Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Alfred Zheng and Version 2 by Alfred Zheng.
Granulocytes (neutrophils, eosinophils, and basophils) are the most abundant circulating cells in the innate immune system. Circulating granulocytes, primarily neutrophils, can cross the endothelial barrier and activate various effector mechanisms to combat invasive pathogens. Eosinophils and basophils also play an important role in allergic reactions and antiparasitic defense. Granulocytes also regulate the immune response, wound healing, and tissue repair by releasing of various cytokines and lipid mediators. The effector mechanisms of granulocytes include the production of reactive oxygen species (ROS), degranulation, phagocytosis, and the formation of DNA-containing extracellular traps.
- granulocytes
- extracellular traps
- mitochondria
- mitochondria-targeted antioxidants
1. Introduction
Granulocytes (neutrophils, eosinophils, and basophils) are the most abundant circulating cells in the innate immune system. These myeloid polymorphonuclear cells are characterized by the presence of specific cytoplasmic granules that can be expelled into the environment upon activation. Circulating granulocytes can cross the endothelial barrier into various tissues and activate numerous effector mechanisms to combat invasive inflammatory pathogens. Eosinophils and basophils also play an important role in allergic (hypersensitive) reactions and antiparasitic defense. Another function of granulocytes is to regulate the immune response, wound healing, and tissue repair by releasing various cytokines and lipid mediators (prostaglandins, leukotrienes, resolvins, platelet activating factor, etc.). The effector mechanisms of granulocytes include the production of reactive oxygen species (ROS), degranulation (resulting in the release of lytic and prooxidant enzymes, as well as histamine), phagocytosis, and the formation of extracellular traps (ETs).
Granulocytes are mainly glycolytic, depending mainly on glycolysis and not oxidative phosphorylation for energy production [1][2][3][4]. Dependence on glycolysis is most pronounced in neutrophils, whereas eosinophils show high metabolic flexibility and use oxidative phosphorylation for effector functions [4]. However, even in neutrophils, mitochondrial ATP production may be important for antimicrobial protection. For example, it has been reported [5] that in N-formylmethionyl-leucyl-phenylalanine (fMLP)-stimulated neutrophils, mitochondrial-produced ATP activates early autocrine purinergic signaling that supports respiratory burst, degranulation, and phagocytosis. Data on the metabolic phenotype of basophils are limited. Sumbayev and colleagues [6] demonstrated that IgE-mediated activation of primary human basophils is accompanied by the accumulation of HIF-1a, which is known to regulate glycolysis. Immune metabolism has been studied in the RBL-2H3 cell line, which shares features of mast cells and basophils. The results of these studies revealed the need for both glycolysis and oxidative phosphorylation in IgE-dependent activation of RBL-2H3 cells [7][8].
All granulocytes have only a small number of mitochondria, and neutrophils have fewer mitochondria than the other cell types. The functionality of mitochondria in granulocytes has long been questioned because the respiration rate in these cells is low and difficult to measure. The mitochondrial membrane potential (ΔΨ), which is an indicator of their functional state, has also not been correctly measured due to the high membrane potential at the plasma membrane. During activation, granulocytes are characterized by strong changes in the potential of the plasma membrane (positive inside) due to the electrogenic activity of NADPH oxidase and the opening of ion channels. The first reliable measurements of ΔΨ became possible after the invention of mitochondrial fluorescent voltage-sensitive probes [9]. In neutrophils, an unusual ΔΨ generation mechanism has been described based on the activity of mitochondrial glycerol-3-phosphate dehydrogenase, which reduces coenzyme Q and stimulates electrogenic electron flow through complexes III and IV of the electron transport chain [10]. It is important to emphasize that the mitochondrial membrane potential is of decisive importance not only for the formation of ATP but also for the transport of Ca2+ into mitochondria and for the reduction of NAD+ by complex I (reverse electron transfer), which is accompanied by the formation of ROS. Mitochondria in granulocytes can form dynamic networks, and a decrease in ΔΨ leads to the fragmentation of elongated organelles. It is assumed that mitochondrial dynamics plays an important role in the regulation of the functions of neutrophils [11], eosinophils [12], basophils [8], and mast cells [13].
The production of reactive oxygen species (ROS) in mitochondria is known as an important regulator of many processes in various cell types. It is catalyzed by redox sites in complex I (NADH:ubiquinone oxidoreductase) and complex III (ubiquinone:cytochrome c oxidoreductase), as well as by several dehydrogenases in the mitochondrial matrix (pyruvate dehydrogenase, 2-oxoglutarate dehydrogenase [14]. In granulocytes, the main source of ROS production is the nonmitochondrial enzyme NADPH oxidase (NOX2). NADPH oxidase is silent in resting granulocytes and is rapidly activated upon stimulation due to phosphorylation by several protein kinases, such as protein kinase C (PKC). Activation of NADPH oxidase, together with degranulation in neutrophils and eosinophils, leads to a massive release of ROS and a strong increase in oxygen consumption (respiratory burst) [15]. Human basophils do not develop respiratory burst in response to various stimuli and do not express NADPH oxidase [16][17]. A much less intense production of mitochondrial ROS (mtROS) may be an important signaling event initiating the activation of NADPH oxidase. The role of mtROS in NOX2 activation, degranulation, and other effector functions of neutrophils and basophils will be discussed in detail in this review. The possible role of mitochondria in NOX2 activation in eosinophils, to the best of our knowledge, has not yet been studied. This task is interesting, since it is known that during a respiratory burst, eosinophils produce two to three times more ROS than neutrophils [18]. At least in part, this difference reflects a more sustained respiratory burst in eosinophils. It has been shown that eosinophils preferentially assemble and activate NOX2 at the plasma membrane, while neutrophils significantly activate it in intracellular vesicles [19]. This difference may be due to the different role of these cells in defense against pathogens: Whereas neutrophils actively phagocytize microbial pathogens and kill them in phagosomes with the help of ROS, eosinophils preferentially release ROS to the outside in the fight against parasites.
The production of cytokines and lipid mediators is not included in the effector functions of granulocytes but is very important in their physiology. In particular, a wide variety of cytokines secreted by neutrophils are involved not only in inflammation but also in immunomodulation, hematopoiesis, angiogenesis, and regeneration [20]. Immunosuppressive neutrophils (largely coinciding with granulocytic/polymorphonuclear myeloid-derived suppressor cells) are of great interest because they may stimulate tumor growth and interfere with cancer immunotherapy [21][22]. Immunosuppression by neutrophils, mediated in part by cytokines, mainly depends on their ability to inhibit T cell proliferation and activation, as well as stimulation of regulatory T cells. Importantly, inhibition of mitochondrial functions has been reported to selectively inhibit neutrophil immunosuppressive functions and stimulate T cell response [23]. Cytokines produced by eosinophils are involved in allergen-induced inflammation and in the promotion of type 2 immune responses [24]. Basophils are the main source of the cytokine IL-4, which plays a crucial role in the regulation of Th2-immune response and allergic inflammation. In addition to IL-4, basophilic granulocytes secrete cytokines such as IL-13, IL-6, TNFα, and TSLP, as well as a number of chemokines [25]. The expression of most cytokines depends on the activity of the transcription factor NF-kB. It was found that in non-immune cells, NF-kB activation depends on the production of mtROS [26], but this possibility has not been studied in granulocytes.
Among the lipid mediators (eicosanoids) produced by granulocytes, the most important role belongs to leukotrienes, which are produced by lipoxygenases that catalyze the insertion of oxygen at positions 5 and 15 of polyunsaturated fatty acids. Leukotriene B4 (LTB4), which is produced by the 5-lipoxygenase (5-LOX) pathway in all granulocytes, regulates neutrophils’ collective behavior as well as various aspects of inflammation (see below). Cysteine-containing leukotrienes, produced by 5-LOX in eosinophils, basophils, and mast cells, are potent bronchoconstrictors and vascular leak stimulators. Eosinophils also express 15-LOX and produce lipoxins and resolvins, which promote the resolution of inflammatory processes [27][28]. The possible role of mitochondria in the production of leukotrienes has not been studied until recently.
All granulocytes are short-lived cells, and the exact values of their half-life in the bloodstream are a matter of controversy even for the most studied of them: neutrophils [29]. Estimates range from 7–9 h to 3.75–5 days. Eosinophils in the blood also have a short half-life of 8 to 18 h, but in tissues it increases to at least 5 days. Moreover, their in vitro lifespan can be extended to 14 days or more with the help of cytokines [30]. The lifespan of basophils in the blood is estimated to be approx. 60 h [31]. Spontaneous apoptosis is critical to the normal physiology of granulocytes, and mitochondria play a central role in orchestrating apoptosis signaling, mainly through the release of several pro-apoptotic proteins into the cytoplasm. It is important to note that granulocyte apoptosis depends on the ROS produced by them [32] or by other cells [33].
2. The Role of Mitochondria in Extracellular Trap Formation
2.1. Neutrophil Extracellular Traps (NETs) and NETosis
Almost two decades ago, Arturo Zychlinsky and co-workers [34] described a new effector function of neutrophils, the release of neutrophil extracellular traps (NETs). NETs consist of decondensed chromatin decorated with antimicrobial proteases from granules, the cytoplasm, and the nucleus. The antimicrobial effect of NETs is due to the restriction of pathogens spreading throughout the organism or even their destruction in situ. Since it was initially shown that NET formation is accompanied by cell death, this process is called NETosis [35]. The formation of NETs, in addition to host defensive function, plays an essential role in the pathogenesis of autoimmune and inflammatory disorders, such as systemic lupus erythematosus, rheumatoid arthritis, small vessel vasculitis, and psoriasis [36][37][38][39][40]. NETs are also involved in thrombosis, a variety of pulmonary pathologies, chronic rhinosinusitis, sepsis, and malignancies [39][40][41].
The release of chromatin was also found in other types of granulocytes, including eosinophils [42] and basophils [17], as well as in mast cells [43], T and B lymphocytes [44], monocytes [45], and macrophages [46].
The formation of NETs can be activated by various physiological stimuli, such as bacteria, fungi, protozoa, viruses, and bacterial products (lipopolysaccharides (LPS)). NETs can be also induced by antibodies and immune complexes, cytokines and chemokines (IL-8, TNF-α, IFN-γ), complement components, etc.
Currently, two fundamentally different forms of NET formation are described: the classical NETosis, and vital NET release, in which cells retain their viability and other effector functions. The formation of vital NETs occurs through the budding of nuclei and the release of vesicles filled with DNA [47]. In addition to nuclear DNA-containing NETs, vital NETs can be formed due to the release of mitochondrial DNA [48][49].
Classic or suicidal NETosis is a multistep sequentially developing process that involves the dissociation of protein complex “azurosomes” located in the membranes of azurophilic granules and containing various enzymes, including neutrophil elastase (NE), cathepsin G, and myeloperoxidase (MPO) [50]. The release of NE and MPO into the cytoplasm and their subsequent migration into the nucleus, accompanied by activation of the histone-citrullinating enzyme peptidylarginine deiminase 4, promote the decondensation of nuclear chromatin [34]. These events culminate in the release of chromatin from the nucleus, rupture of the cell membrane, and NET release [34].
The signaling pathways leading to NETosis can include the activation of various isoforms of PKC [51][52][53], cyclin-dependent kinases 4/6 [54], Raf-MEK-ERK-signaling cascade [55], and Src-family tyrosine kinases [56], but the detailed picture remains unclear.
NETosis induced by various stimuli depends on ROS generated by NADPH oxidase [34]. However, some stimuli, including several microorganisms [57][58], monosodium urate crystals [59], activated platelets or platelet-derived microparticles [60], complement components [61], cytokines [62], and soluble immune complexes [63] induce NETosis independently of NOX2-derived ROS. NOX2-independent NETosis also can be induced by calcium ionophores A23187 and ionomycin [64]. Importantly, this form of NETosis still requires ROS, and these are mtROS [6[5][65]. In particular, in neutrophils isolated from the blood of patients with X-linked CGD lacking functional NOX2, the formation of NETs in response to A23187 was prevented by mitochondria-targeted antioxidant SkQ1 [66] and therefore depends on the enhanced generation of mtROS without the participation of NADPH oxidase. At the same time, NETosis induced by A23187 in neutrophils from healthy donors was suppressed by both SkQ1 and specific NADPH oxidase inhibitors such as apocynin and VAS2870 [66], indicating that mtROS can stimulate NETosis via the activation of NADPH oxidase.
As shown in the study [66], mtROS generation induced by both fMLP and A23187 depends on the opening of mPTP. This phenomenon remained unnoticed for a long time, probably due to the fact that the most well-known mPTP inhibitor, cyclosporin A, is also an inhibitor of cytoplasmic phosphatase calcineurin, which is involved in the transmission of numerous Ca2+-dependent signals [67]. Interestingly, fMLP did not cause significant decrease in the mitochondrial membrane potential and mitochondrial swelling characteristic for the prolonged opening of mPTP. This mode of mPTP opening has been described for both isolated mitochondria [67] and cell models [68]. Ca2+ overload caused by A23187 in neutrophils presumably leads to long-term opening of the mPTP, as revealed by the significant swelling of mitochondria that precedes chromatin decondensation and destruction of the nuclear envelope [67]. The regulation of effector functions of neutrophils by the mPTP opening and mitochondrial ROS is illustrated by the scheme (Figure 1).
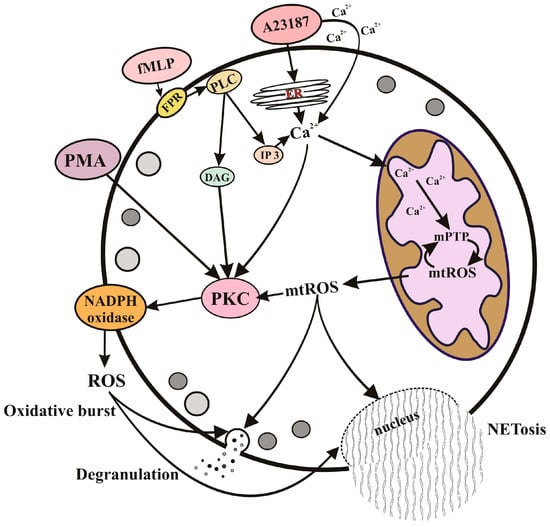
Figure 1. Regulation of effector functions of neutrophils by mitochondrial ROS. Degranulation, oxidative burst, and NETosis can be stimulated by pathogens through specific receptors (such as the specific G protein-coupled fMLP receptor) or by direct Ca2+ mobilization (as modeled with the calcium ionophore A23187). The subsequent accumulation of Ca2+ in the mitochondrial matrix leads to the opening of nonselective mitochondrial pores (mPTP) and the formation of mitochondrial ROS (mtROS). mtROS released into the cytosol can activate NADPH oxidase (NOX2), degranulation, and NETosis through protein kinase C (PKC) activation, as well as through several unidentified signaling pathways. ROS produced by NOX2 also stimulate degranulation and NETosis. Receptor-dependent activation includes stimulation of phospholipase C (PLC), which catalyzes the synthesis of inositol triphosphate (IP3) and diacylglycerol (DAG). DAG is a powerful stimulus for PKC, so this branch of neutrophil activation is independent of mitochondria. This is modeled by the synthetic analogue of DAG, phorbol 12-myristate 13-acetate (PMA), which stimulates the effector functions of neutrophils independently of mitochondria.
Recently, Dunham-Snary and co-workers [69]. W reported that functional mitochondria from healthy donor neutrophils are involved in the defense against Staphylococcus aureus infection. The authors showed that inhibition of electron transport chain complex III impairs infection-induced mtROS formation, NET formation (both vital and lytic), and bacterial killing. In addition, the authors described a surprising mechanism of bacterial engulfment involving mitochondria, which formed lasso-like structures around pathogens prior to phagocytosis [70].
2.2. The Mechanisms of Eosinophil and Basophil Extracellular Trap Formation
Eosinophils, like neutrophils, can release DNA-containing fibrils upon stimulation, which have been termed eosinophil extracellular traps (EETs). In eosinophils, two different mechanisms of EET formation have been described. One of them (EETosis) leads to cell death similar to NETosis and depends on ROS generated by NADPH oxidase [70]. Another mechanism for EET release has been studied in detail by Simon and colleagues [42][48] and is based on the rapid extrusion of mitochondrial DNA (mtDNA). This mechanism is independent of cell death and is preceded by degranulation [71]. Vital release of EETs also depends on the activation of NADPH oxidase as well as on PI3K-dependent signaling [72]. The possible role of mtROS in both mechanisms of ET formation was not studied.
It has recently been reported that the physiological activation of human and mouse basophils can also lead to the rapid formation of extracellular DNA fibrils [17], so-called basophil extracellular traps (BETs). It was shown that basophils release mtDNA rather than nuclear DNA, and this process is similar to vital NETosis and depends on ROS. Mitochondria-targeted antioxidant MitoQ inhibits BET formation, indicating that mtROS are critical [17]. Since NADPH oxidase is absent in basophils [16][17], mitochondria are probably the only source of ROS that controls the formation of BETs.
References
- Karnovsky, M.L. The Metabolism of Leukocytes. Semin. Hematol. 1968, 5, 156–165.
- Furuno, T.; Ohyama, N.; Nakanishi, M. The Relation between Degranulation and Rapid Metabolic Responses in RBL-2H3 Cells. Biol. Pharm. Bull. 1999, 22, 310–312.
- Rodríguez-Espinosa, O.; Rojas-Espinosa, O.; Moreno-Altamirano, M.M.B.; López-Villegas, E.O.; Sánchez-García, F.J. Metabolic Requirements for Neutrophil Extracellular Traps Formation. Immunology 2015, 145, 213–224.
- Porter, L.; Toepfner, N.; Bashant, K.R.; Guck, J.; Ashcroft, M.; Farahi, N.; Chilvers, E.R. Metabolic Profiling of Human Eosinophils. Front. Immunol. 2018, 9, 1404.
- Bao, Y.; Ledderose, C.; Seier, T.; Graf, A.F.; Brix, B.; Chong, E.; Junger, W.G. Mitochondria Regulate Neutrophil Activation by Generating ATP for Autocrine Purinergic Signaling. J. Biol. Chem. 2014, 289, 26794–26803.
- Sumbayev, V.V.; Nicholas, S.A.; Streatfield, C.L.; Gibbs, B.F. Involvement of Hypoxia-Inducible Factor-1 HiF(1alpha) in IgE-Mediated Primary Human Basophil Responses. Eur. J. Immunol. 2009, 39, 3511–3519.
- Sharkia, I.; Erlich, T.H.; Landolina, N.; Assayag, M.; Motzik, A.; Rachmin, I.; Kay, G.; Porat, Z.; Tshori, S.; Berkman, N.; et al. Pyruvate Dehydrogenase Has a Major Role in Mast Cell Function, and Its Activity Is Regulated by Mitochondrial Microphthalmia Transcription Factor. J. Allergy Clin. Immunol. 2017, 140, 204–214.e8.
- Pavlyuchenkova, A.N.; Zinovkin, R.A.; Makievskaya, C.I.; Galkin, I.I.; Chelombitko, M.A. Mitochondria-Targeted Triphenylphosphonium-Based Compounds Inhibit FcεRI-Dependent Degranulation of Mast Cells by Preventing Mitochondrial Dysfunction through Erk1/2. Life Sci. 2022, 288, 120174.
- Korchak, H.M.; Rich, A.M.; Wilkenfeld, C.; Rutherford, L.E.; Weissmann, G. A Carbocyanine Dye, DiOC6(3), Acts as a Mitochondrial Probe in Human Neutrophils. Biochem. Biophys. Res. Commun. 1982, 108, 1495–1501.
- van Raam, B.J.; Sluiter, W.; de Wit, E.; Roos, D.; Verhoeven, A.J.; Kuijpers, T.W. Mitochondrial Membrane Potential in Human Neutrophils Is Maintained by Complex III Activity in the Absence of Supercomplex Organisation. PLoS ONE 2008, 3, e2013.
- Zheng, X.; Chen, M.; Meng, X.; Chu, X.; Cai, C.; Zou, F. Phosphorylation of Dynamin-Related Protein 1 at Ser616 Regulates Mitochondrial Fission and Is Involved in Mitochondrial Calcium Uniporter-Mediated Neutrophil Polarization and Chemotaxis. Mol. Immunol. 2017, 87, 23–32.
- Bonjour, K.; Palazzi, C.; Silva, T.P.; Malta, K.K.; Neves, V.H.; Oliveira-Barros, E.G.; Neves, I.; Kersten, V.A.; Fortuna, B.T.; Samarasinghe, A.E.; et al. Mitochondrial Population in Mouse Eosinophils: Ultrastructural Dynamics in Cell Differentiation and Inflammatory Diseases. Front. Cell Dev. Biol. 2022, 10, 836755.
- Zhang, B.; Alysandratos, K.-D.; Angelidou, A.; Asadi, S.; Sismanopoulos, N.; Delivanis, D.-A.; Weng, Z.; Miniati, A.; Vasiadi, M.; Katsarou-Katsari, A.; et al. Human Mast Cell Degranulation and Preformed TNF Secretion Require Mitochondrial Translocation to Exocytosis Sites: Relevance to Atopic Dermatitis. J. Allergy Clin. Immunol. 2011, 127, 1522–1531.e8.
- Grivennikova, V.G.; Vinogradov, A.D. Mitochondrial Production of Reactive Oxygen Species. Biochemistry 2013, 78, 1490–1511.
- Thomas, D.C. The Phagocyte Respiratory Burst: Historical Perspectives and Recent Advances. Immunol. Lett. 2017, 192, 88–96.
- de Boer, M.; Roos, D. Metabolic Comparison between Basophils and Other Leukocytes from Human Blood. J. Immunol. 1986, 136, 3447–3454.
- Morshed, M.; Hlushchuk, R.; Simon, D.; Walls, A.F.; Obata-Ninomiya, K.; Karasuyama, H.; Djonov, V.; Eggel, A.; Kaufmann, T.; Simon, H.-U.; et al. NADPH Oxidase-Independent Formation of Extracellular DNA Traps by Basophils. J. Immunol. 2014, 192, 5314–5323.
- Petreccia, D.C.; Nauseef, W.M.; Clark, R.A. Respiratory Burst of Normal Human Eosinophils. J. Leukoc. Biol. 1987, 41, 283–288.
- Lacy, P.; Abdel-Latif, D.; Steward, M.; Musat-Marcu, S.; Man, S.F.P.; Moqbel, R. Divergence of Mechanisms Regulating Respiratory Burst in Blood and Sputum Eosinophils and Neutrophils from Atopic Subjects. J. Immunol. 2003, 170, 2670–2679.
- LeSuer, W.E.; Kienzl, M.; Ochkur, S.I.; Schicho, R.; Doyle, A.D.; Wright, B.L.; Rank, M.A.; Krupnick, A.S.; Kita, H.; Jacobsen, E.A. Eosinophils Promote Effector Functions of Lung Group 2 Innate Lymphoid Cells in Allergic Airway Inflammation in Mice. J. Allergy Clin. Immunol. 2023, 152, 469–485.e10.
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-Derived Cytokines: Facts beyond Expression. Front. Immunol. 2014, 5, 508.
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-Derived Suppressor Cells Coming of Age. Nat. Immunol. 2018, 19, 108–119.
- Zhao, Y.; Rahmy, S.; Liu, Z.; Zhang, C.; Lu, X. Rational Targeting of Immunosuppressive Neutrophils in Cancer. Pharmacol. Ther. 2020, 212, 107556.
- Cheng, G.; Hardy, M.; Topchyan, P.; Zander, R.; Volberding, P.; Cui, W.; Kalyanaraman, B. Mitochondria-Targeted Hydroxyurea Inhibits OXPHOS and Induces Antiproliferative and Immunomodulatory Effects. iScience 2021, 24, 102673.
- Miyake, K.; Shibata, S.; Yoshikawa, S.; Karasuyama, H. Basophils and Their Effector Molecules in Allergic Disorders. Allergy 2021, 76, 1693–1706.
- Zakharova, V.V.; Pletjushkina, O.Y.; Galkin, I.I.; Zinovkin, R.A.; Chernyak, B.V.; Krysko, D.V.; Bachert, C.; Krysko, O.; Skulachev, V.P.; Popova, E.N. Low Concentration of Uncouplers of Oxidative Phosphorylation Decreases the TNF-Induced Endothelial Permeability and Lethality in Mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 968–977.
- Yamada, T.; Tani, Y.; Nakanishi, H.; Taguchi, R.; Arita, M.; Arai, H. Eosinophils Promote Resolution of Acute Peritonitis by Producing Proresolving Mediators in Mice. FASEB J. 2011, 25, 561–568.
- Ogawa, M.; Ishihara, T.; Isobe, Y.; Kato, T.; Kuba, K.; Imai, Y.; Uchino, Y.; Tsubota, K.; Arita, M. Eosinophils Promote Corneal Wound Healing via the 12/15-Lipoxygenase Pathway. FASEB J. 2020, 34, 12492–12501.
- Koenderman, L.; Tesselaar, K.; Vrisekoop, N. Human Neutrophil Kinetics: A Call to Revisit Old Evidence. Trends Immunol. 2022, 43, 868–876.
- Park, Y.M.; Bochner, B.S. Eosinophil Survival and Apoptosis in Health and Disease. Allergy Asthma Immunol. Res. 2010, 2, 87–101.
- Ohnmacht, C.; Voehringer, D. Basophil Effector Function and Homeostasis during Helminth Infection. Blood 2009, 113, 2816–2825.
- Wedi, B.; Straede, J.; Wieland, B.; Kapp, A. Eosinophil Apoptosis Is Mediated by Stimulators of Cellular Oxidative Metabolisms and Inhibited by Antioxidants: Involvement of a Thiol-Sensitive Redox Regulation in Eosinophil Cell Death. Blood J. Am. Soc. Hematol. 1999, 94, 2365–2373.
- Ilmarinen, P.; Moilanen, E.; Kankaanranta, H. Mitochondria in the Center of Human Eosinophil Apoptosis and Survival. Int. J. Mol. Sci. 2014, 15, 3952–3969.
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535.
- Steinberg, B.E.; Grinstein, S. Unconventional Roles of the NADPH Oxidase: Signaling, Ion Homeostasis, and Cell Death. Sci. STKE 2007, 2007, e11.
- Vorobjeva, N.V.; Pinegin, B.V. Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Health and Disease. Biochemistry 2014, 79, 1286–1296.
- Pinegin, B.; Vorobjeva, N.; Pinegin, V. Neutrophil Extracellular Traps and Their Role in the Development of Chronic Inflammation and Autoimmunity. Autoimmun. Rev. 2015, 14, 633–640.
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2018, 18, 134–147.
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190.
- Vorobjeva, N.V. Neutrophil Extracellular Traps: New Aspects. Moscow Univ. Biol. Sci. Bull. 2020, 75, 173–188.
- Svistushkin, V.M.; Nikiforova, G.N.; Vorobjeva, N.V.; Dekhanov, A.S.; Dagil, Y.A.; Bredova, O.Y.; Eremeeva, K.V. Neutrophil extracellular traps in the pathogenesis of chronic rhinosinusitis. Vestn. Otorinolaringol. 2021, 86, 105–112.
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like Release of Mitochondrial DNA by Eosinophils Contributes to Antibacterial Defense. Nat. Med. 2008, 14, 949–953.
- von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-Independent Antimicrobial Activity of Mast Cells by Means of Extracellular Trap Formation. Blood 2008, 111, 3070–3080.
- Ingelsson, B.; Söderberg, D.; Strid, T.; Söderberg, A.; Bergh, A.-C.; Loitto, V.; Lotfi, K.; Segelmark, M.; Spyrou, G.; Rosén, A. Lymphocytes Eject Interferogenic Mitochondrial DNA Webs in Response to CpG and Non-CpG Oligodeoxynucleotides of Class C. Proc. Natl. Acad. Sci. USA 2018, 115, E478–E487.
- Granger, V.; Faille, D.; Marani, V.; Noël, B.; Gallais, Y.; Szely, N.; Flament, H.; Pallardy, M.; Chollet-Martin, S.; de Chaisemartin, L. Human Blood Monocytes Are Able to Form Extracellular Traps. J. Leukoc. Biol. 2017, 102, 775–781.
- Chow, O.A.; von Köckritz-Blickwede, M.; Bright, A.T.; Hensler, M.E.; Zinkernagel, A.S.; Cogen, A.L.; Gallo, R.L.; Monestier, M.; Wang, Y.; Glass, C.K.; et al. Statins Enhance Formation of Phagocyte Extracellular Traps. Cell Host Microbe 2010, 8, 445–454.
- Yipp, B.G.; Kubes, P. NETosis: How Vital Is It? Blood 2013, 122, 2784–2794.
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable Neutrophils Release Mitochondrial DNA to Form Neutrophil Extracellular Traps. Cell Death Differ. 2009, 16, 1438–1444.
- Li, T.; Zhang, Z.; Li, X.; Dong, G.; Zhang, M.; Xu, Z.; Yang, J. Neutrophil Extracellular Traps: Signaling Properties and Disease Relevance. Mediators Inflamm. 2020, 2020, 9254087.
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A Myeloperoxidase-Containing Complex Regulates Neutrophil Elastase Release and Actin Dynamics during NETosis. Cell Rep. 2014, 8, 883–896.
- Gray, R.D.; Lucas, C.D.; MacKellar, A.; Li, F.; Hiersemenzel, K.; Haslett, C.; Davidson, D.J.; Rossi, A.G. Activation of Conventional Protein Kinase C (PKC) Is Critical in the Generation of Human Neutrophil Extracellular Traps. J. Inflamm. 2013, 10, 12.
- Vorobjeva, N.V.; Vakhlyarskaya, S.S.; Chernyak, B.V. The Role of Protein Kinase C Isoforms in the Formation of Neutrophil Extracellular Traps. Moscow Univ. Biol. Sci. Bull. 2022, 77, 112–118.
- Vorobjeva, N.; Dagil, Y.; Pashenkov, M.; Pinegin, B.; Chernyak, B. Protein Kinase C Isoforms Mediate the Formation of Neutrophil Extracellular Traps. Int. Immunopharmacol. 2023, 114, 109448.
- Amulic, B.; Knackstedt, S.L.; Abu Abed, U.; Deigendesch, N.; Harbort, C.J.; Caffrey, B.E.; Brinkmann, V.; Heppner, F.L.; Hinds, P.W.; Zychlinsky, A. Cell-Cycle Proteins Control Production of Neutrophil Extracellular Traps. Dev. Cell 2017, 43, 449–462.e5.
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK Pathway Is Required for Neutrophil Extracellular Trap Formation. Nat. Chem. Biol. 2011, 7, 75–77.
- Vorobjeva, N.V. Participation of Non-Receptor Src-Family Tyrosine Kinases in the Formation of Neutrophil Extracellular Traps. Mosc. Univ. Biol. Sci. Bull. 2023, 78, 8–13.
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus Aureus. J. Immunol. 2010, 185, 7413–7425.
- Parker, H.; Dragunow, M.; Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Requirements for NADPH Oxidase and Myeloperoxidase in Neutrophil Extracellular Trap Formation Differ Depending on the Stimulus. J. Leukoc. Biol. 2012, 92, 841–849.
- Arai, Y.; Nishinaka, Y.; Arai, T.; Morita, M.; Mizugishi, K.; Adachi, S.; Takaori-Kondo, A.; Watanabe, T.; Yamashita, K. Uric Acid Induces NADPH Oxidase-Independent Neutrophil Extracellular Trap Formation. Biochem. Biophys. Res. Commun. 2014, 443, 556–561.
- Pieterse, E.; Rother, N.; Yanginlar, C.; Gerretsen, J.; Boeltz, S.; Munoz, L.E.; Herrmann, M.; Pickkers, P.; Hilbrands, L.B.; van der Vlag, J. Cleaved N-Terminal Histone Tails Distinguish between NADPH Oxidase (NOX)-Dependent and NOX-Independent Pathways of Neutrophil Extracellular Trap Formation. Ann. Rheum. Dis. 2018, 77, 1790–1798.
- de Bont, C.M.; Boelens, W.C.; Pruijn, G.J.M. NETosis, Complement, and Coagulation: A Triangular Relationship. Cell. Mol. Immunol. 2019, 16, 19–27.
- Tatsiy, O.; McDonald, P.P. Physiological Stimuli Induce PAD4-Dependent, ROS-Independent NETosis, With Early and Late Events Controlled by Discrete Signaling Pathways. Front. Immunol. 2018, 9, 2036.
- Chen, K.; Nishi, H.; Travers, R.; Tsuboi, N.; Martinod, K.; Wagner, D.D.; Stan, R.; Croce, K.; Mayadas, T.N. Endocytosis of Soluble Immune Complexes Leads to Their Clearance by FcγRIIIB but Induces Neutrophil Extracellular Traps via FcγRIIA In Vivo. Blood 2012, 120, 4421–4431.
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; von Bernuth, H.; Zychlinsky, A. Diverse Stimuli Engage Different Neutrophil Extracellular Trap Pathways. Elife 2017, 6, e24437.
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil Extracellular Traps Enriched in Oxidized Mitochondrial DNA Are Interferogenic and Contribute to Lupus-like Disease. Nat. Med. 2016, 22, 146–153.
- Vorobjeva, N.; Galkin, I.; Pletjushkina, O.; Golyshev, S.; Zinovkin, R.; Prikhodko, A.; Pinegin, V.; Kondratenko, I.; Pinegin, B.; Chernyak, B. Mitochondrial Permeability Transition Pore Is Involved in Oxidative Burst and NETosis of Human Neutrophils. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165664.
- Bernardi, P.; Rasola, A.; Forte, M.; Lippe, G. The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol. Rev. 2015, 95, 1111–1155.
- Dumas, J.F.; Argaud, L.; Cottet-Rousselle, C.; Vial, G.; Gonzalez, C.; Detaille, D.; Leverve, X.; Fontaine, E. Effect of Transient and Permanent Permeability Transition Pore Opening on NAD(P)H Localization in Intact Cells. J. Biol. Chem. 2009, 284, 15117–15125.
- nullDunham-Snary, K.J.; Surewaard, B.G.; Mewburn, J.D.; Bentley, R.E.; Martin, A.Y.; Jones, O.; Al-Qazazi, R.; Lima, P.A.; Kubes, P.; Archer, S.L. Mitochondria in Human Neutrophils Mediate Killing of Staphylococcus Aureus. Redox Biol. 2022, 49, 102225.
- nullUeki, S.; Melo, R.C.N.; Ghiran, I.; Spencer, L.A.; Dvorak, A.M.; Weller, P.F. Eosinophil Extracellular DNA Trap Cell Death Mediates Lytic Release of Free Secretion-Competent Eosinophil Granules in Humans. Blood 2013, 121, 2074–2083.
- nullGermic, N.; Fettrelet, T.; Stojkov, D.; Hosseini, A.; Horn, M.P.; Karaulov, A.; Simon, D.; Yousefi, S.; Simon, H.-U. The Release Kinetics of Eosinophil Peroxidase and Mitochondrial DNA Is Different in Association with Eosinophil Extracellular Trap Formation. Cells 2021, 10, 306.
- nullGermic, N.; Stojkov, D.; Oberson, K.; Yousefi, S.; Simon, H.-U. Neither Eosinophils nor Neutrophils Require ATG5-Dependent Autophagy for Extracellular DNA Trap Formation. Immunology 2017, 152, 517–525.
More
