Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Zeinab Ezzeddine.
Salmonella enterica (S. enterica) serovars Enteritidis and Typhimurium are the main causes of bacterial gastroenteritis worldwide. This Gram-negative rods bacterium possesses several virulence factors that enable it to survive the host’s nutritional immunity. Toxins and metallophores are among these factors. Heavy metals, in particular, are essential for the survival of all living organisms including bacteria.
- Salmonella enterica
- bacterial gastroenteritis
- metallophores
1. Introduction
The Gram-negative bacterium Salmonella enterica (S. enterica) is a rod-shaped, intracellular pathogen. This zoonotic pathogen poses a serious threat to both human and animal health worldwide [1]. It is a major factor in both morbidity and mortality for people all around the world. Salmonella species can infect a diverse range of birds, reptiles, and mammals, including humans [2]. Salmonella Typhi (S. Typhi) and S. Paratyphi cause typhoid fever, a systemic febrile illness only affecting humans. The other numerous NTS serovars such as S. Typhimurium and S. Enteritidis infect many different hosts and result in diarrheal disease. NTS also causes severe, extra-intestinal, invasive bacteremia, referred to as invasive NTS (iNTS) disease [3]. Annually, Salmonella causes ~200 million to over 1 billion infections worldwide, with 93 million cases of gastroenteritis and 155,000 deaths, and 85% of illnesses which are food-linked [4]. Following ingestion, S. enterica (S. enterica serovar Typhimurium and S. enterica serovar Enteritidis) invade the intestinal epithelium in the colon and ileum, thus causing sepsis or spreading to systemic locations and creating a neutrophilic gastroenteritis. In normal adult hosts most serovars do not spread hematogenously, creating sepsis.
Salmonella is spread via the ingestion of contaminated food or water (fecal–oral transmission) [5]. The symptoms of enteric salmonellosis include fever, nausea, vomiting, and diarrhea, and they are typically self-limiting. Treatment is required at all ages for enteric fever caused by S. Typhi, which usually does not cause gastroenteritis. Treatment should also be given to infants, malnourished people, and immunosuppressed individuals, to prevent complications of hematogenous dissemination.
Typhoid fever symptoms include fever, headache, lethargy, and anorexia, with intestinal symptoms only occurring in about one third of cases [6]. Sub-Saharan Africa and people with impaired immune systems are experiencing the emergence of an invasive nontyphoidal type of illness (iNTS) [7]. Invasive cases may cause potentially fatal bloodstream infections. Since antibiotic therapy can be life-saving for certain systemic infections, antimicrobial resistance is a serious issue. Resistance to antibiotics develops in both pathogenic and commensal bacteria within the treated host as a result of antibiotics abuse in both people and animals [8]. When it comes to zoonotic germs that are found in food, humans get infected when they consume poorly cooked meat from infected animals, and poorly cooked eggs or from foods that have been contaminated during processing or retail [9]. Only a subset of antibiotics is effective for treating S. enterica infections, as those that do not achieve adequate concentrations of macrophages are ineffective. Resistance can also spread through environmental factors like water or wildlife or through direct animal contact (as with pets) [10].
2. Siderophores
Because iron is a cofactor needed for vital processes including energy production and DNA replication, iron sequestration provides an efficient antimicrobial defense. Salmonella-infected macrophages enhance iron export, thus Salmonella multiplication is restricted by low iron levels in macrophages, emphasizing the significance of defining Salmonella’s iron acquisition mechanisms in these iron-starved environments. Enterobactin and salmochelin, two catecholate siderophores, are secreted by Salmonella to obtain iron [26][11].2.1. Enterochelin (Enterobactin)
It is known that macrophages increase a variety of iron-regulatory proteins (such as ferroportin) to fight bacterial infection [26][11]. These proteins prevent pathogens from accessing the labile iron pool [27][12]. To sequester iron from the host iron-binding proteins, Salmonella is prompted by the lack of iron to express enterobactin (Ent), a catecholate siderophore with a high affinity for ferric iron (Kd of 10−49 M) [23,24][13][14]. Siderophores are small iron chelators that facilitate iron transport into the bacterial cells. A triscatechol derivative of a cyclic triserine lactone gives rise to the siderophore enterobactin [28][15]. The structure of Ent is illustrated in Figure 1.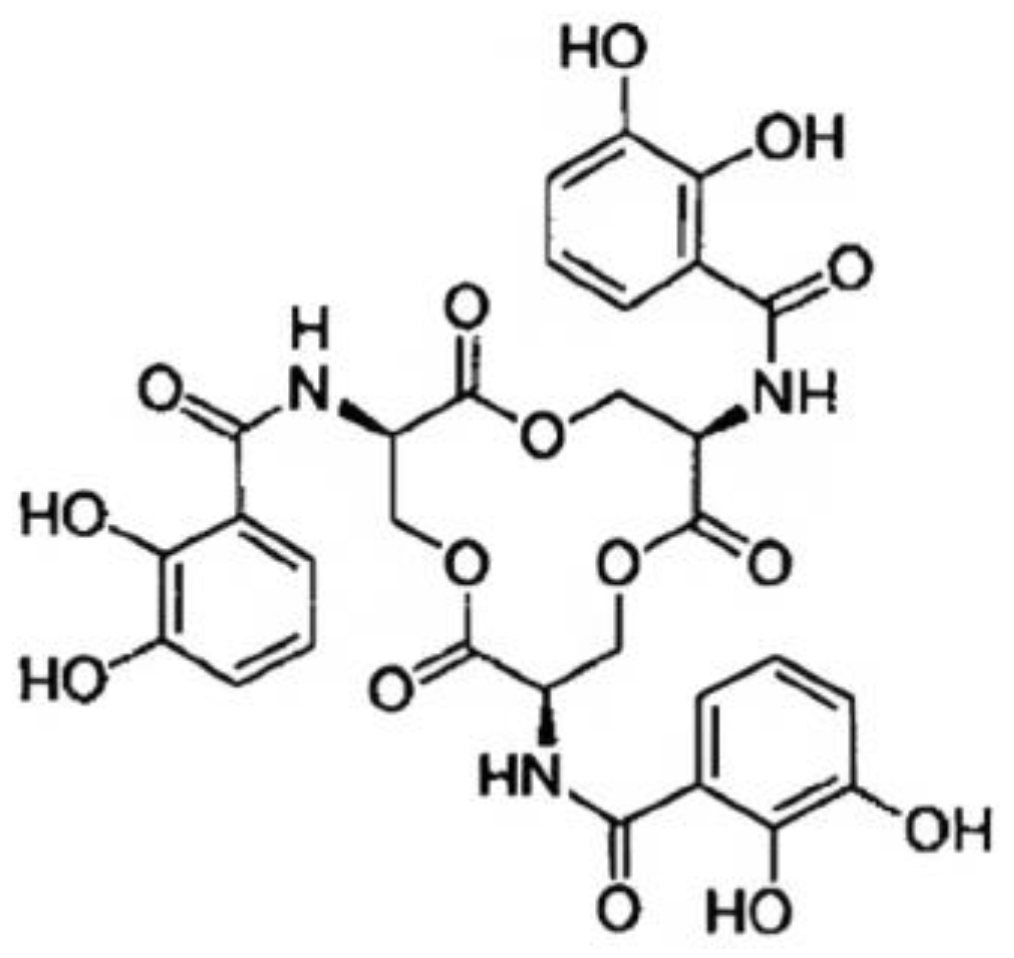
Figure 1. Enterobactin’s structural illustration displaying the catechol, amide linkage, and triserine ring components.
The Biosynthesis of Enterobactin
Thirteen proteins that are present in numerous species of Gram-negative enteric bacteria, including E. coli, S. enterica, Shigella dysenteriae (S. dysenteriae), and Klebsiella pneumoniae (K. pneumoniae) are encoded by a 24-kb gene cluster [29][16]. These thirteen proteins work together to produce, transport, and process the siderophore enterobactin. The operon entABCDEFH is involved in the biosynthesis of enterobactin from chorismic acid, and EntS and TolC are involved in Ent export through the cytoplasmic membrane. The two-module nonribosomal peptide synthetase (NRPS), which consists of EntE, EntB, and EntF, produces Ent (2,3-dihydroxybenzoylserine trilactone) from 2,3-dihydroxybenzoic acid (DHB) and serine [30][17]. EntD catalyzes the post-translational 4′-phosphopantetheinylation of EntB and EntF, a process necessary for the covalent attachment of assembly line intermediates during the production of Ent [31,32][18][19]. EntC, EntB, and EntA operate sequentially to divert the principal metabolite chorismate to DHB. Ent is exported across the inner membrane by EntS [33][20], a significant facilitator-subfamily exporter, and through the outer membrane by a TolC-dependent mechanism after being synthesized in the cytoplasm [34][21]. Given its very high affinity for Fe3+ (Kd = 10−35 M at physiological pH, [35][22]), Ent may successfully compete for Fe3+ binding outside of the cell with all known protein and small-molecule ligands. The Fe3+–Ent complex is then transported into the cell through the TonB-dependent outer membrane Ent-specific porin FepA [36[23][24],37], escorted through the periplasm by FepB, and pumped into the cytoplasm through the two-protein inner membrane channel FepDG into the cytoplasm via FepC-catalyzed ATP hydrolysis [38][25] (Figure 2). Before the tightly bound ferric iron can be transported to intracellular iron carriers, Fe3+ from Ent must first be released by the esterase Fes activity [39][26], which must also enzymatically degrade its trilactone scaffold to three equivalents of DHB-Ser. The iron-dependent repressor Fur (Fe uptake regulation), which acts as a sensor for intracellular iron by dissociating from its DNA-binding site when iron is deficient [40][27], regulates transcription of the entire Ent system. As a result, when iron levels are low, the transcription of the Ent synthesis, export, and import genes is stimulated, and it is suppressed when iron levels are high.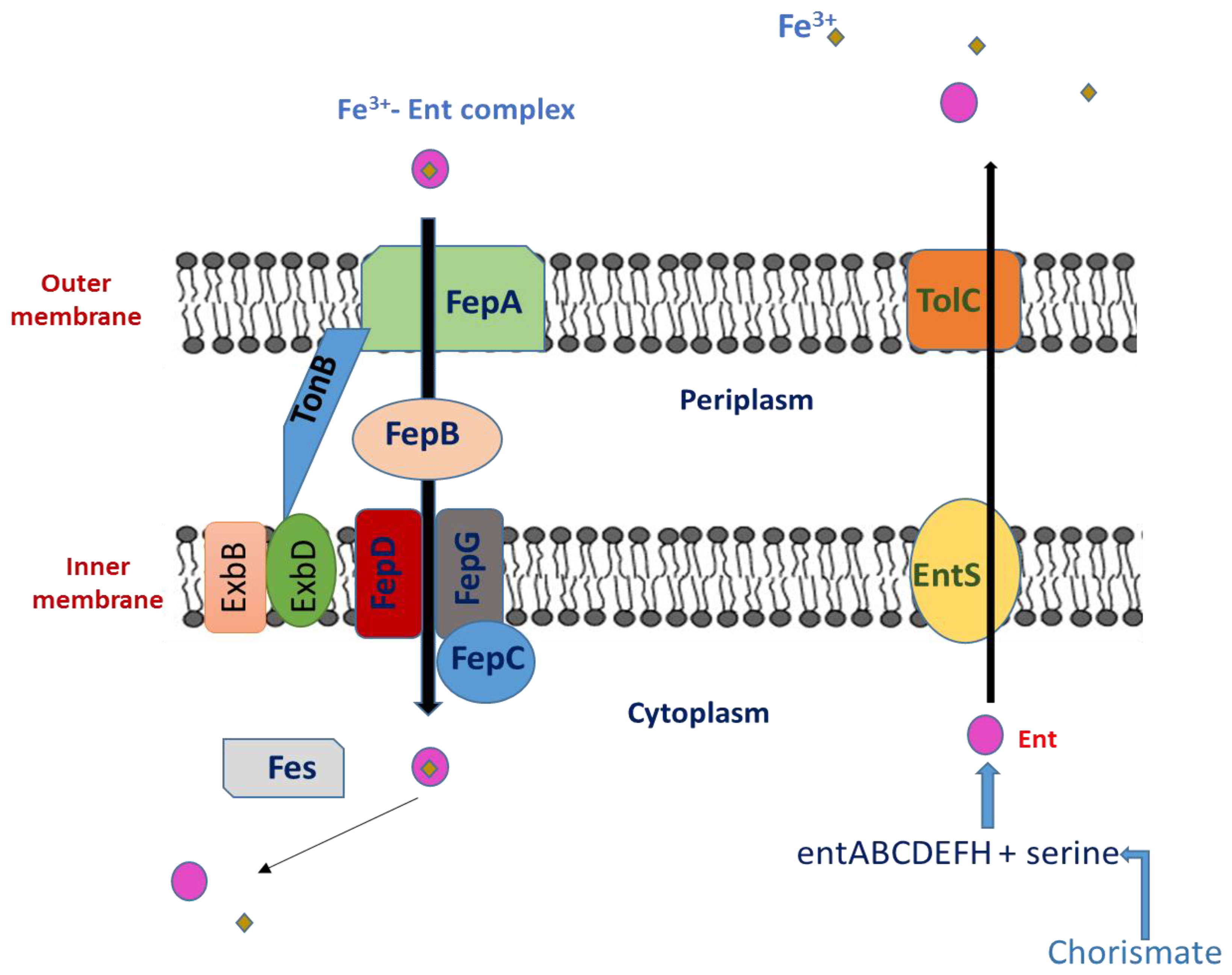
Figure 2. Ent export and Ent–Fe3+ complex import system in Salmonella.
2.2. Interaction between Ent and the Immune System
2.2.1. Effect of Ent on Macrophages
Iron is essential for the redox activity of heme proteins expressed in both immune and non-immune cells. A study was conducted by Yeoh et al. to investigate the potential impact of enterobactin’s iron chelation on macrophage nitrosative and immunological responses. Ent was found to reduce the LPS-induced release of cytokines (such as serum amyloid A, IL-6, and lipocalin 2) and nitrite in macrophages in a dose-dependent manner [41][28]. Ent also suppressed the mRNA and protein expression of inducible nitric oxide synthase (iNOS; a heme protein) that is stimulated by LPS [26][11]. They also investigated if Ent might shield the intracellular pathogen Salmonella enterica spp. typhimurium in the gentamycin protection assay to show the physiological significance of these results. Ent increased the expression of iNOS (mRNA, protein) and Arginase-1 (mRNA), but it decreased the nitrite levels in Salmonella-infected macrophages. More critically, Salmonella eradication by macrophages treated with Ent was compromised. The addition of exogenous Ent significantly increased the longevity of both strains, and Ent-sufficient Salmonella also outlived their isogenic Ent-deficient counterparts. However, Ent’s inhibitory activities were negated when saturated with iron (1:1 ratio), demonstrating that Ent must be in its iron-free form to effectively inhibit macrophages. These data support the idea that Ent protects bacteria against immunological reactions from macrophages in addition to facilitating bacterial iron absorption [42,43][29][30].2.2.2. The Interaction between Siderocalin and Enterobactin
Withholding necessary iron from invading germs has long been recognized as a critical host defensive mechanism [44][31]. Increasing the expression of transferrin, lactoferrin receptors, and ferritin and decreasing the level of extracellular iron in serum are common bacteriostatic responses. The mammalian protein Siderocalin (Scn), also known as Lcn2, neutrophil-gelatinase-associated lipocalin, 24p3, or uterocalin, is used in a more focused approach [36][23]. Scn is generated and released in response to the activation of innate immune receptors, such as Toll-like receptors, in a variety of cell types. Scn is naturally present in neutrophil granules. Successful pathogens generate alternate or modified siderophores that Scn does not bind toin order to circumvent this defense. This supplements the overall antibacterial iron-depletion response and prevents major early bacteremia [45][32]. Numerous microbes produce the classic 2,3-catecholate siderophore enterobactin (Ent), which was the first recognized target of Scn [45][32]. The protein binds to and sequesters the ferric siderophore complex with an affinity for [Fe3(Ent)]3−, acting as a growth inhibitor of pathogens that solely rely on Ent-mediated iron uptake similar to that of FepA, the cognate outer membrane receptor [46][33]. Scn is distinct from other host iron-binding proteins in that it is selective for iron intended for bacterial usage as a ferric siderophore complex and does not chelate iron directly. Scn has been linked to innate immunity as well as kidney development [47][34]. As an iron-donating molecule, it transports iron to the cytoplasm, where it may activate or repress genes that respond to iron [48][35]. Therefore, Scn may be a substitute for transferrin in the transport of iron and be crucial for the development of tissues and organs. Scn may also function at a stage of development before transferrin circulation and the expression of transferrin receptors are established [49][36].2.3. Salmochelins
In addition to Ent, Salmonella secretes other siderophores salmochelins (SX, S1, S2 and S4). Lipocalin-2, a siderophore-capturing protein, is secreted by macrophages in response to gamma interferon (IFN-γ) during infection [50,51][37][38]. Enterobactin is bound by lipocalin-2, while salmochelin is not. The latter is a glycosylated enterobactin derivative that favors Salmonella’s ability to grow specifically in the intestine [49][36]. Moreover, Ent is bound to serum albumin thus its effectiveness decreases [52][39]. In addition, the hydrophobicity of its three catechol acyl ‘arms’, which allow Ent to partition into lipid bilayers, further reduces the amount of Fe3+–Ent available for bacterial cell import [53][40].Salmochelin Biosynthesis
Salmochelins are enterobactin-related substances that contain a 5-C-glucosylated 2,3-dihydroxybenzoyl residue (DHB) (Figure 3). A twofold β-C-glucosylated enterobactin analogue is the main substance of salmochelin S4 [54][41]. Salmochelin S2 has one unglycosylated DHB-serine moiety at the C-terminal end. Additionally, salmochelin SX is a monomeric DHB(glucosyl)-serine molecule, and salmochelin S1 is a dimer with a DBH(glucosyl)-seryl-DHB-serine constitution [54][41].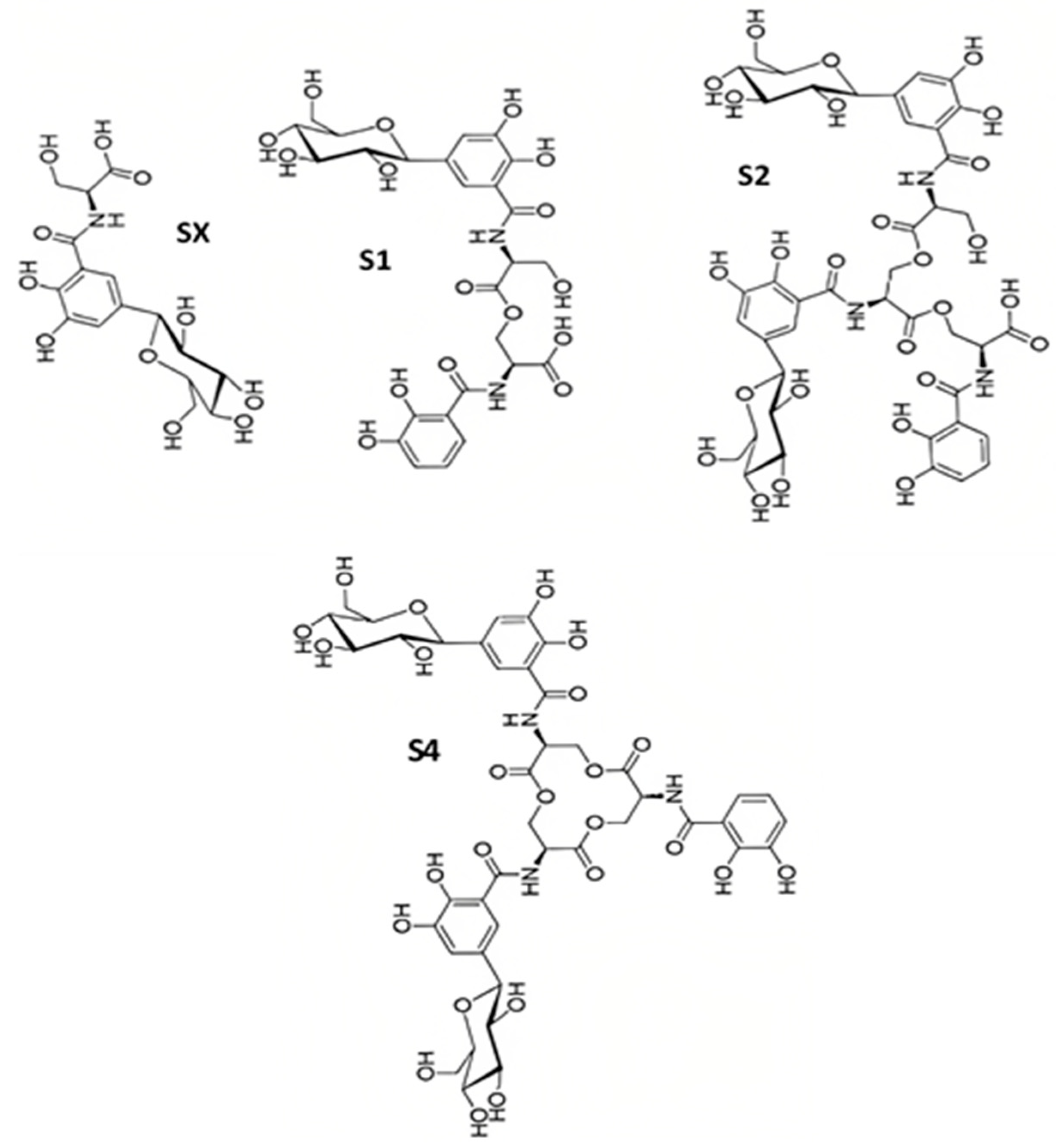
Figure 3. The structures of different salmochelins SX, S1, S2, and S4 secreted by S. enterica.
References
- Fardsanei, F.; Dallal, M.M.S.; Douraghi, M.; Memariani, H.; Bakhshi, B.; Salehi, T.Z.; Nikkhahi, F. Antimicrobial resistance, virulence genes and genetic relatedness of Salmonella enterica serotype Enteritidis isolates recovered from human gastroenteritis in Tehran, Iran. J. Glob. Antimicrob. Resist. 2018, 12, 220–226.
- Lou, L.; Zhang, P.; Piao, R.; Wang, Y. Salmonella Pathogenicity Island 1 (SPI-1) and Its Complex Regulatory Network. Front. Cell. Infect. Microbiol. 2019, 9, 270.
- Takaya, A.; Yamamoto, T.; Tokoyoda, K. Humoral Immunity vs. Salmonella. Front. Immunol. 2020, 10, 3155.
- He, Y.; Wang, J.; Zhang, R.; Chen, L.; Zhang, H.; Qi, X.; Chen, J. Epidemiology of foodborne diseases caused by Salmonella in Zhejiang Province, China, between 2010 and 2021. Front. Public Health 2023, 11, 1127925.
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella pathogenicity and host adaptation in chicken-associated serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607.
- Herekar, F.; Sarfaraz, S.; Imran, M.; Ghouri, N.; Shahid, S.; Mahesar, M. Clinical spectrum and outcomes of patients with different resistance patterns of Salmonella enterica. Pak. J. Med. Sci. 2022, 38, 356–361.
- Rabsch, W.; Tschäpe, H.; Bäumler, A.J. Non-typhoidal salmonellosis: Emerging problems. Microbes Infect. 2001, 3, 237–247.
- Uche, I.V.; MacLennan, C.A.; Saul, A. A systematic review of the incidence, risk factors and case fatality rates of invasive nontyphoidal Salmonella (iNTS) disease in Africa (1966 to 2014). PLoS Negl. Trop. Dis. 2017, 11, e0005118.
- Ezzeddine, Z.; Ghssein, G. Towards new antibiotics classes targeting bacterial metallophores. Microb. Pathog. 2023, 29, 06221.
- Awada, R.; Ghssein, G.; Roz, A.; Farhat, M.; Nehme, N.; Hassan, H.F. Prevalence of Campylobacter spp. in broilers in North Lebanon. Vet. World 2023, 16, 322–328.
- Saha, P.; Xiao, X.; Yeoh, B.S.; Chen, Q.; Katkere, B.; Kirimanjeswara, G.S.; Vijay-Kumar, M. The bacterial siderophore enterobactin confers survival advantage to Salmonella in macrophages. Gut Microbes 2019, 10, 412–423.
- Fischbach, M.A.; Lin, H.; Liu, D.R.; Walsh, C.T. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat. Chem. Biol. 2006, 2, 132–138.
- Ghssein, G.; Ezzeddine, Z. The Key Element Role of Metallophores in the Pathogenicity and Virulence of Staphylococcus aureus: A Review. Biology 2022, 11, 1525.
- Ghssein, G.; Matar, S.F. Chelating Mechanisms of Transition Metals by Bacterial Metallophores “Pseudopaline and Staphylopine”: A Quantum Chemical Assessment. Computation 2018, 6, 56.
- Loomis, L.D.; Raymond, K.N. Solution equilibria of enterobactin and metal enterobactin complexes. Inorg. Chem. 1991, 30, 906–911.
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588.
- Gehring, A.M.; Mori, I.; Walsh, C.T. Reconstitution and characterization of the Escherichia coli enterobactin synthetase from EntB, EntE, and EntF. Biochemistry 1998, 37, 2648–2659.
- Walsh, C.; Liu, J.; Rusnak, F.; Sakaitani, M. Molecular studies on enzymes in chorismate metabolism and the enterobactin biosynthetic pathway. Chem. Rev. 1990, 90, 1105–1129.
- Lambalot, R.H.; Gehring, A.M.; Flugel, R.S.; Zuber, P.; LaCelle, M.; Marahiel, M.A.; Walsh, C.T. A new enzyme superfamily—The phosphopantetheinyl transferases. Chem. Biol. 1996, 3, 923–936.
- Furrer, J.L.; Sanders, D.N.; Hook-Barnard, I.G.; McIntosh, M.A. Export of the siderophore enterobactin in Escherichia coli: Involvement of a 43 kDa membrane exporter. Mol. Microbiol. 2002, 44, 1225–1234.
- Bleuel, C.; Große, C.; Taudte, N.; Scherer, J.; Wesenberg, D.; Krauß, G.J.; Grass, G. TolC is involved in enterobactin efflux across the outer membrane of Escherichia coli. J. Bacteriol. 2005, 187, 6701–6707.
- Crosa, J.H.; Mey, A.R.; Payne, S.M. Iron Transport in Bacteria; ASM Press: Washington, DC, USA, 2004.
- Annamalai, R.; Jin, B.; Cao, Z.; Newton, S.M.; Klebba, P.E. Recognition of ferric catecholates by FepA. J. Bacteriol. 2004, 186, 3578–3589.
- Buchanan, S.K.; Smith, B.S.; Venkatramani, L.; Xia, D.; Esser, L.; Palnitkar, M.; Deisenhofer, J. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 1999, 6, 56–63.
- Faraldo-Gomez, J.D.; Sansom, M.S. Acquisition of siderophores in gram-negative bacteria. Nat. Rev. Mol. Cell Biol. 2003, 4, 105–116.
- Lin, H.; Fischbach, M.A.; Liu, D.R.; Walsh, C.T. In vitro characterization of salmochelin and enterobactin trilactone hydrolases IroD, IroE, and Fes. J. Am. Chem. Soc. 2005, 127, 11075–11084.
- Hantke, K. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 2001, 4, 172–177.
- Yeoh, B.S.; Saha, P.; Xiao, X.; Singh, V.; Vijay-Kumar, M. Enterobactin, a metallophore, mitigates the immune responses of macrophages. J. Immunol. 2017, 198 (Suppl. S1), 113–121.
- Ong, S.T.; Ho, J.Z.S.; Ho, B.; Ding, J.L. Iron-withholding strategy in innate immunity. Immunobiology 2006, 211, 295–314.
- Devireddy, L.R.; Teodoro, L.R.; Richard, F.A.; Green, M.R. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science 2001, 293, 829–834.
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 2002, 10, 1033–1043.
- Yang, J.; Mori, K.; Li, J.Y.; Barasch, J. Iron, lipocalin, and kidney epithelia. Am. J. Phys. Renal Phys. 2003, 285, F9–F18.
- Yang, J.; Goetz, D.H.; Li, J.Y.; Wang, W.; Mori, K.; Setlik, D.; Du, T.; Erdjument-Bromage, H.; Tempst, P.; Strong, R.K.; et al. An iron delivery pathway mediated by a lipocalin. Mol. Cell 2002, 10, 1045–1056.
- Kaplan, J. Mechanisms of cellular iron acquisition: Another iron in the fire. Cell 2002, 111, 603–606.
- Flo, T.H.; Smith, K.D.; Sato, S.; Rodriguez, D.J.; Holmes, M.A.; Strong, R.K.; Akira, S.; Aderem, A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004, 432, 917–921.
- Nairz, M.; Fritsche, G.; Brunner, P.; Talasz, H.; Hantke, K.; Weiss, G. Interferon-gamma limits the availability of iron for intramacrophage Salmonella typhimurium. Eur. J. Immunol. 2008, 38, 1923–1936.
- Raffatellu, M.; George, M.D.; Akiyama, Y.; Hornsby, M.J.; Nuccio, S.P.; Paixao, T.A.; Butler, B.P.; Chu, H.; Santos, R.L.; Berger, T.; et al. Lipocalin-2resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 2009, 5, 476–486.
- Hantke, K.; Nicholson, G.; Rabsch, W.; Winkelmann, G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. USA 2003, 100, 3677–3682.
- Konopka, K.; Neilands, J.B. Effect of serum albumin on siderophore-mediated utilization of transferrin iron. Biochemistry 1984, 23, 2122–2127.
- Luo, M.; Lin, H.; Fischbach, M.A.; Liu, D.R.; Walsh, C.T.; Groves, J.T. Enzymatic tailoring of the bacterial siderophore enterobactin alters membrane partitioning and iron acquisition. ACS Chem. Biol. 2006, 1, 29–32.
- Bister, B.; Bischoff, D.; Nicholson, G.J.; Valdebenito, M.; Schneider, K.; Winkelmann, G.; Hantke, K.; Süssmuth, R.D. The structure of salmochelins: C-glucosylated enterobactins of Salmonella enterica. Biometals 2004, 17, 471–481.
- Bäumler, A.J.; Tsolis, R.M.; van der Velden, A.W.M.; Stojiljkovic, I.; Anic, S.; Heffron, F. Identification of a new iron regulated locus of Salmonella typhi. Gene 1996, 183, 207–213.
- Müller, S.I.; Valdebenito, M.; Hantke, K. Salmochelin, the long-overlooked catecholate siderophore of Salmonella. Biometals 2009, 22, 691–695.
- Fischbach, M.A.; Lin, H.; Liu, D.R.; Walsh, C.T. In vitro characterization of IroB, a pathogen-associated C-glycosyltransferase. Proc. Natl. Acad. Sci. USA 2005, 102, 571–576.
- Crouch, M.L.V.; Castor, M.; Karlinsey, J.E.; Kalhorn, T.; Fang, F.C. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2008, 67, 971–983.
- Bäumler, A.J.; Norris, T.L.; Lasco, T.; Voight, W.; Reissbrodt, R.; Rabsch, W.; Heffron, F. IroN, a novel outer membrane siderophore receptor characteristic of Salmonella enterica. J. Bacteriol. 1998, 180, 1446–1453.
- Zhu, M.; Valdebenito, M.; Winkelmann, G.; Hantke, K. Functions of the siderophore esterases IroD and IroE in iron-salmochelin utilization. Microbiology 2005, 151, 2363–2372.
- Lim, D.; Kim, K.; Song, M.; Jeong, J.H.; Chang, J.H.; Kim, S.R.; Hong, C.; Im, S.S.; Park, S.H.; Lee, J.C.; et al. Transcriptional regulation of Salmochelin glucosyltransferase by Fur in Salmonella. Biochem. Biophys. Res. Commun. 2020, 529, 70–76.
- Balbontín, R.; Villagra, N.; Pardos de la Gándara, M.; Mora, G.; Figueroa-Bossi, N.; Bossi, L. Expression of IroN, the salmochelin siderophore receptor, requires mRNA activation by RyhB small RNA homologues. Mol. Microbiol. 2016, 100, 139–155.
- Zhang, Z.; Gosset, G.; Barabote, R.; Gonzalez, C.S.; Cuevas, W.A.; Saier, M.H. Functional interactions between the carbon and iron utilization regulators, Crp and Fur, in Escherichia coli. J. Bacteriol. 2005, 187, 980–990.
More
