We present an overview of the current state of knowledge on the SARS-CoV-2 and COVID-19 pandemic. In addition to an overview of the epidemiological, clinical, and radiological features of SARS-CoV-2, we also summarize possible therapeutic options currently under investigation and the future outlook for the disease. Whereas the trials on SARS-CoV-2 genome-based specific vaccines and therapeutic antibodies are currently being tested, this solution is more long-term, as they require thorough testing of their safety. On the other hand, the repurposing of the existing therapeutic agents previously designed for other virus infections and pathologies happens to be the only practical approach as a rapid response measure to the emergent pandemic. The current pandemic emergency will be a trigger for more systematic drug repurposing design approaches based on big data analysis. Further on, regression analytical review is presented on the virological and evolutionary history of SARS-CoV viruses.
- COVID-19
- SARS-CoV-2
- pneumonia
- ACE2
- clinical trials
1. General Clinical Features of COVID-19
The usual symptoms of COVID-19 include fever (83–98%), cough (59–82%), shortness of breath (19–55%), and muscle ache (11–44%), which are similar to those of SARS and MERS. [1] Some patients may have sore throat, rhinorrhea, headache and confusion a few days before the onset of fever, indicating that fever is a critical symptom, but not the only initial manifestation of infection. [1] The pattern of fever has not yet been fully understood. A small proportion of patients had hemoptysis [2][3], and a number of cases were found relatively asymptomatic. [4] COVID-19 patients may have normal or lower white blood cell counts, lymphopenia, or thrombocytopenia, with the increased C-reactive protein level. [1][2][3] People who have fever and upper respiratory tract symptoms with leukopenia or lymphopenia should be suspected for this disease, especially for patients with travel history to the endemic area or close exposure record.
However, the clinical course of COVID-19 pneumonia exhibits a broad spectrum of severity and progression patterns. In some patients, dyspnea develops within a median of 8 days after the onset of illness (range of 5–13 days), while in others, respiratory distress may be absent. [2] Around 3–29% patients may need the admission to the intensive care unit. Severely ill patients may have poor disease course of rapid progression to multiple organ dysfunction and even death [1][2], and those who have shortness of breath and hypoxemia can quickly progress into acute respiratory distress syndrome (ARDS), severe sepsis with shock, and even multiple organ dysfunction within one week. [3][5] ARDS was observed to develop in 17–29% of hospitalized patients approximately 8 days after symptoms onset, and the global mortality rate reached approximately 5.4% [2].
It is also worth noting that the gastrointestinal symptoms of COVID-19 may be caused by the direct viral damage to the intestine rather than the immunopathogenic response to the lung infection of the host. Since angiotensin-converting enzyme 2 (ACE2), the main cellular receptor of SARS-CoV-2 is expressed in the human gastrointestinal epithelial cells, it is believed that the viral shedding at the gastrointestinal tract and fecal–oral transmission is highly plausible. [6] Indeed, it was reported that the rectal swabs showed positive results even after the nasopharyngeal tests were constitutively negative [7]. Besides, the live virus was also detected in stool samples of diseased patients. This evidence strongly indicate that stool can be contagious for a long time after the discharge of patients based on two negative nasopharyngeal swabs. Thus, adding rectal swabs to the discharge criteria should be considered for the prevention of both nosocomial and community spread of COVID-19.
Aside from the gastrointestinal symptoms, a retrospective study of 214 patients in China reported that 5.6 % of patients experienced hypogeusia and 5.1 % experienced hyposmia [8]. Though the loss of olfaction during SARS-CoV-2 infection could be explained by the swelling of the nasal mucosa, a larger population of patients should be included to determine whether hypogeusia and hyposmia could be a common neurological manifestation of COVID-19. Nevertheless, hyposmia and hypogeusia are now being recommended as the early warning signs and an indication for early self-isolation.
2. Radiological Features of COVID-19
The radiological examinations, including chest X-ray (CXR) and chest computed tomography (CT) scan, are important for early detection and treatment of COVID-19 [9]. The imaging findings of COVID-19 pneumonia mimic influenza, SARS-CoV, and MERS-CoV pneumonia [10][11][12][13][14]. The primary Wuhan study revealed that upon diagnosis, 74 [75%] patients showed bilateral pneumonia, and the remaining 25 [25%] patients showed unilateral pneumonia. [1] In addition, 14 [14%] patients showed multiple mottling and ground-glass opacities [1]. In the subsequent study, it was reported that the predominant pattern of abnormality observed was peripheral (44 [54%]), ill-defined (66 [81%]), and mainly involved the right lower lobes (225 [27%] of 849 affected segments) [1]. Bilateral multiple consolidation usually occurs in more severe cases [9].
Chest CT is more efficient in detecting pneumonia at the early stages of COVID-19. However, the imaging findings of COVID-19 pneumonia on chest CT are variable and nonspecific [15][16][17]. The most common patterns of COVID-19 on chest CT scans include multiple GGO lesions (56.4%), and bilateral patchy shadowing (51.8%), and the other patterns consist of local patchy shadowing (28.1%), and interstitial abnormalities (4.4%). Severe cases tend to yield more prominent radiologic findings on chest CT scan, such as more bilateral patchy shadowing (82%), more multiple GGO lesions (60%), and more local patchy shadowing (55.1%) than non-severe cases. No CXR or chest CT abnormality was identified in 17.9% of non-severe cases and 2.9% of severe cases [1][2][18]. Pure GGO lesions can be found in the early stages. Focal or multifocal GGO lesions may progress into consolidation or GGO lesions with superimposed interlobular/intralobular septal thickening as crazy-paving pattern during disease progression, and the expansion of consolidation represented disease progression [19][2019][2119]. Pure consolidative lesions were relatively less common. Pulmonary cavitary lesion, pleural effusion, and lymphadenopathy are rarely reported [19][20][21][19][22].
However, interestingly, it was also reported that asymptomatic patients could show early CT changes [23]. Conversely, as mentioned earlier, another study has shown positive RT-PCR results for SARS-CoV-2 in the absence of CT changes [24]. Despite the limited number of cases available for thorough radiographic study, we can observe the trend of varied presentations of COVID-19 pneumonia. Asymptomatic patients showing positive CT findings undoubtedly pose challenges for the current diagnostic protocol, especially those patients who have false-negative RT-PCR results.
Moreover, different radiographic patterns are seen as the COVID-19 progresses. Typically, after the first to second week of the onset, lesions progress to bilateral diffused pattern with consolidations. By contrast, both ground-glass opacification and consolidation were present relatively early in SARS [5]. This again could be indicative of the significant difference in diagnostic sensitivity between these two diseases, especially at early or asymptomatic stage. In conclusion, correlating imaging features with clinical and laboratory findings to assess patients may be essential to facilitate early diagnosis of COVID-19 pneumonia (Figure 1).
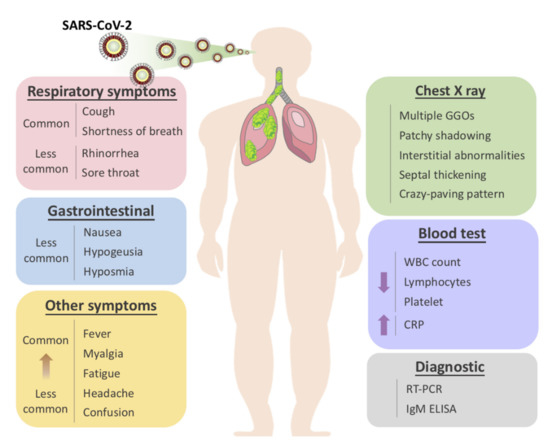
Figure 1. Overview of symptomatic, radiological and laboratory characteristics of COVID-19.
2. Virological Features
Even though SARS-CoV viruses are popularly believed to be a form of respiratory syndrome, by which it was named, evidence exists that there is a structural similarity between HIV-1 gp41 and SARS-CoV Spike 2 proteins [36]. This feature in fusion peptide continues to mutate in SARS-CoV-2 and its variants [37][38]. Further questions have been raised on the categorical rationale on the virological features of SARS-CoV series that it is too over-lengthed for single-strand virus, and corresponds better to the negative-sense paramyxovirus [39].
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Dawei Wang; Bo Hu; Chang Hu; Fangfang Zhu; Xing Liu; Jing Zhang; Binbin Wang; Hui Xiang; Zhenshun Cheng; Yong Xiong; Yan Zhao; Yirong Li; Xinghuan Wang; Zhiyong Peng; Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061, 10.1001/jama.2020.1585.
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New Engl. J. Med. 2020.
- Jasper Fuk-Woo Chan; Shuofeng Yuan; Kin-Hang Kok; Kelvin Kai-Wang To; Hin Chu; Jin Yang; Fanfan Xing; Jieling Liu; Cyril Chik-Yan Yip; Rosana Wing-Shan Poon; Hoi-Wah Tsoi; Simon Kam-Fai Lo; Kwok-Hung Chan; Vincent Kwok-Man Poon; Wan-Mui Chan; Jonathan Daniel Ip; Jian-Piao Cai; Vincent Chi-Chung Cheng; Honglin Chen; Christopher Kim-Ming Hui; Kwok-Yung Yuen; A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet 2020, 395, 514-523, 10.1016/s0140-6736(20)30154-9.
- D. Paraskevis; E.G. Kostaki; G. Magiorkinis; G. Panayiotakopoulos; G. Sourvinos; S. Tsiodras; Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infection, Genetics and Evolution 2020, 79, 104212, 10.1016/j.meegid.2020.104212.
- Hindson, J. COVID-19: Faecal–oral transmission? Nat. Rev. Gastroenterol. Hepatol. 2020, 1.
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 1–4.
- Mao, L.; Wang, M.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; Miao, X.; Hu, Y.; et al. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: A Retrospective Case Series Study. Ssrn Electron. J. 2020.
- Zu, Z.Y.; Di Jiang, M.; Xu, P.P.; Chen, W.; Ni, Q.; Lua, G.; Zhang, L.J. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology 2020, 200490.
- Malainou, C.; Herold, S; Influenza. Internist 2019, 60, 1004-1011.
- Emmie De Wit; Neeltje Van Doremalen; Darryl Falzarano; Vincent Munster; SARS and MERS: recent insights into emerging coronaviruses. Nature Reviews Genetics 2016, 14, 523-534, 10.1038/nrmicro.2016.81.
- Hui, D.S.; Zumla, A; Severe Acute Respiratory Syndrome. Infect. Dis. Clin. North Am. 2019, 33, 869–889.
- Jasper Fuk-Woo Chan; Susanna K.P. Lau; Kelvin Kai-Wang To; Vincent C. C. Cheng; Patrick C.Y. Woo; Kwok-Yung Yuen; Middle East Respiratory Syndrome Coronavirus: Another Zoonotic Betacoronavirus Causing SARS-Like Disease. Clinical Microbiology Reviews 2015, 28, 465-522, 10.1128/CMR.00102-14.
- Chan, J.F.-W.; Li, K.S.; To, K.K.-W.; Cheng, V.C.; Chen, H.; Yuen, K.-Y; Is the discovery of the novel human betacoronavirus 2c EMC/2012 (HCoV-EMC) the beginning of another SARS-like pandemic. J. Infect. 2012, 65, 477–489.
- Jeffrey P. Kanne; Chest CT Findings in 2019 Novel Coronavirus (2019-nCoV) Infections from Wuhan, China: Key Points for the Radiologist. Radiology 2020, 295, 16-17, 10.1148/radiol.2020200241.
- Kim, H. Outbreak of novel coronavirus (COVID-19): What is the role of radiologists? Eur. Radiol. 2020, 1–2.
- Lee, K.S; Pneumonia Associated with 2019 Novel Coronavirus: Can Computed Tomographic Findings Help Predict the Prognosis of the Disease. Korean J. Radiol. 2020, 21, 257–258.
- Nanshan Chen; Min Zhou; Xuan Dong; Jieming Qu; Fengyun Gong; Yang Han; Yang Qiu; Jingli Wang; Ying Liu; Yuan Wei; Jia'an Xia; Ting Yu; Xinxin Zhang; Li Zhang; Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 2020, 395, 507-513, 10.1016/S0140-6736(20)30211-7.
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiolpgy 2020, 200370.Fengxiang Song; Nannan Shi; Fei Shan; Zhiyong Zhang; Jie Shen; Hongzhou Lu; Yun Ling; Yebin Jiang; Yuxin Shi; Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology 2020, 295, 210-217, 10.1148/radiol.2020200274.
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A; et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 202–207.Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiolpgy 2020, 200370.
- Fengxiang Song; Nannan Shi; Fei Shan; Zhiyong Zhang; Jie Shen; Hongzhou Lu; Yun Ling; Yebin Jiang; Yuxin Shi; Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A; et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 210-217, 10.1148/radiol.2020200274., 202–207.
- Pan, Y.; Guan, H.; Zhou, S.; Wang, Y.; Li, Q.; Zhu, T.; Hu, Q.; Xia, L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): A study of 63 patients in Wuhan, China. Eur. Radiol. 2020, 1–4.
- Heshui Shi; Xiaoyu Han; Nanchuan Jiang; Yukun Cao; Osamah Alwalid; Jin Gu; Yanqing Fan; Chuansheng Zheng; Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. The Lancet Infectious Diseases 2020, 20, 425-434, 10.1016/s1473-3099(20)30086-4.
- Lan, L.; Xu, D.; Ye, G.; Xia, C.; Wang, S.; Li, Y.; Xu, H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA 2020.
- Lam, T.T.-Y.; Shum, M.H.-H.; Zhu, H.-C.; Tong, Y.-G.; Ni, X.-B.; Liao, Y.-S.; Wei, W.; Cheung, W.Y.-M.; Li, W.-J.; Li, L.-F.; et al. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature 2020, 1–6.
- Ignatius T.S. Yu; Yuguo Li; Tze-Wai Wong; Wilson W S Tam; Andy Chan; Joseph H.W. Lee; D.Y.C. Leung; Tommy Ho; Evidence of Airborne Transmission of the Severe Acute Respiratory Syndrome Virus. New England Journal of Medicine 2004, 350, 1731-1739, 10.1056/nejmoa032867.
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. New Engl. J. Med. 2020.
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020.
- Zhang, T.; Wu, Q.; Zhang, Z; Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Current Biology 2020, 30, 1346-1351.
- Müller, N.L.; Ooi, G.C.; Khong, P.-L.; Nicolaou, S; Severe Acute Respiratory Syndrome: Radiographic and CT Findings. Am. J. Roentgenol. 2003, 181, 3-8.
- Narinder Paul; Heidi Roberts; Jagdish Butany; Taebong Chung; Wayne Gold; Sangeeta Mehta; Eli Konen; Anuradha Rao; Yves Provost; Harry H. Hong; Leon Zelovitsky; Gordon L. Weisbrod; Radiologic Pattern of Disease in Patients with Severe Acute Respiratory Syndrome: The Toronto Experience. RadioGraphics 2004, 24, 553-563, 10.1148/rg.242035193.
- Lee, E.Y.P.; Ng, M.-Y.; Khong, P.-L. COVID-19 pneumonia: What has CT taught us? Lancet Infect. Dis. 2020, 20, 384–385.
- Das, K.M.; Lee, E.Y.; Langer, R.D.; Larsson, S.G. Middle East Respiratory Syndrome Coronavirus: What Does a Radiologist Need to Know? Am. J. Roentgenol. 2016, 206, 1193–1201.
- Gu, J.; Han, B.; Wang, J. COVID-19: Gastrointestinal Manifestations and Potential Fecal–Oral Transmission. Gastroenterology 2020.
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q; Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412.
- Xue Wu Zhang; Yee Leng Yap; Structural similarity between HIV-1 gp41 and SARS-CoV S2 proteins suggests an analogous membrane fusion mechanism. J. Mol. Struct. THEOCHEM 2004, 677, 73-76.
- Alex L. Lai; Jack H. Freed; SARS-CoV-2 Fusion Peptide has a Greater Membrane Perturbating Effect than SARS-CoV with Highly Specific Dependence on Ca2+. J. Mol. Biol. 2021, 433, 166946-166946.
- Meng, B.; Abdullahi, A; Ferreira, I. A. T. M.; Goonawardane, N.; Saito, A.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706-714.
- Pachankis, Y.I. Theoretical Strategies in SARS-CoV-2 Human Host Treatment. Journal of Clinical and Medical Images 2023, 6, 1-4.
