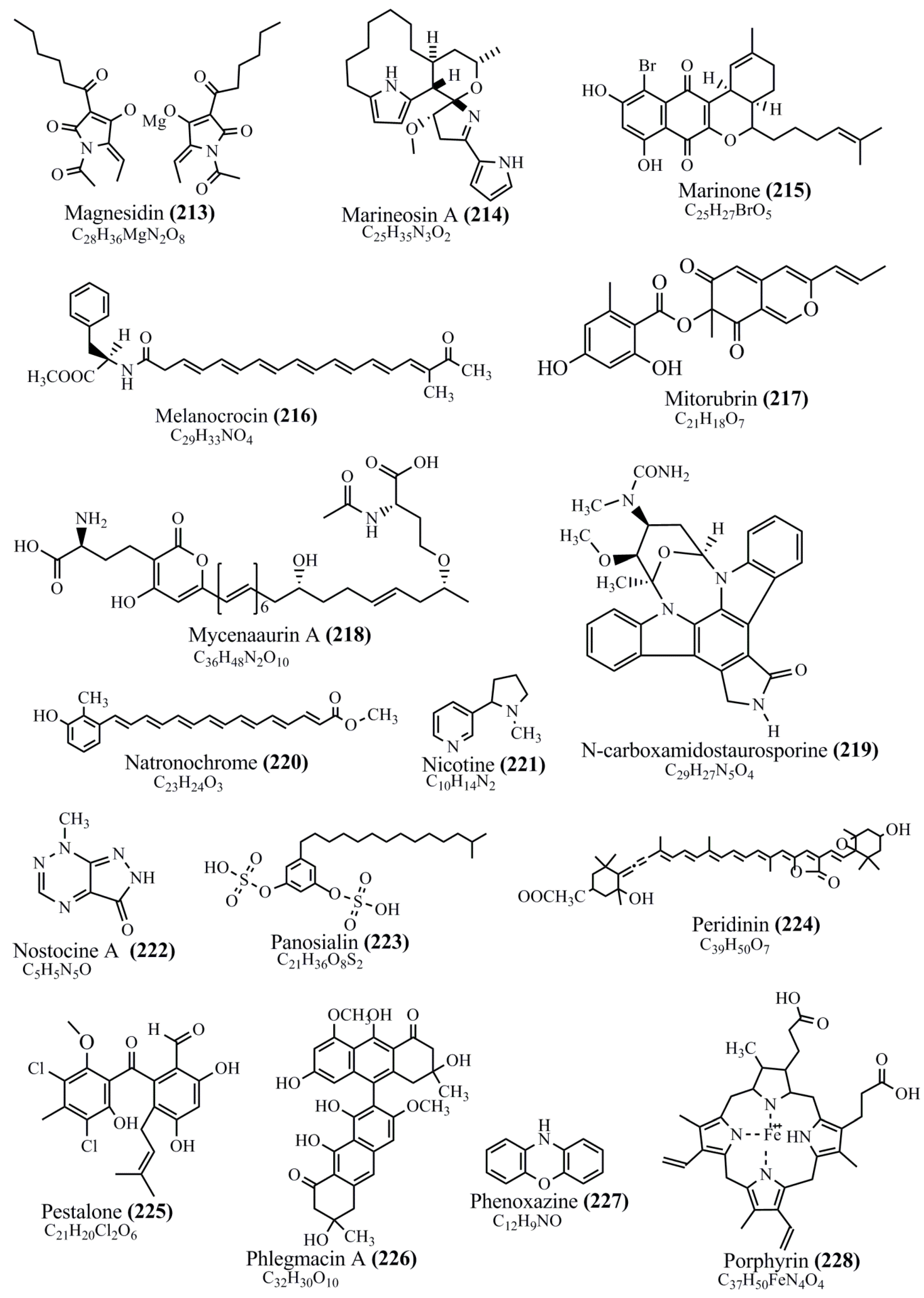
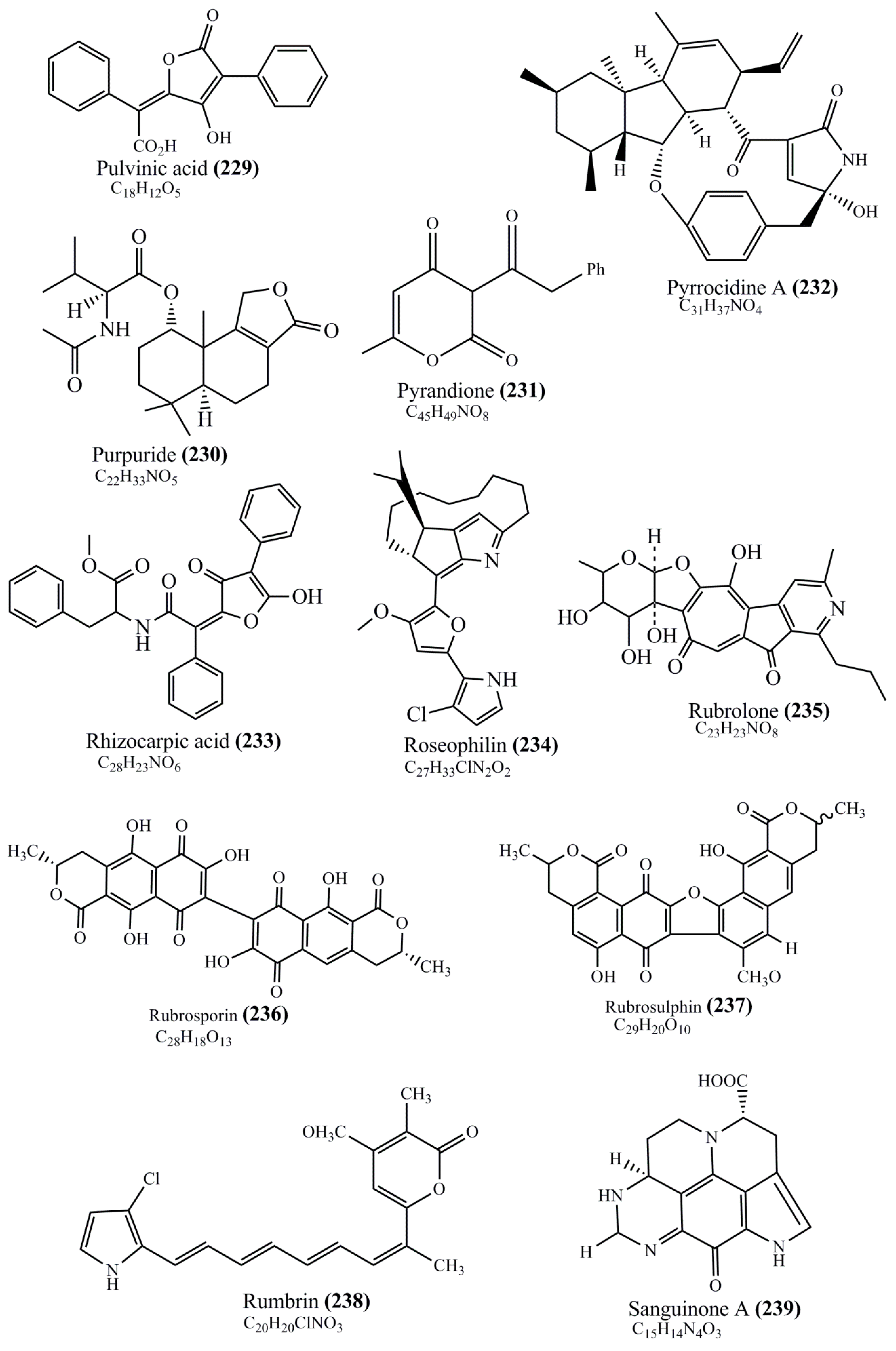
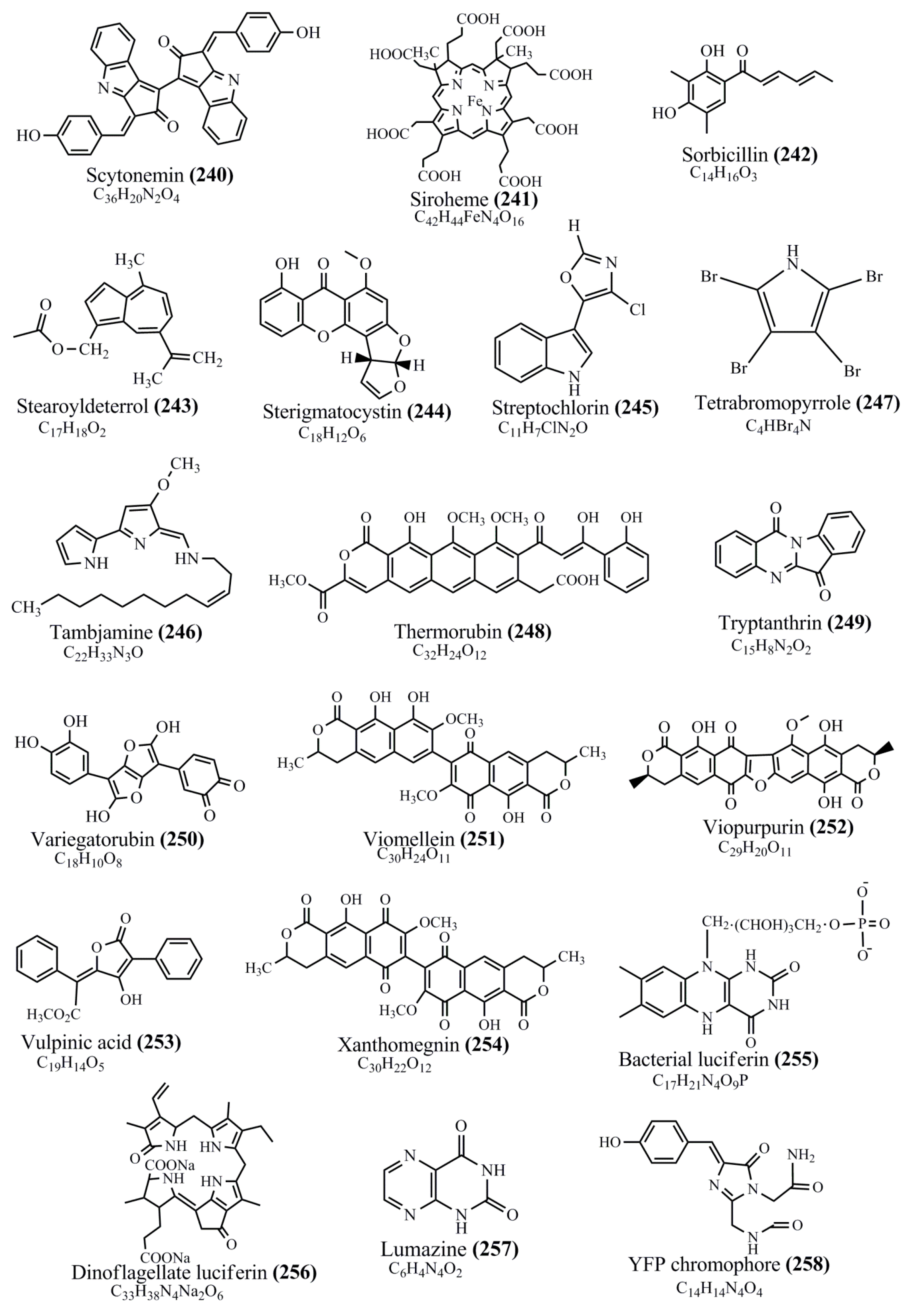

Figure 1.
Chemical structures of various pigments.
3. Brief Historical Note on Microbial Pigments
In 1879, a natural yellow pigment called “lactoflavin” was obtained from milk. In 1932, a yellow dye from aqueous yeast extracts was fractioned by Warburg and Christian. Afterwards, Karrer and Kuhn elucidated the yellow pigment called riboflavin, and both of them were endowed with the Nobel Prizes in chemistry for Karrer and Kuhn 1937 and 1938, respectively [22]. In the early 1970s, the purple pigment bacteriorhodopsin of Halobacterium was discovered [23]. Several pigmented non-photosynthetic bacteria and fungi were isolated during 1934 and 1976 by Ingraham and Baumann (both of them had conducted a systematic survey of carotenoid-producing non-photosynthetic bacteria in the 1930s) and Valadon respectively [24]. Monascus pigments are the well0 known natural food colorants known around the world since 1884 [25]. In Asia, for more than 10 decades, monascus red pigments appear to be used as food colorants to red pot-roast lamb and red rice koji [21]. So far, a total of 65 different monascus pigmented compounds have been reported, and some of which possessing antimicrobial, anticancer, and anti-obesity activities were recently well reviewed [26]. In 1934, ZoBell and Feltham found that 69.4% of bacterial colonies grown on agar medium inoculated with seawater and marine sediment were chromogenic. An infallible literature summary carried out by ZoBell in 1946 shows that many of marine bacterial species which spoil fish appeared to be pigmented [27]. Zeaxanthin producing Flavobacterium was isolated during the mid-1960s by scientists at Hoffmann-La Roche [21]. In 1964, thermorubin, a red pigment, was first isolated from a mildly thermophilic soil actinomycetes Thermoactinomyces antibioticus [28].4. Host Pigmented Compounds Said to Be of Microbial Origin
Dolastatin, a well-known antitumor compound isolated from different marine invertebrate species like sea hares and molluscs, has recently been found to actually have originated from their symbiotically associated marine cyanobacteria [13]. Pigments produced by some marine plants, invertebrates, and vertebrates such as seagrass, sponges, corals, molluscs, and tunicates are indeed produced by their epibiotic bacteria [29]. Some of the compounds such as Tambajamine, a yellow pigment molecule isolated from sponges and bryozoans, are believed to originate from endobiotic or epibiotic Pseudoalteromonas [30]. Tambjamines isolated from bryozoans (Bugula dentata and Sessibugula translucens), nudibranchs, and ascidians (Atapozoa sp.) have been found to be produce by Streptomyces sp., Pseudoalteromonas tunicate, and Serratia marcescens [31][32][31,32].5. Ecology and Habitats of Pigmented Microorganisms
A plethora of research articles have reported the isolation of pigmented microorganisms like bacteria, fungi, and yeast from terrestrial as well as marine milieus. They are distributed in different geographical conditions, from polar regions to tropical environments and from aerial to deep-sea regions. It is believed that microorganisms from different geographical regions are known to tolerate harsh conditions by producing pigments. Some of the pigmented microbes such as bacteria (e.g., Stenotrophomonas) and yeast (e.g., Rhodotorula) from terrestrial environment are found to enter coastal environments through discharges from hospitals and domestic sewages, thereby adapting to marine environment. Literature survey indicates that pigmented bacteria could be divided into two categories of true marine pigmented bacteria—primarily of marine origin and adaptive pigmented bacteria—originated from terrestrial ecosystem and survive and proliferate in coastal environment (Figure 1). Irrespective of the common occurrence of PB in terrestrial environment, marine pigmented microbes are gaining more attention due to their varied bioactive pigment compounds. Recent studies have been diverted to investigate marine microbial pigments as novel chromogenic compounds for biotechnological and industrial application. The occurrence of PB in a marine environment is found to vary according to geographical and nutritional conditions. Apparently, the diversity of pigmented heterotrophic bacteria (PHB) is less in abundance when compared to the enormous diversity of marine heterotrophic bacteria (MHB). Green and blue pigments are rare colors produced by microorganisms. The colony forming units (CFU) of PHB may vary depending on sampling site, seasonal variation, and availability of nutrients. Occurrence of high frequency of pigmented bacteria is noticed in air–water interfaces [33], glaciers [34], ice cores [35], bacterioneuston (sea surface microlayer) and underlying waters [36], salt lakes [37], deepsea hydrothermal vents [38], and abyssal hot springs (e.g., Thermus). Recently, various pigmented bacterial communities have been isolated from lava caves [39]. P. aeruginosa, a pigmented bacterium, has been reported to isolate from the wounds skin of humans and animals. These PBs are reported to be isolated from different marine niches such as seawater, marine sediment, seagrass, sponge, mussel, sea cucumber [40], algal mats, corals, freshwater, athalassohaline lagoon, marine solar saltern, microbial mats in Antarctic lakes, oil contaminated soil, nonsaline alkaline groundwater, and sea ice (e.g., Algoriphagus) [41] (Figure 2).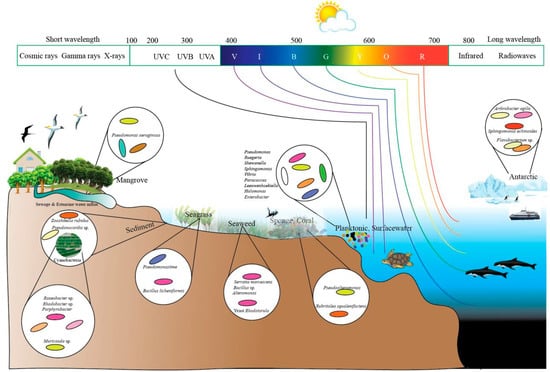
Figure 2.
Distribution of marine pigmented microorganisms in different niches.
