Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Chiara Villa.
Amyotrophic lateral sclerosis (ALS) is a devastating progressive neurodegenerative disorder characterized by selective loss of lower and upper motor neurons (MNs) in the brain and spinal cord, resulting in paralysis and eventually death due to respiratory insufficiency. Pathological protein aggregates are also a feature of ALS, and occur in the form of ubiquitinated inclusions in neurons and glia.
- amyotrophic lateral sclerosis
- aggregation
- neurodegenerative diseases
1. Pathological Protein Aggregation Involved in ALS
As previously mentioned, protein aggregates are a pathological feature of several neurodegenerative diseases, including extracellular plaques of Aβ and intracellular neurofibrillary aggregates of tau protein in AD, or Lewy bodies in PD [26][1]. Pathological protein aggregates are also a feature of ALS, and occur in the form of ubiquitinated inclusions in neurons and glia (Figure 1).
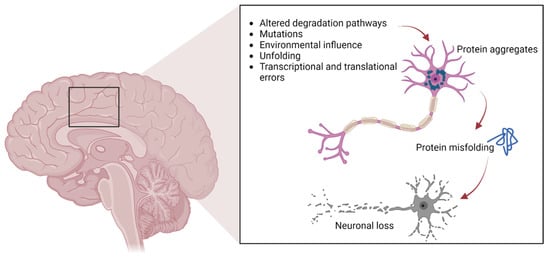
Figure 1. The pathways involved in protein aggregation in neuronal cells, leading to neuronal death typically found in neurodegenerative diseases.
Such inclusions contain different proteins, some of which may have an intrinsic tendency to aggregate following genetic mutations (SOD1, TDP-43, FUS), whereas others may simply be trapped in the aggregates [13,14,15,16,17,27][2][3][4][5][6][7]. In particular, cysteine-mediated aggregates of mutant SOD1 (mutSOD1) have been observed in ALS MNs, with the wt form of SOD1 also present, thus demonstrating the strong affinity and co-aggregation of the two forms of the protein [28][8]. It is widely believed that the toxic functions of mutSOD1 and other proteins typical of ALS are related to their tendency to aggregate. This hypothesis is supported by the presence of intracellular cytoplasmic inclusions, as well as mitochondrial inclusions rich in SOD1 identified in cell models and in animal spinal MNs, as well as in MNs of patients with ALS [27][7].
Numerous experimental theories have been proposed to explain how the aggregation of mutant proteins contributes to cellular toxicity in ALS patients. These suggestions have included the ability to sequester proteins necessary for the normal functioning of the MN [29][9], the ability to reduce the activity of the proteasome, which is essential for correct protein turnover, and the ability to inhibit the correct functioning of specific cellular organelles, such as mitochondria, by internal or external aggregation [30][10]. Cell degeneration depends on the sensitivity of MNs to the aggregation of proteins in the mutant form, supported by the observation thatTDP-43 and FUS also aggregate in patient tissues and ALS models in the same manner as SOD1 [31,32][11][12]. Indeed, anatomopathological observations of tissues derived from ALS patients show that these two proteins aggregate as cytoplasmic inclusions (positive for ubiquitin, but negative for SOD1) and that the mutations that affect them seem to increase the degree of aggregation [33][13]. Furthermore, it has been shown that the aggregation of FUS and TDP-43 is based on a conserved low-complexity domain (LCD), also called a prion-like domain (PrLD) [34][14], and that such aggregates can also sequester wt proteins and in their native form [10][15]. The LCD can mediate the liquid–liquid phase separation (LLPS) and therefore the formation of stress granules (SGs), which are cytoplasmic condensates lacking a membrane, composed primarily of RNA and RNA-binding proteins (RBPs), generated under unfavorable environmental conditions. SGs may serve to protect RNA from degradation by inhibiting the initiation of mRNA translation at the cellular level and starting the synthesis of cytoprotective proteins. They are intrinsically dynamic and dissolve quickly upon stress removal [35][16]. TDP-43 mislocalization and aggregation are observed in approximately 97% of all ALS cases, including all sALS cases, whereas SOD1 (2%) and FUS (1%) inclusions are associated with the remaining cases [36][17]. Not only are ubiquitin-immunoreactive inclusions most frequently reported in all forms of ALS, but the aggregates may also be reactive to p62, a protein that participates in sequestosome formation and autophagy [37][18].
To prevent the formation of protein aggregates, the cells are equipped with protein quality control systems, and the presence of chaperone proteins ensures that proteins in their native form fold correctly. These can intervene to avoid misfolding and therefore restore the proteins to the correct conformation shape: the UPS or the ALP. Dysfunction of these systems causes the formation of protein aggregates [38][19]. In this context, genetic screening has identified disease-associated mutations in many of the proteins identified within ALS inclusions [5][20]. These findings imply that aggregates are not merely a disease marker but are also strongly linked to the etiology and pathomechanisms of neurodegeneration.
2. SOD1
In 1993, Rosen et al. first described eleven disease-associated mutations in the SOD1 gene located on chromosome 21 [39][21], which encodes for the Cu/Zn superoxide dismutase, a cytoplasmic enzyme responsible for the catabolism of superoxide radicals to hydrogen peroxide and molecular oxygen [40][22]. SOD1 is ubiquitously expressed, highly conserved, and represents ~1% of all cytoplasmic proteins. It is a 32 kDa polypeptide of 153 amino acids, composed of a binding site with a zinc atom and another for a copper atom (Figure 2). This protein is predominantly localized in the cytoplasm of cells, although it is also apparently present in the intramembrane space of the mitochondria, nucleus, lysosomes, and peroxisomes [41][23]. Its role is to convert superoxide anions, which are toxic to the cell, into peroxides of hydrogen and oxygen [42][24]. SOD1 also exerts pro-oxidant activity including peroxidation with the production of hydroxyl radicals and nitration of tyrosines [43][25]. The ubiquitinated form of this protein is widely expressed and constitutes about 0.5–0.8% of soluble proteins in the human brain [44][26]. To date, over 200 mutations in SOD1 have been identified accounting for approximately 20% of fALS patients [45,46][27][28], characterized by inter-family and intra-family variability in phenotype with respect to severity of symptoms, age of onset, and disease duration [46][28]. The most common mutations are G93A, A4V, H46R, D90A, inherited as dominant traits, except for the latter that also shows a recessive inheritance pattern of transmission, but only in Scandinavian populations [32,47][12][29].
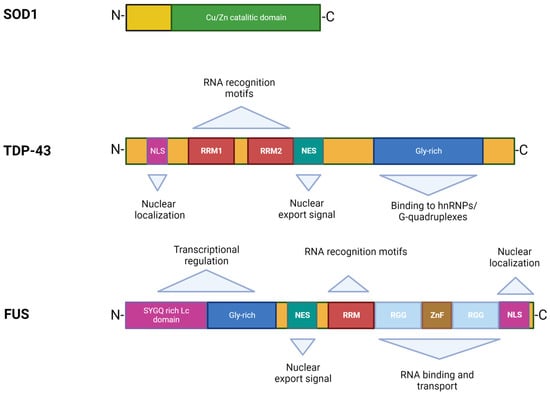
Figure 2.
Structures and functional domains of SOD1, TDP-43, and FUS proteins.
The two principal critical features of SOD1 mediated cytotoxicity are misfolding and protein aggregation. This means that disease onset is driven by mutant protein that is synthesized inside MNs. MutSOD1 protein interacts specifically with neurofilament light chain mRNA and the dynein–dynactin complex, thus inducing cytoskeletal defects or altering axonal transport [47][29]. Furthermore, it has an increased tendency to form aggregate-prone monomers, and the degree of instability correlates inversely with survival time, suggesting that increased propensity to aggregation may be the unifying common denominator for different SOD1 mutations [47][29]. Researchers identified misfolded SOD1 in MNs in a subset of patients with sALS without SOD1 mutations, thus suggesting a role for wt SOD1 in sALS, possibly after secondary (oxidative) modification [48,49][30][31]. Finally, the aggregation and spread of mutant SOD1 has been demonstrated in cultured cells [50][32], and its seeding ability via a prion-like mechanism illustrated using spinal cord homogenate [51][33].
There are currently no explanations for how mutSOD1 causes disease. Initially it was hypothesized that the mutations impair the enzymatic activity of the protein, causing increased levels of reactive oxygen species (ROS) with consequent oxidative stress and death of neuronal cells [52][34]. More recent studies have shown that mutant protein forms maintain their catalytic activity intact with no apparent causal relationship between residual enzyme activity, clinical progression, and disease phenotype [53][35].
The mutSOD1 protein accumulates in the oligomeric form and produces cytoplasmic aggregates, which can cause neuronal cell death by sequestering other cytoplasmic proteins required for neuronal survival, blocking the UPS with consequent loss of chaperone proteins, destruction of mitochondria, and the blockade of cytoskeletal or axonal transport [54][36].
3. TDP-43
TDP-43 was first isolated in 1995, when researchers observed its ability to bind the transactivation response region (TAR) of HIV DNA, hence the name TAR DNA binding protein [55][37]. It was subsequently found in the human brain and in several cell culture systems [56][38]. TDP-43 is a highly conserved protein across different species and shows ubiquitous expression in humans and rodents with a predominant localization in the nucleus. This protein consists of 414 amino acids and has a molecular weight of 43 kDa, encoded by the TARDBP gene located on chromosome 1, a member of a heterogeneous family of proteins that bind RNA, known as hnRNP (heterogeneous ribonucleoprotein) [57][39]. From a structural point of view, the protein contains an N-terminal region, a nuclear localization signal (NLS), two RNA recognition motifs (RRM1 and RRM2) which exhibit an export signal nuclear (NES), and a C-terminal region comprising a glycine-rich LCD which mediates the interaction with other proteins belonging to the hnRNP family (Figure 2) [57][39]. Mutations affecting the TDP-43 protein represent about 5% of sALS and about 3% of fALS cases [58][40] as well as patients affected by frontotemporal dementia (FTD) [13,14][2][3]. The majority of TARDBP mutations are clustered mainly within the glycine-rich C terminal, and have a crucial role in nucleocytoplasmic shuttling, aggregation propensity, and protein–protein interaction [59][41]. The cellular function of TDP-43 remains unknown, but different studies have shown that this protein plays a fundamental role in a number of biological activities, including regulation of gene transcription, control of splicing processes, and maintaining the stability of mRNA [60][42]. Protein levels are strictly controlled through a self-regulation system, with particular involvement of the C-terminal region, further supporting the suggestion that mutations in this domain can interfere with the homeostatic control process by altering the recruitment of complexes required for self-regulation [61][43].
In pathological conditions, this protein tends to form ubiquitinated inclusions in the central nervous system (CNS), in particular the hippocampus, neocortex, and spinal cord. A further feature that distinguishes it from the wt form is its localization: insoluble protein aggregates tend to form in the cytoplasm in the neurons of patients with either pathology, resulting in a decrease of TDP-43 protein in the nucleus, in contrast to healthy subjects where it is expressed in the nucleus [13][2]. In addition to ubiquitination, hyperphosphorylation of TDP-43 is important for protein aggregation in ALS pathogenesis. In particular, in samples from ALS patients TDP-43 appears to be hyperphosphorylated at the C-terminal level, thus favoring its aggregation [62][44]. According to the findings of Braak and colleagues, pTDP-43 is widespread throughout the CNS, particularly in the agranular neocortex, and in spinal and bulbar MNs, where the presence of pTDP-43 makes it impossible to distinguish between the two types of MNs [63][45]. Furthermore, hyperphosphorylation of TDP-43 has been clearly linked to cell death in areas of the CNS, in relation to disease progression [64][46]. Another study also demonstrated that pTDP-43 aggregates led to the death of dopaminergic neurons in the substantia nigra [65][47].
Regardless of the presence of genetic mutations, the aberrant localization of TDP-43 in the cytoplasm of neurons in ALS patients appears to be linked to a pathogenetic mechanism associated with a loss of function in nuclear protein responsible for the regulation of mRNA transcription and splicing processes [60][42]. The formation of cellular inclusions of TDP-43 induces toxicity in the cell, and in this case the protein acquires a toxic function (gain of function) [66,67,68][48][49][50].
Furthermore, the accumulation of TDP-43 within ubiquitinated inclusions (UBIs) can lead to the altered regulation of genes or factors involved in the degradation processes of intracellular proteins, thus contributing to TDP-43 proteinopathy.
TDP-43 levels are strictly regulated by an intrinsic autoregulatory pathway, as demonstrated by observations that inactivation of one copy of the gene does not reduce protein mRNA levels in mice. Autoregulation is believed to be mediated by TDP-43 dependent splicing of an intron in the 3′ UTR region of its own mRNA, and splicing of this gene leads to unstable RNA which undergoes decay [69][51]. Furthermore, overexpression of wt human TDP-43 in neurons results in neurodegeneration accompanied by decreased locomotor activity, motor impairment, shorter lifespan, and MN loss [70][52].
TDP-43 is primarily cleaved by caspase 3 into two extremely aggregation-prone fragments with molecular weights of 25 kDa and 35 kDa, namely TDP-25 and TDP-35, respectively [71][53]. These fragments derive from the entire wt TDP-43 protein chain, induce greater toxicity, and contribute to the loss of function of TDP-43. Therefore, they must be efficiently cleared from cells to prevent their aggregation and the sequestration of other important neuronal components. The clearance of aberrant or misfolded proteins is mediated by the protein quality control system (PQC) [72][54]. This system is composed of chaperone and co-chaperone proteins that recognize and bind damaged proteins and direct them towards degradation processes.
As mentioned previously, the LCD sequence of TDP-43 controls its ability to undergo LLPS [73][55], leading to the formation of insoluble aggregates [74][56]. In particular, when aggregate-prone TDP-43 is more concentrated, the critical concentration for LLPS is exceeded, making it difficult to maintain protein homeostasis by preventing aggregation [62][44]. Further evidence of the importance of proper interactions between TDP-43 and LLPS comes from a recent study by Gao et al. performed on LLPS-deficient TDP-43 mice, showing that low levels of TDP-43 and LLPS led to the alteration of neuronal cells [75][57].
4. FUS
The discovery of TDP-43 mutations in ALS rapidly led to the identification of mutations in another RNA binding protein, namely FUS [16[5][6],17], accounting for 4–5% of fALS and 1% of sALS forms associated with young age at onset and short survival time [5,76][20][58]. The FUS gene is located on chromosome 16 and encodes for a 526-amino acid protein of 75 kDa in weight, showing a structure similar to that of TDP-43 (Figure 2) [77,78][59][60]. Although FUS was initially identified as a component of a fusion oncogene resulting from a chromosomal translocation observed in liposarcomas [60][42], this protein also plays a role in RNA processes [79][61]. FUS is widely expressed in most human tissues [80][62] and is primarily localized at the nucleus, although it shuttles to the cytoplasm to mediate a wide range of cellular processes including DNA repair, genomic stability, transcriptional regulation, splicing, transport, and maturation of mRNAs [60,81][42][63]. Specifically in the CNS, FUS regulates mRNA transport towards the dendrites and supports synaptic plasticity upon activation of glutamate receptors [82][64].
As with TARDBP, the majority of pathogenic mutations in FUS map to the C terminal within the NLS region of the protein that regulates interaction with transportin-1 [83][65], thereby interfering with the nuclear–cytoplasmic balance of FUS [78,82][60][64]. Accordingly, this protein exhibits mainly nuclear localization under healthy conditions, but abnormal cytoplasmic aggregates have been found in the brains and spinal cords of ALS patients with FUS mutations [16,57,82][5][39][64]. Therefore, the toxic effects of FUS seem to be related to its aberrant cytoplasmic localization that possibly disrupts nucleocytoplasmic transport [84][66]. This evidence is supported by a study in Drosophila where deletion of the nuclear export signal reduced the toxicity of mutant FUS [85][67].
Furthermore, authors have reported that FUS can undergo phase transition by LCD sequence [86][68] and formation of GSs [80][62], similar to TDP-43 [75][57]. Study in vitro demonstrated that ALS mutations may accelerate the kinetics of phase separation and exacerbate the transition of FUS from liquid to solid phase [86][68]. Further studies confirmed that an aberrant phase transition is reflected in the molecular mechanism underpinning ALS pathogenesis [87,88,89,90][69][70][71][72].
Interestingly, neuropathological examination of tissues from patients harboring FUS mutations showed increased cytoplasmic FUS staining, FUS-immunoreactive dystrophic neurites, and cytoplasmic inclusions in lower MNs [16,17][5][6]. These mislocalized immunoreactive FUS inclusions were strikingly non-reactive for TDP-43, suggesting that neurodegenerative processes driven by FUS are independent from TDP-43 mislocalization.
References
- Lansbury, P.T.; Lashuel, H.A. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature 2006, 443, 774–779.
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133.
- Arai, T.; Hasegawa, M.; Akiyama, H.; Ikeda, K.; Nonaka, T.; Mori, H.; Mann, D.; Tsuchiya, K.; Yoshida, M.; Hashizume, Y.; et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006, 351, 602–611.
- Bruijn, L.I.; Houseweart, M.K.; Kato, S.; Anderson, K.L.; Anderson, S.D.; Ohama, E.; Reaume, A.G.; Scott, R.W.; Cleveland, D.W. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 1998, 281, 1851–1854.
- Kwiatkowski, T.J., Jr.; Bosco, D.A.; Leclerc, A.L.; Tamrazian, E.; Vanderburg, C.R.; Russ, C.; Davis, A.; Gilchrist, J.; Kasarskis, E.J.; Munsat, T.; et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 2009, 323, 1205–1208.
- Vance, C.; Rogelj, B.; Hortobágyi, T.; De Vos, K.J.; Nishimura, A.L.; Sreedharan, J.; Hu, X.; Smith, B.; Ruddy, D.; Wright, P.; et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 2009, 323, 1208–1211.
- Cozzolino, M.; Ferri, A.; Carrì, M.T. Amyotrophic lateral sclerosis: From current developments in the laboratory to clinical implications. Antioxid. Redox Signal. 2008, 10, 405–443.
- Guareschi, S.; Cova, E.; Cereda, C.; Ceroni, M.; Donetti, E.; Bosco, D.A.; Trotti, D.; Pasinelli, P. An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proc. Natl. Acad. Sci. USA 2012, 109, 5074–5079.
- Parker, S.J.; Meyerowitz, J.; James, J.L.; Liddell, J.R.; Crouch, P.J.; Kanninen, K.M.; White, A.R. Endogenous TDP-43 localized to stress granules can subsequently form protein aggregates. Neurochem. Int. 2012, 60, 415–424.
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. 2021, 60, 9215–9246.
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929.
- Mathis, S.; Goizet, C.; Soulages, A.; Vallat, J.M.; Masson, G.L. Genetics of amyotrophic lateral sclerosis: A review. J. Neurol. Sci. 2019, 399, 217–226.
- Sun, Z.; Diaz, Z.; Fang, X.; Hart, M.P.; Chesi, A.; Shorter, J.; Gitler, A.D. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol. 2011, 9, e1000614.
- King, O.D.; Gitler, A.D.; Shorter, J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012, 1462, 61–80.
- Blokhuis, A.M.; Groen, E.J.; Koppers, M.; van den Berg, L.H.; Pasterkamp, R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013, 125, 777–794.
- Asadi, M.R.; Sadat Moslehian, M.; Sabaie, H.; Jalaiei, A.; Ghafouri-Fard, S.; Taheri, M.; Rezazadeh, M. Stress Granules and Neurodegenerative Disorders: A Scoping Review. Front. Aging Neurosci. 2021, 13, 650740.
- Ling, S.C.; Polymenidou, M.; Cleveland, D.W. Converging mechanisms in ALS and FTD: Disrupted RNA and protein homeostasis. Neuron 2013, 79, 416–438.
- Ma, S.; Attarwala, I.Y.; Xie, X.Q. SQSTM1/p62: A Potential Target for Neurodegenerative Disease. ACS Chem. Neurosci. 2019, 10, 2094–2114.
- Kocaturk, N.M.; Gozuacik, D. Crosstalk Between Mammalian Autophagy and the Ubiquitin-Proteasome System. Front. Cell Dev. Biol. 2018, 6, 128.
- Taylor, J.P.; Brown, R.H., Jr.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206.
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X.; et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62.
- Buettner, G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anticancer Agents Med. Chem. 2011, 11, 341–346.
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606.
- Kaur, S.J.; McKeown, S.R.; Rashid, S. Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene 2016, 577, 109–118.
- Rowland, L.P.; Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001, 344, 1688–1700.
- Pardo, C.A.; Xu, Z.; Borchelt, D.R.; Price, D.L.; Sisodia, S.S.; Cleveland, D.W. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc. Natl. Acad. Sci. USA 1995, 92, 954–958.
- Abel, O.; Shatunov, A.; Jones, A.R.; Andersen, P.M.; Powell, J.F.; Al-Chalabi, A. Development of a Smartphone App for a Genetics Website: The Amyotrophic Lateral Sclerosis Online Genetics Database (ALSoD). JMIR Mhealth Uhealth 2013, 1, e18.
- Berdyński, M.; Miszta, P.; Safranow, K.; Andersen, P.M.; Morita, M.; Filipek, S.; Żekanowski, C.; Kuźma-Kozakiewicz, M. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 2022, 12, 103.
- Andersen, P.M. Amyotrophic lateral sclerosis associated with mutations in the CuZn superoxide dismutase gene. Curr. Neurol. Neurosci. Rep. 2006, 6, 37–46.
- Robberecht, W.; Philips, T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013, 14, 248–264.
- Bosco, D.A.; Morfini, G.; Karabacak, N.M.; Song, Y.; Gros-Louis, F.; Pasinelli, P.; Goolsby, H.; Fontaine, B.A.; Lemay, N.; McKenna-Yasek, D.; et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat. Neurosci. 2010, 13, 1396–1403.
- Grad, L.I.; Guest, W.C.; Yanai, A.; Pokrishevsky, E.; O’Neill, M.A.; Gibbs, E.; Semenchenko, V.; Yousefi, M.; Wishart, D.S.; Plotkin, S.S.; et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc. Natl. Acad. Sci. USA 2011, 108, 16398–16403.
- Chia, R.; Tattum, M.H.; Jones, S.; Collinge, J.; Fisher, E.M.; Jackson, G.S. Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis. PLoS ONE 2010, 5, e10627.
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310.
- Radunović, A.; Delves, H.T.; Robberecht, W.; Tilkin, P.; Enayat, Z.E.; Shaw, C.E.; Stević, Z.; Apostolski, S.; Powell, J.F.; Leigh, P.N. Copper and zinc levels in familial amyotrophic lateral sclerosis patients with CuZnSOD gene mutations. Ann. Neurol. 1997, 42, 130–131.
- Ticozzi, N.; Tiloca, C.; Morelli, C.; Colombrita, C.; Poletti, B.; Doretti, A.; Maderna, L.; Messina, S.; Ratti, A.; Silani, V. Genetics of familial Amyotrophic lateral sclerosis. Arch. Ital. Biol. 2011, 149, 65–82.
- Ou, S.H.; Wu, F.; Harrich, D.; García-Martínez, L.F.; Gaynor, R.B. Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J. Virol. 1995, 69, 3584–3596.
- Kawakami, I.; Arai, T.; Hasegawa, M. The basis of clinicopathological heterogeneity in TDP-43 proteinopathy. Acta Neuropathol. 2019, 138, 751–770.
- Lagier-Tourenne, C.; Polymenidou, M.; Cleveland, D.W. TDP-43 and FUS/TLS: Emerging roles in RNA processing and neurodegeneration. Hum. Mol. Genet. 2010, 19, R46–R64.
- Andersen, P.M.; Al-Chalabi, A. Clinical genetics of amyotrophic lateral sclerosis: What do we really know? Nat. Rev. Neurol. 2011, 7, 603–615.
- Khosravi, B.; Hartmann, H.; May, S.; Möhl, C.; Ederle, H.; Michaelsen, M.; Schludi, M.H.; Dormann, D.; Edbauer, D. Cytoplasmic poly-GA aggregates impair nuclear import of TDP-43 in C9orf72 ALS/FTLD. Hum. Mol. Genet. 2017, 26, 790–800.
- Law, W.J.; Cann, K.L.; Hicks, G.G. TLS, EWS and TAF15: A model for transcriptional integration of gene expression. Brief. Funct. Genom. 2006, 5, 8–14.
- Ayala, Y.M.; De Conti, L.; Avendaño-Vázquez, S.E.; Dhir, A.; Romano, M.; D’Ambrogio, A.; Tollervey, J.; Ule, J.; Baralle, M.; Buratti, E.; et al. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J. 2011, 30, 277–288.
- Gruijs da Silva, L.A.; Simonetti, F.; Hutten, S.; Riemenschneider, H.; Sternburg, E.L.; Pietrek, L.M.; Gebel, J.; Dötsch, V.; Edbauer, D.; Hummer, G.; et al. Disease-linked TDP-43 hyperphosphorylation suppresses TDP-43 condensation and aggregation. EMBO J. 2022, 41, e108443.
- Braak, H.; Brettschneider, J.; Ludolph, A.C.; Lee, V.M.; Trojanowski, J.Q.; Del Tredici, K. Amyotrophic lateral sclerosis—A model of corticofugal axonal spread. Nat. Rev. Neurol. 2013, 9, 708–714.
- Brettschneider, J.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.; Van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013, 74, 20–38.
- Brettschneider, J.; Arai, K.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Lee, E.B.; Kuwabara, S.; Shibuya, K.; Irwin, D.J.; Fang, L.; et al. TDP-43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord. Acta Neuropathol. 2014, 128, 423–437.
- Mackenzie, I.R.; Rademakers, R. The role of transactive response DNA-binding protein-43 in amyotrophic lateral sclerosis and frontotemporal dementia. Curr. Opin. Neurol. 2008, 21, 693–700.
- Vanden Broeck, L.; Callaerts, P.; Dermaut, B. TDP-43-mediated neurodegeneration: Towards a loss-of-function hypothesis? Trends Mol. Med. 2014, 20, 66–71.
- Ederle, H.; Dormann, D. TDP-43 and FUS en route from the nucleus to the cytoplasm. FEBS Lett. 2017, 591, 1489–1507.
- Polymenidou, M.; Lagier-Tourenne, C.; Hutt, K.R.; Huelga, S.C.; Moran, J.; Liang, T.Y.; Ling, S.C.; Sun, E.; Wancewicz, E.; Mazur, C.; et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat. Neurosci. 2011, 14, 459–468.
- Li, Y.; Ray, P.; Rao, E.J.; Shi, C.; Guo, W.; Chen, X.; Woodruff, E.A., 3rd; Fushimi, K.; Wu, J.Y. A Drosophila model for TDP-43 proteinopathy. Proc. Natl. Acad. Sci. USA 2010, 107, 3169–3174.
- Zhang, Y.J.; Xu, Y.F.; Dickey, C.A.; Buratti, E.; Baralle, F.; Bailey, R.; Pickering-Brown, S.; Dickson, D.; Petrucelli, L. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J. Neurosci. 2007, 27, 10530–10534.
- Crippa, V.; Cicardi, M.E.; Ramesh, N.; Seguin, S.J.; Ganassi, M.; Bigi, I.; Diacci, C.; Zelotti, E.; Baratashvili, M.; Gregory, J.M.; et al. The chaperone HSPB8 reduces the accumulation of truncated TDP-43 species in cells and protects against TDP-43-mediated toxicity. Hum. Mol. Genet. 2016, 25, 3908–3924.
- Conicella, A.E.; Zerze, G.H.; Mittal, J.; Fawzi, N.L. ALS Mutations Disrupt Phase Separation Mediated by α-Helical Structure in the TDP-43 Low-Complexity C-Terminal Domain. Structure 2016, 24, 1537–1549.
- Alberti, S.; Hyman, A.A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 196–213.
- Gao, J.; Wang, L.; Ren, X.; Dunn, J.R.; Peters, A.; Miyagi, M.; Fujioka, H.; Zhao, F.; Askwith, C.; Liang, J.; et al. Translational regulation in the brain by TDP-43 phase separation. J. Cell Biol. 2021, 220, e202101019.
- Liu, Z.J.; Lin, H.X.; Liu, G.L.; Tao, Q.Q.; Ni, W.; Xiao, B.G.; Wu, Z.Y. The investigation of genetic and clinical features in Chinese patients with juvenile amyotrophic lateral sclerosis. Clin. Genet. 2017, 92, 267–273.
- Sreedharan, J.; Blair, I.P.; Tripathi, V.B.; Hu, X.; Vance, C.; Rogelj, B.; Ackerley, S.; Durnall, J.C.; Williams, K.L.; Buratti, E.; et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 2008, 319, 1668–1672.
- Lanson, N.A., Jr.; Pandey, U.B. FUS-related proteinopathies: Lessons from animal models. Brain Res. 2012, 1462, 44–60.
- Zhao, M.; Kim, J.R.; van Bruggen, R.; Park, J. RNA-Binding Proteins in Amyotrophic Lateral Sclerosis. Mol. Cells 2018, 41, 818–829.
- Andersson, M.K.; Ståhlberg, A.; Arvidsson, Y.; Olofsson, A.; Semb, H.; Stenman, G.; Nilsson, O.; Aman, P. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 2008, 9, 37.
- Deng, H.; Gao, K.; Jankovic, J. The role of FUS gene variants in neurodegenerative diseases. Nat. Rev. Neurol. 2014, 10, 337–348.
- Da Cruz, S.; Cleveland, D.W. Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr. Opin. Neurobiol. 2011, 21, 904–919.
- Chook, Y.M.; Süel, K.E. Nuclear import by karyopherin-βs: Recognition and inhibition. Biochim. Biophys. Acta 2011, 1813, 1593–1606.
- Dormann, D.; Rodde, R.; Edbauer, D.; Bentmann, E.; Fischer, I.; Hruscha, A.; Than, M.E.; Mackenzie, I.R.; Capell, A.; Schmid, B.; et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 2010, 29, 2841–2857.
- Lanson, N.A., Jr.; Maltare, A.; King, H.; Smith, R.; Kim, J.H.; Taylor, J.P.; Lloyd, T.E.; Pandey, U.B. A Drosophila model of FUS-related neurodegeneration reveals genetic interaction between FUS and TDP-43. Hum. Mol. Genet. 2011, 20, 2510–2523.
- Patel, A.; Lee, H.O.; Jawerth, L.; Maharana, S.; Jahnel, M.; Hein, M.Y.; Stoynov, S.; Mahamid, J.; Saha, S.; Franzmann, T.M.; et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 2015, 162, 1066–1077.
- Hofweber, M.; Hutten, S.; Bourgeois, B.; Spreitzer, E.; Niedner-Boblenz, A.; Schifferer, M.; Ruepp, M.D.; Simons, M.; Niessing, D.; Madl, T.; et al. Phase Separation of FUS Is Suppressed by Its Nuclear Import Receptor and Arginine Methylation. Cell 2018, 173, 706–719.e713.
- Luo, F.; Gui, X.; Zhou, H.; Gu, J.; Li, Y.; Liu, X.; Zhao, M.; Li, D.; Li, X.; Liu, C. Atomic structures of FUS LC domain segments reveal bases for reversible amyloid fibril formation. Nat. Struct. Mol. Biol. 2018, 25, 341–346.
- Murray, D.T.; Kato, M.; Lin, Y.; Thurber, K.R.; Hung, I.; McKnight, S.L.; Tycko, R. Structure of FUS Protein Fibrils and Its Relevance to Self-Assembly and Phase Separation of Low-Complexity Domains. Cell 2017, 171, 615–627.e616.
- Yoshizawa, T.; Ali, R.; Jiou, J.; Fung, H.Y.J.; Burke, K.A.; Kim, S.J.; Lin, Y.; Peeples, W.B.; Saltzberg, D.; Soniat, M.; et al. Nuclear Import Receptor Inhibits Phase Separation of FUS through Binding to Multiple Sites. Cell 2018, 173, 693–705.e622.
More
