2. Natural Pigments
Natural pigments are naturally derived pigments synthesized mainly by plants, animals, and microbes
[5][9][5,9]. Most of the natural pigments used for different purposes since ancient times are produced from plants, such as annatto, grapes, indigo, beetroot, turmeric, madder, saffron, etc.
[10][11][10,11]. However, the process of pigment production from plants may not be a good option because of various problems, such as season dependency, loss of vulnerable plant species due to their extensive use, variations in color shades and intensity, expensive production, and issues related to stability and solubility
[2].
Nowadays, microorganisms, including bacteria, fungi, and algae, have been shown to be an excellent alternative source of natural pigments. For the large-scale production of pigments, microorganisms are more suitable, due to a clear understanding of their cultural techniques, processing, and ease of handling. Natural pigments from microbes, especially from bacteria and fungi, have been reported worldwide by many researchers
[1][10][12][13][14][15][16][17][18][19][20][1,10,12,13,14,15,16,17,18,19,20]. Many bacterial species have been reported to possess potential for pigment production
[10][21][22][23][10,21,22,23], but their pathogenic nature as well as associated toxicity have blocked production and commercialization. This eventually opened a new avenue for producing pigments from fungi and for their various applications.
3. Fungal Pigments
Fungi have been shown to be a good and readily available alternative source of natural pigments
[1][20][24][25][26][1,20,24,25,26]. Fungi have immense advantages over plants such as season-independent pigment production, easy and fast growth in a cheap culture medium, production of pigments with different color shades and of more stable, soluble pigments, and easy processing
[10][27][10,27]. Fungi belonging to the
Monascaceae,
Trichocomaceae,
Nectriaceae,
Hypocreaceae,
Pleosporaceae,
Cordycipitaceae,
Xylariaceae,
Chaetomiaceae,
Sordariaceae,
Chlorociboriaceae,
Hyaloscyphaceae,
Hymenochaetaceae,
Polyporaceae,
Ophiostomataceae,
Tremellaceae,
Herpotrichiellaceae, and
Tuberaceae families have been described as potent pigment producers
[8][12][20][25][26][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][8,12,20,25,26,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. These fungi are known to synthesize a variety of pigments as secondary metabolites. They are prolific producers of pigments belonging to several chemical classes, such as carotenoids, melanins, azaphilones, flavins, phenazines, quinones, monascin, violacein, indigo, etc.
[16][25][26][46][47][48][49][16,25,26,46,47,48,49].
The use of
Monascus pigments for the production of red mold rice (ang-kak) is the oldest recorded use of fungal pigments by humans. Certain species of
Monascus, viz.,
Monascus ruber and
Monascus purpureus, have been reported to be good potential producers of pigments worldwide. Studies have shown the potential of the red pigment produced by
M. ruber as an important food colorant as well as food additive
[50][51][50,51]. Many new pigments produced by
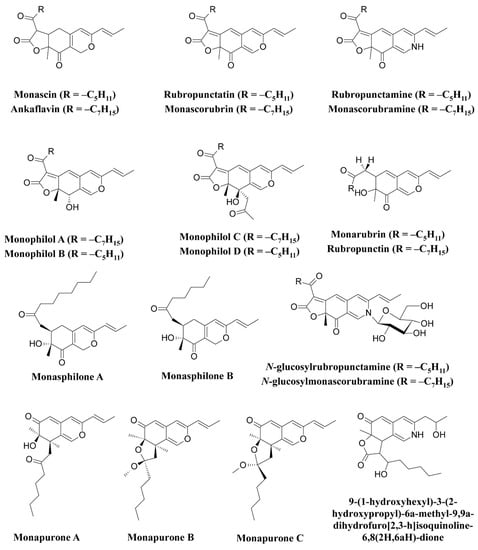
M. ruber, such as
N-glucosylrubropunctamine,
N-glucosylmonascorubramine, monarubrin, rubropunctin, etc., have been discovered (
Figure 1)
[52][53][54][52,53,54]. Recently, researchers revealed the first detailed biosynthetic pathway of
Monascus azophilone pigments (MonAzPs) in
M. ruber M7, based on targeted gene knockouts, heterologous gene expression, as well as in vitro enzymatic and chemical reactions
[55]. Along with
M. ruber,
M. purpureus was also reported to produce a variety of novel pigments, such as monapurone A–C, monasphilone A–B, monapilol A–D, and 9-(1-hydroxyhexyl)-3-(2-hydroxypropyl)-6a-methyl-9,9a-dihydrofuro[2,3-h] isoquinoline-6,8 (2H,6aH)-dione (
Figure 1)
[56][57][58][59][56,57,58,59]. Another study reports on the physicochemical (pH, light, and heat stability) properties of the red pigment of
M. purpureus [60].
Figure 1.
Pigments reported from
Monascus
species (
M. ruber
and
Along with
Monascus, many species of
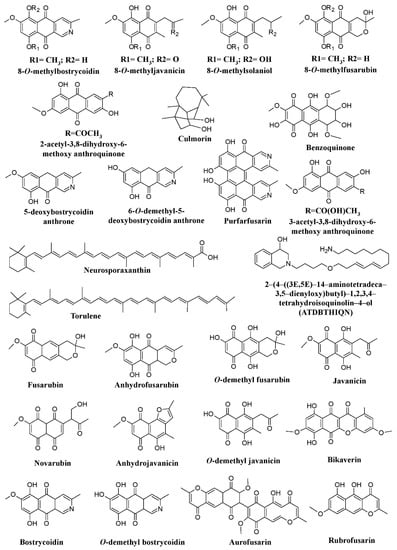
Fusarium have been reported for their capability to produce pigments. Studies have reported pigments such as bikaverin, nor-bikaverin, fusarubins, some naphthoquinone (8
-O-methybostrycoidin, 8-
O-methylfusarubin, 8-
O-methylnectriafurone, 8-
O-methyl-13-hydroxynorjavanicin, 8-
O-methylanhydrofusarubinlactol, and 13-hydroxynorjavanicin), and a novel isoquinoline-type, pigment 2-(4-((3E,5E)-14-aminotetradeca-3,5-dienyloxy)butyl)-1,2,3,4-tetrahydroisoquinolin-4-ol (ATDBTHIQN), from
Fusarium fujikuroi (formerly known as
Fusarium moniliforme) (
Figure 2)
[25][61][62][25,63,65]. Similarly, differently colored naphthoquinones [bostrycoidin, 9-
O-methylfusarubin, 5-
O-methyljavanicin, 8-
O-methylbostrycoidin, 1,4-naphthalenedione-3,8-dihydroxy-5,7-dimethoxy-2-(2-oxopropyl), 5-
O-methylsolaniol, and 9-
O-methylanhydrofusarubin], two anthraquinones compounds [2-acetyl-3,8-dihydroxy-6-methoxy anthraquinone and 2-(1-hydroxyethyl)-3,8-dihydroxy-6-methoxy anthraquinone], and polyketide pigment (bikaverin) were reported from
Fusarium oxysporum (
Figure 2)
[25][47][63][64][25,47,64,67]. Another species of
Fusarium,
Fusarium graminearum, has been found to produce a variety of pigments such as 5-deoxybostrycoidin anthrone, 6-
O-dimethyl- 5-deoxybostrycoidin anthrone, purpurfusarin, 6-
O-demethyl-5-deoxybostrycoidin, 5-deoxybostrycoidin, and aurofusarin (
Figure 2)
[25][63][65][66][25,64,66,121].
Figure 2.
Pigments from fungal genera of Nectriaceae (
Fusarium
,
Fusicolla
, and
A red pigment aurofusarin has been found to be produced by many species of Fusarium such as Fusarium culmorum, Fusarium sporotrichioides, Fusarim. acuminatum, Fusarium avenaceum, Fusarium poae, Fusarium crookwellens, Fusarium pseudograminearum, Fusarium sambucinum, and Fusarium tricinctum. Bikaverin has been reported to be produced by Fusarium lycopersici, and Fusarium vasinfectum. Fusarium solani and Fusarium verticillioides (currently known as F. fujikuroi) have been described to produce both aurofusarin and bikaverin (
Figure 2)
[25]. Similarly, benzoquinone has been reported from Fusarium sp. JN158 (
Figure 2)
[67][68]. A study has shown that the synthesis of major Fusarium carotenoids (neurosporaxanthin and β-carotene) is induced by light via transcriptional induction of the structural genes carRA, carB, carT, and carD
[43]. Similarly, other members of the fungal family Nectriaceae, such as Albonectria rigidiuscula and Fusicolla aquaeductuum (formerly known as Fusarium decemcellulare and Fusarium aquaeductuum respectively) were reported for their pigment production potential (
Figure 2)
[43][63][43,64]. Recently, the biosynthetic pathway of chrysogine mediated by two-module non-ribosomal peptide synthetase (NRPS) gene cluster was discovered in Fusarium graminearum in which enhanced chrysogine production was observed upon overexpression of NRPS14
[68][122].
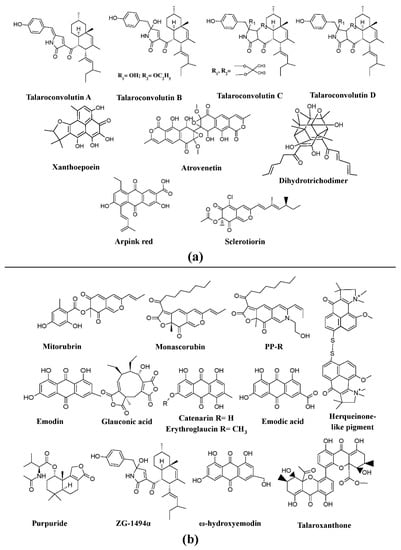
Many investigations report
Penicillium as potent producers of pigment
[25][69][70][71][72][25,61,96,97,98], such as arpink red
TM (first commercial red colorant), talaroconvolutins A–D, sclerotiorin, xanthoepocin, atrovenetin, and dihydrotrichodimerol discovered from
Penicillum oxalicum var.
armeniaca,
Penicillum convolutum (formerly known as
Talaromyces convolutes),
Penicillum mallochii,
Penicillum simplicissimum,
Penicillum melinii, and
Penicillum flavigenum, respectively (
Figure 3a)
[41][73][74][75][76][41,91,93,94,123]. An uncharacterized red pigment has been reported from
Penicillium miczynskii [77][71]. Besides, many other
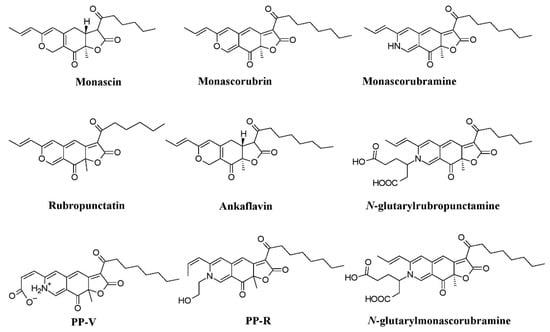
Monascus-like pigments such as PP-V [(10Z)-12-carboxylmonascorubramine] and PP-R [(10Z)-7-(2-hydroxyethyl)-monascorubramine] have been reported from
Penicillium (
Figure 4)
[78][95]. A biosynthetic pathway for the yellow pigment chrysogine from
Penicillium chrysogenum has been proposed recently
[79][92].
Figure 3. Pigments from the genera
Penicillium and
Talaromyces. (
a) Different pigments produced by
Penicillium species, re-drawn from
[41][73][74][75][76][41,91,93,94,123]. (
b) Various pigments produced by
Talaromyces species, re-drawn from
[80][81][82][83][100,101,107,109].
Figure 4.
Monascus
–like azaphilone pigments of
Penicillium
and
Talaromyces species, re-drawn from [25][78][84]. species, re-drawn from [25,95,106].
Talaromyces spp. have been reported as a source of pigments by many researchers. The pigment production ability of
Talaromyces purpureogenus (formerly known as
Penicillium purpureogenum) was evaluated by many researchers
[85][86][87][102,104,105]. Studies report the production of a herqueinone-like pigment from
Talaromyces marneffei (formerly known as
Penicillium marneffei),
Monascus-like azaphilone pigments (
N-glutarylmonascorubramine and
N-glutarylrubropunctamine) from
Talaromyces purpureogenus (formerly known as
Penicillium purpureogenum), industrially important red pigments (mitorubrin, monascorubrin, PP-R, glauconic acid, purpuride, and ZG-1494α) from
Talaromyces atroroseus, trihydroxyanthraquinones (emodin, erythroglaucin, and catenarin) from
Talaromyces stipitatus, and a xanthone dimer (talaroxanthone) from
Talaromyces sp. (
Figure 3b)
[80][81][82][83][88][100,101,103,107,109]. An uncharacterized red pigment was discovered from
Talaromyces siamensis under submerged fermentation
[77][71]. Moreover, other species of
Talaromyces, Talaromyces aculeatus,
Talaromyces atroroseus,
Talaromyces albobiverticillius,
Talaromyces cnidii,
Talaromyces coalescens,
Talaromyces pinophilus,
Talaromyces purpurogenus,
Talaromyces funiculosus,
Talaromyces amestolkiae,
Talaromyces ruber,
Talaromyces stollii, and
Talaromyces verruculosus have been reported to have the ability to produce
Monascus-like azaphilone pigments (
Figure 4)
[25][84][25,106].
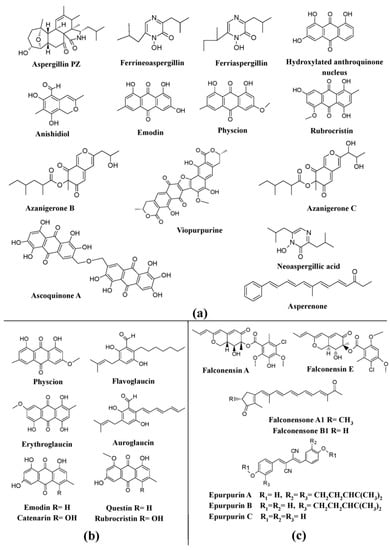
Several members of the genus
Aspergillus, such as
Aspergillus niger, have been known to synthesize a wide variety of pigments, such as aspergillin, asperenone, azaphilones (azanigerones A–F), and melanin (
Figure 5a)
[25][89][90][91][25,110,114,115].
Aspergillus nidulans was reported to produce ascoquinone A, norsolorinic acid, and melanin
[25][92][93][25,112,113], whereas
Aspergillus fumigatus was reported to produce melanin and melanin-like pigments
[25][94][25,111]. In addition, a variety of other pigments such as asperenone, anishidiol, neoaspergillic acid, sterigmatocystin, and an uncharacterized yellow pigment have been discovered from
Aspergillus nishimurae,
Aspergillus awamori,
Aspergillus sclerotiorum,
Aspergillus versicolor, and
Aspergillus terreus, respectively
[25][73][89][95][96][25,91,110,116,118]. Many other species of
Aspergillus such as
Aspergillus glaucus,
Aspergillus cristatus, and
Aspergillus repens have been reported to produce a variety of hydroxyanthraquinone pigments, emodin, physcion, questin, erythroglaucin, catenarin, and rubrocristin; while
Aspergillus melleus,
Aspergillus ochraceus,
Aspergillus sulphureus, and
Aspergillus westerdijkiae have been described to be major producers of polyketide-based pigments (rubrosulfin, viomellein, viopurpurin, and xanthomegnin) (
Figure 5a)
[25]. In addition to this, other pigments such as ferriaspergillin, ferrineoaspergillin, and an uncharacterized yellow pigment have also been reported from the genus
Aspergillus (
Figure 5a)
[97][98][119,120].
Figure 5. Pigments from the genus
Aspergillus and its teleomorphic genera. (
a) Structures of pigments produced by
Aspergillus species. (
b) Pigments produced by species of
Eurotium (teleomorph of
Aspergillus). (
c) Pigments produced by species of
Emericella (teleomorph of
Aspergillus), re-drawn from
[25].
Certain teleomorphic species of
Aspergillus have been described as producers of a variety of pigments. Some of the well-known azaphilone pigments such as falconensins A–H, zeorin, falconensones A1 and B2 have been reported from
Emericella falconensis and
Emericella fruticulosa (currently known as
Aspergillus falconensis and
Aspergillus fruticulosus, respectively), epurpurins A-C from
Emericella purpurea (currently known as
Aspergillus purpureus), and the pigment sterigmatocystin from
Emericella rugulosus,
Emericella parvathecia, and
Emericella nidulans (currently known as
Aspergillus rugulosus,
Aspergillus parvathecia, and
Aspergillus nidulans) (
Figure 5c). Similarly, other
Aspergillus spp. such as
Aspergillus amstelodami,
Aspergillus chevalieri,
Aspergillus glaucus,
Aspergillus umbrosus,
Aspergillus spiculosus,
Aspergillus glaber,
Aspergillus echinulatum,
Aspergillus tonophilus,
Aspergillus intermedius,
Aspergillus leucocarpus,
Aspergillus ruber, and
Aspergillus cristatus (which were formerly known as
Eurotium amstelodami,
Eurotium chevalieri,
Eurotium herbariorum,
Eurotium umbrosum,
Eurotium spiculosum,
Eurotium spiculosum,
Eurotium echinulatum,
Eurotium tonophilum,
Eurotium intermedium,
Eurotium leucocarpum,
Eurotium rubrum, and
Eurotium cristatum, respectively) have also been reported to produce pigments such as physcion, erythroglaucin, flavoglaucin, auroglaucin, catenarin, rubrocristin, and emodin (
Figure 5b)
[25].
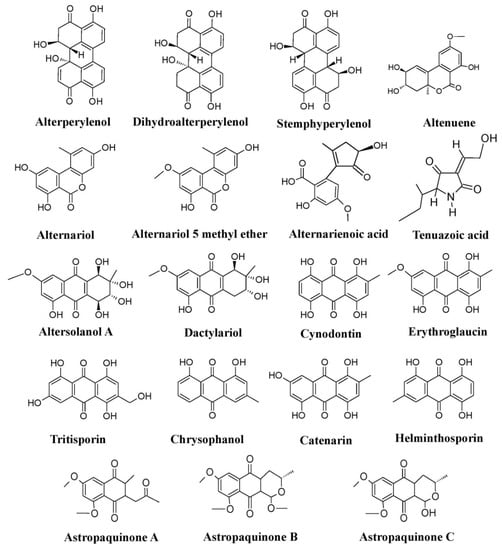
Members of different genera of the fungal family Pleosporaceae (
Alternaria,
Curvularia,
Pyrenophora, etc.) have immense potential for pigment production. Species of
Alternaria such as
Alternaria alternata,
Alternaria solani,
Alternaria porri, and
Alternaria tomatophila have been reported to produce a variety of pigments such as dactylariol, alterperylenol, dihydroalterperylenol, alternariol, alternariol-5-methyl ether, altenuene, alternarienoic acid, tenuazoic acid, stemphyperylenol, and altersolanol A (
Figure 6)
[25][99][100][101][25,76,77,78]. Also, other members of the Pleosporaceae,
Curvularia and
Pyrenophora, have been known to produce different types of pigments, e.g.,
Curvularia lunata produces hydroxyanthraquinone pigments such as chrysophanol, cynodontin, helminthosporin, erythroglaucin, and catenarin, whereas different species of
Pyrenophora such as
Pyrenophora teres,
Pyrenophora graminea,
Pyrenophora tritici-repentis,
Pyrenophora grahamii,
Pyrenophora dictyoides, and
Pyrenophora chaetomioides (which were previously known as
Drechslera teres,
Drechslera graminea,
Drechslera tritici-repentis,
Drechslera phlei,
Drechslera dictyoides,
Drechslera avenae, respectively) have also been reported to produce hydroxyanthraquinone pigments such as cynodontin, erythroglaucin, catenarin, helminthosporin, and tritisporin (
Figure 6)
[25][69][25,61].
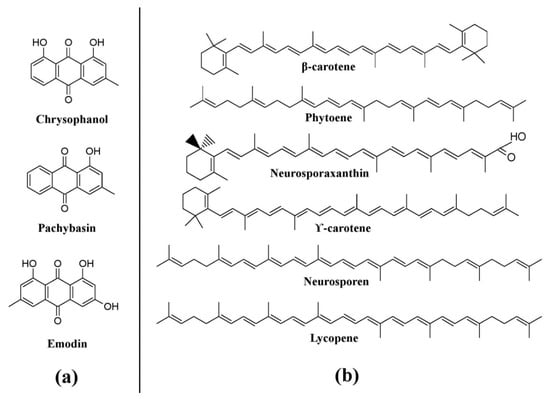
Trichoderma, a well-known bio-control agent, has been known to produce a variety of pigments
[25][102][25,124]. Several hydroxyanthraquinones such as pachybasin, chrysophanol, emodin, T22 azaphilone, 1-hydroxy-3-methyl-anthraquinone, 2,4,5,7-tetrahydroxyanthraquinone, 1,3,6,8-tetrahydroxyanthraquinone, and 1,8-dihydroxy-3-methyl-anthraquinone, have been reported from different species of
Trichoderma (
Trichoderma harzianum,
Trichoderma polysporum,
Trichoderma viride, and
Trichoderma aureoviride) (
Figure 7a)
[25], whereas
Trichoderma afrharzianum,
Trichoderma pyramidale, and
Trichoderma sp. 1 are reported to produce uncharacterized yellow pigments in submerged fermentation
[77][71]. Studies have also revealed that certain species of
Neurospora, such as
Neurospora crassa,
Neurospora sitophila, and
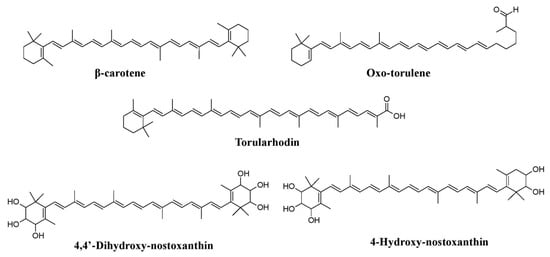
Neurospora intermedia produce a variety of carotenoids such as phytoene, β-carotene, γ-carotene, lycopene, neurosporene, and neurosporaxanthin (
Figure 7b)
[25][26][103][25,26,90].
Figure 6.
Pigments produced by members of the fungal family Pleosporaceae (species of
Alternaria
,
Curvularia
,
Astrosphaeriella
, and
Figure 7.
Pigments from other fungi. (
a
) Pigments from
Trichoderma
species, based on [25]. ( b
) Pigments from
Neurospora species, re-drawn from [25][103]. species, re-drawn from [25,90].
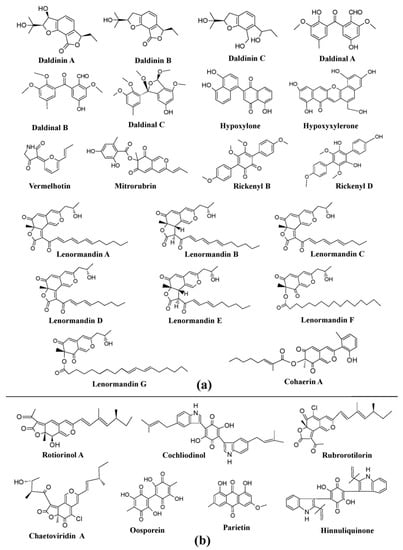
Many genera of the Xylariaceae family, such as
Daldinia,
Hypoxylon,
Jackrogersella, etc., have a great capability to synthesize pigments of very diverse colors and hues
[25]. A variety of interesting pigments such as BNT (1,1ˊ-Binaphthalene-4,4ˊ-5,5́-tetrol), daldinol, daldinal A–C, and daldinin A–C have been reported from different species of
Daldinia, such as
Daldinia bambusicola, D
aldinia caldariorum,
Daldinia concentrica,
Daldinia eschscholzii,
Daldinia childiae,
Daldinia clavata,
Daldinia fissa,
Daldinia grandis,
Daldinia lloydi,
Daldinia loculata,
Daldinia petriniae,
Daldinia singularis (
Figure 8a). Similarly, several cohaerin variants (cohaerin A–K), multiformin A, and sassafrins D have been obtained from
Jackrogersella cohaerens (formerly known as
Annulohypoxylon cohaerens) (
Figure 8a). Besides this, several species of
Hypoxylon were declared to produce diverse pigments e.g.,
Hypoxylon fragiforme (hypoxyxylerone, cytochalasin H, fragiformins A–B, and mitorubrin),
Hypoxylon howeanum (mitorubrin and azaphilones),
Hypoxylon lechatii (vermelhotin and hypoxyvermelhotins A–C),
Hypoxylon fuscum (daldinin A–C),
Hypoxylon fulvo-sulphureum (mitorubrinol derivatives),
Hypoxylon sclerophaeum (hypoxylone),
Hypoxylon rickii (rickenyl B and D),
Hypoxylon lenormandii and
Hypoxylon jaklitschii (lenormandins A-G),
Hypoxylon rubiginosum (mitorubrin, rubiginosin, and hypomiltin) (
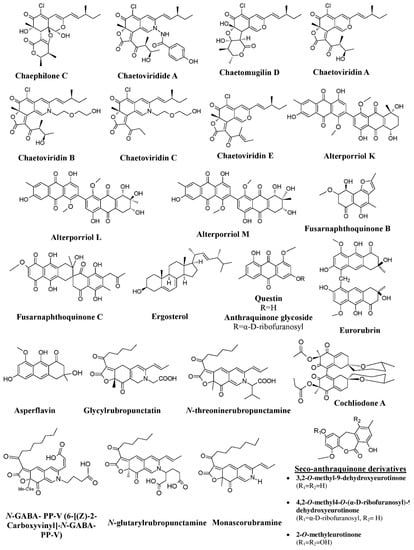
Figure 8a). Members of the Chaetomiaceae family also exhibit potential of pigment production.
Chaetomium cupreum has been mentioned to produce red azaphilone pigments, oosporein, rotiorinols A–C, rubrorotiorin, whereas
Chaetomium globosum produces yellow azaphilone pigments (chaetoviridins A–D), chaetoglobin A–B, chaetomugilins A–F, and cochliodinol (
Figure 8b). Production of parietin (hydroxyanthraquinone pigment) has also been revealed from the
Achaetomium sp. (
Figure 8b)
[25].
Figure 8. Pigments from the fungi of Xylariaceae and Chaetomiaceae families. (
a) Pigments from members of the Xylariaceae family (species of
Daldinia,
Hypoxylon, and
Jackrogersella), re-drawn from
[25]. (
b) Pigments from members of the Chaetomiaceae family (species of
Chaetomium and
Achaetomium) and Hypoxylaceae, re-drawn from
[25][104][25,84].
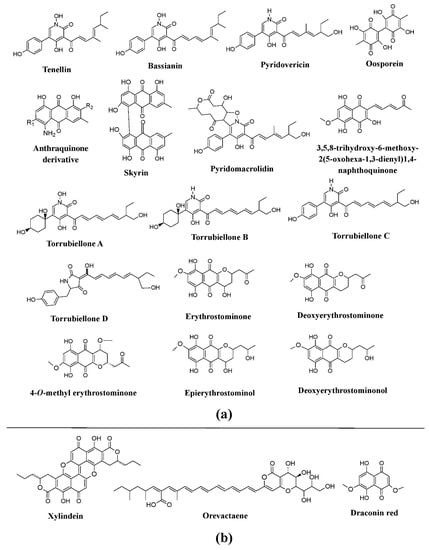
Also, the genera belonging to the family Cordycipitaceae such as
Torrubiella,
Cordyceps,
Beauveria,
Hyperdermium, and
Lecanicillium have been revealed to be promising producers of bioactive pigments, e.g., tenellin and bassianin are reported from
Beauveria bassiana and
Beauveria brongniartii (formerly known as
Beauveria tenella), pyridovericin and pyridomacrolidin from
Beauveria bassiana, torrubiellones A–D from the genus
Torubiella, oosporein from
Lecanicillium aphanocladii, whereas anthraquinone-related compounds are reported from
Cordyceps farinosa (formerly known as
Isaria farinosa) (
Figure 9a)
[41][105][106][107][108][41,73,74,75,125]. Similarly, the pigments erythrostominone, 4-
O-methyl erythrostominone, deoxyerythrostominone, deoxyerythrostominol, epierythrostominol, and 3,5,8-TMON (3,5,8-trihydroxy-6-methoxy-2-(5-oxohexa-1,3-dienyl)-1,4-naphthoquinone) have been reported from
Ophiocordyceps unilateralis (formerly known as
Cordyceps unilateralis), and skyrin from
Hyperdermium bertonii (
Figure 9a)
[25].
Figure 9. Pigments from the fungi of the Cordycipitaceae family and some other group. (
a) Pigments from members of the families Cordycipitaceae (species of
Beauveria,
Torrubiella,
Cordyceps,
Hyperdermium, and
Lecanicillium) and Ophiocordycipitaceae (
Ophiocordyceps sp.), re-drawn from
[25][41][105][106][107][108][25,41,73,74,75,125]. (
b) Pigments known from other groups of fungi (species of
Chlorociboria,
Scytalidium, and
Epicoccum), re-drawn from
[37][41][37,41].
Apart from this, studies have reported the production of the pigment xylindein from
Chlorociboria aeruginosa and
Chlorociboria aeruginascens, draconin red from
Scytalidium cuboideum, and a yellow pigment from
Scytalidiium ganodermophthorum and
Scytalidium lignicola. Other pigments, such as orevactaene produced from
Epicoccum nigrum, emodin, ω-hydroxyemodin, and emodic acid from
Hamigera avellanea (formerly known as
Talaromyces avellaneus) are also known (
Figure 3b,
Figure 9b)
[33][36][37][39][41][83][33,36,37,39,41,109]. Recently, fungi such as
Sanghuangporus baumii and
Clonostachys intermedia have been found to produce a yellow pigment under submerged fermentation
[77][71]. Production of melanin was reported from different groups of fungi such as
Phyllosticta capitalensis,
Xylaria polymorpha,
Trametes versicolor,
Inonotus hispidus,
Oxyporus populinus,
Fomes fomentarius,
Exophiala dermatitidis, Tuber melanosporum, Sporothrix schenckii, and
Cryptococcus neoformans [29][34][35][44][109][110][111][29,34,35,44,80,81,83]. Similarly, a study has shown the possible industrial application of the red pigment produced by
Paecilomyces sinclairii [112][126]. Besides filamentous fungi, certain genera of yeasts (
Rhodotorula,
Sporidiobolus,
Sporobolomyces and
Xanthophyllomyces) have also been known as pigment producers. Different species of
Rhodotorula (Rhodotorula glutinis,
Rhodotorula mucilaginosa (syn. Rhodotorula rubra),
Rhodotorula babjevae,
Rhodotorula toruloides Rhodotorula graminis),
Sporidiobolus (Sporidiobolus pararoseus,
Sporidiobolus johnsonii), and
Sporobolomyces (
Sporobolomyces uberrimus,
Sporobolomyces salmonicolor) have been reported to be prolific producers of torulin and torularhodin
[113][127]. Researchers have discovered pigments such as β-carotene, torulene, and torularhodin from
Rhodotorula glutini and multi-hydroxy carotenoids (4,4′-dihydroxy-nostoxanthin and 4-hydroxy-nostoxanthin) from
Xanthophyllomyces dendrorhous (
Figure 10)
[13][114][13,128].
Figure 10.
Pigments reported from yeasts such as
Rhodotorula glutini
and
Xanthophyllomyces dendrorhous, re-drawn from [13][114]. , re-drawn from [13,128].
In addition to terrestrial fungi, marine fungi are also very good producers of a variety of unique pigments having promising therapeutic and industrial applications
[115][116][129,130]. Studies on marine fungi by many researchers have reported a wide range of pigments and hues, e.g., a variety of anthraquinone pigments [asperflavin, 2-
O-methyleurotinone, questin, eurorubrin, 2-
O-methyl-9-dehydroxyeurotinone, 2-
O-methyl- 4-
O-(α-D-ribofuranosyl)-9-dehydroxyeurotinone, and 6, 3-
O-(α-D-ribofuranosyl)-questin] from the mangrove endophytic fungus
A. ruber (formerly known as
Eurotium rubrum), fusarnaphthoquinones B and fusarnaphthoquinones C from the sea fan-derived fungi
Fusarium species, and bianthraquinone derivatives (alterporriol K, alterporriol L, and alterporriol M) from mangrove endophytic
Alternaria sp. (
Figure 11)
[117][118][119][69,79,117]. Researchers have also investigated the red pigment production from mangrove fungus
Penicillium sp. and a yellow pigment production from the marine sponge-associated fungus
Trichoderma parareesei [120][121][70,99].
Figure 11. Pigments produced by marine fungal isolates, re-drawn from [117][118][119][122][123]. Pigments produced by marine fungal isolates, re-drawn from [69,72,79,82,117].
Also, many studies have revealed the production of polyketide pigments (
N-threonine rubropunctamine) and chlorinated azaphilone pigments (chaephilone-C, chaetoviridides-A, chaetoviridides-B, chaetoviridides-C) from marine fungal isolates of
Talaromyces spp. and
Chaetomium sp., respectively (
Figure 11)
[122][123][72,82]. A recent study has reported a novel pigment,
N-GABA-PP-V (6-[(Z)-2-Carboxyvinyl]-
N-GABA-PP-V), along with
N-threonine-monascorubramine,
N-glutaryl-rubropunctamine, and PP-O from the marine-derived fungus
Talaromyces albobiverticillius (
Figure 11)
[124][131]. Many antarctic fungi have also been discovered to produces pigments of different chemical classes and characteristics. A number of yeast and filamentous fungi isolated from the different samples collected from Antarctic regions have been reported to produce a variety of pigments with different colors
[125][86].