Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mohammad Harun-Ur-Rashid and Version 2 by Alfred Zheng.
Exploring bio-inspired nanomaterials (BINMs) and incorporating them into micro/nanodevices represent a significant development in biomedical applications. Nanomaterials, engineered to imitate biological structures and processes, exhibit distinctive attributes such as exceptional biocompatibility, multifunctionality, and unparalleled versatility. The utilization of BINMs demonstrates significant potential in diverse domains of biomedical micro/nanodevices, encompassing biosensors, targeted drug delivery systems, and advanced tissue engineering constructs.
- bio-inspired nanomaterials
- micro/nanodevices
- biomedical applications
- nanotechnology
- nano-biotechnology
- biomimetic polymers
- microfabrication
1. Introduction
Bio-inspired nanomaterials (BINMs), alternatively referred to as biomimetic nanomaterials (BNMs), are a class of materials that are intentionally engineered and manufactured to replicate the intricate structures, functionalities, or mechanisms observed in natural biological systems [1][2][3][1,2,3]. These advancements offer a novel trajectory for the field of material science, facilitating the creation of materials possessing unique characteristics that can effectively tackle a wide range of scientific, technological, and environmental obstacles through successful applications in multidimensional sectors, including medicine and healthcare [4], biotechnology and bioengineering [5], energy [6], environment [7], material science [8], robotics [9][10][9,10], and many more [11][12][13][11,12,13]. Various biological entities, including proteins, DNA [14][15][14,15], cells [16], and complete organisms [17], can serve as sources of inspiration for these materials. Using DNA’s self-assembling properties has facilitated the construction of shapes and patterns at the nanoscale level [18]. The adhesive characteristics exhibited by gecko feet have served as a source of inspiration for developing sophisticated adhesive materials [19]. Furthermore, the self-cleaning and hydrophobic characteristics exhibited by the lotus leaf have prompted advancements in creating self-cleaning surfaces and coatings with water-repellent properties [20]. The interdisciplinary field of BINMs integrates principles from biology, chemistry, physics, and material science. A bottom-up approach is often employed, commencing at the atomic or molecular level and progressing upward. This is juxtaposed with the conventional top-down methodology, wherein the initial focus is on a larger system that is subsequently deconstructed into smaller constituent parts. Various techniques can be employed to fabricate BINMs, such as molecular self-assembly, in which molecules autonomously organize themselves into desired structures. Another method is biosynthesis, which involves utilizing biological organisms such as bacteria, fungi, or plants to synthesize nanomaterials. The field of BINMs holds significant potential for scientific investigation; however, it is not devoid of inherent obstacles. One of the primary obstacles lies in the capacity to regulate the synthesis and assembly processes of these materials in order to attain the intended properties [21]. There exist additional concerns regarding the potential environmental and health ramifications associated with these nanomaterials, necessitating the need for further examination and comprehensive testing prior to their widespread implementation [22]. Notwithstanding these challenges, BINMs constitute a captivating and burgeoning area of investigation. The advancement of our knowledge in the fields of biology and nanotechnology is expected to enhance the possibilities for the development of novel and influential BINMs.
With the advancement and comprehension of BINMs, an opportunity arises to investigate the pragmatic utilization of these materials in the configuration of micro/nanodevices. Micro/nanodevices, as their nomenclature implies, are miniature devices that operate at the micro- or nanolevel. The significance of micro/nanodevices has increased substantially due to their potential to enhance capabilities in diverse sectors, including medicine, environmental monitoring, electronics, and energy production. These devices provide an unparalleled degree of control and accuracy at a minuscule level, enabling us to devise and develop solutions to previously insurmountable obstacles. The potential for developing novel and influential micro/nanodevices using BINMs is anticipated to grow due to technological advancements and improved comprehension of biological systems. Through the utilization of the distinct characteristics exhibited by these nanomaterials in the form of nanocomposite gels [23][24][25][26][23,24,25,26] and films [27][28][29][27,28,29], structural colored nanomaterials [30][31][32][30,31,32], organo-metallic nanomaterials [33], molecular machines [34], and nanobiosensors [35] have found widespread application and have replaced mainly more conventional bulk materials in a variety of sectors [36][37][38][39][40][41][36,37,38,39,40,41] as well as in theoretical inquiries [42]. Researchers and practitioners can fabricate devices that imitate or draw inspiration from biological systems to execute targeted functions, frequently surpassing the efficiency and efficacy of conventional devices. Medicine and healthcare are highly significant domains for applying micro/nanodevices [43][44][43,44]. Environmental monitoring is a field that extensively utilizes micro/nanodevices [45]. These encompass sensors capable of detecting various environmental pollutants, even in exceedingly low concentrations. These devices can continuously monitor air and water quality, thereby offering significant data that can be utilized to safeguard the environment. The electronics and computing sector represents a significant domain in which micro/nanodevices are widely used [46]. Modern electronic and computing devices rely on various components, such as transistors found in computers and sensors present in smartphones, which collectively serve as the fundamental infrastructure for these technologies. By further reducing the size of these devices and enhancing their operational capabilities, it is possible to develop electronic devices that are more potent and consume less energy. Micro/nanodevices are paramount in energy production and storage [47]. Nanostructured materials have been employed to improve the efficiency of solar cells, fuel cells, and batteries, among other applications. These devices have the potential to enhance energy efficiency, mitigate expenses, and foster the adoption of renewable energy sources. Although the prospect of micro/nanodevices is vast, there are still obstacles to overcome in manufacturing, integration, reliability, and safety. Current investigations in BINMs and their utilization in micro/nanodevices are actively tackling these obstacles, thus laying the foundation for a novel epoch in diverse industries.
The convergence of BINMs and micro/nanodevices is driving a transformative shift across various academic fields, with a particular emphasis on biomedicine. The convergence described in this context capitalizes on the distinctive characteristics of BINMs, which are derived from biological systems, to optimize the functionality of micro/nanodevices. These devices, in turn, provide a pragmatic framework for implementing these nanomaterials. The inherent characteristic of BINMs, known as the “bottom-up” approach, is highly compatible with the micro/nanoscale. This compatibility facilitates the formation of intricate structures through the process of self-assembly. The advantageous collaboration between nanomaterials and biological systems in biomedicine is of great significance, as it allows for the customization of nanomaterials to enhance their interaction capabilities with biological entities. One example of improving targeted drug delivery systems involves the integration of nanomaterials into micro/nanodevices, thereby enabling the accurate administration of drugs to particular cells or tissues. Moreover, developing micro/nanosensors with high sensitivity is feasible, thereby improving the capability for early disease detection and accurate environmental monitoring. Although this interdisciplinary field offers significant prospects, it is important to acknowledge the persistent challenges associated with the control of nanomaterial synthesis and assembly, their integration into devices, and the assurance of safety and efficacy in practical applications. However, the potential advantages signify a promising outlook for this convergence.
2. Biomedical Applications of Bio-inspired Nanomaterials in Micro/Nanodevices
When the many uses of bio-inspired nanomaterials in micro/nanodevices are examined, a wide range of biological uses is found. From improving drug delivery methods to coming up with new ways to treat patients, these new materials are paving the way for big changes in healthcare. Drug delivery involves complicated systems, nanotheranostics for combined therapy and diagnostics, gene therapy for genetic disorders, and the creativity of self-propelled active nanovehicles and biohybrid micro/nanomotors that can move through complex biological environments. Bio-inspired nanobiosensors that can identify molecules in a complex way add to these achievements. Also, bio-inspired organ-on-a-chip technology gives us new ways to test drugs, and cancer-on-a-chip models change how people study cancer. Bio-inspired wound healing dressing mats and antimicrobial surfaces, such as those made from structure-oriented peptides, metal/metal oxide NPs, and chitosan, show how bio-inspired nanomaterials have a wide range of uses in medicinal applications. Bacteriophage-based antimicrobial surfaces use the power of nature to fight bacterial diseases. This all-around look at bio-inspired nanomaterials shows their importance and opens up a new era of opportunities for biomedical progress in micro/nanodevices. Figure 1 summarizes the biomedical applications of BINMs in micro/nanodevices.
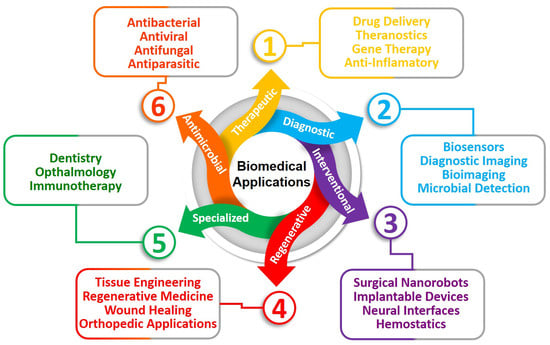
Figure 1.
Biomedical applications of BINMs in micro/nanodevices.
2.1. Drug Delivery and Therapeutic Applications
2.1.1. Drug Delivery Systems
In the field of medicine, BINMs have made major advancements, particularly in the creation of sophisticated drug delivery systems. These nanomaterials can potentially address major difficulties in targeted medication delivery because they were created and constructed to resemble biological structures and processes. Micro- and nanodevices made from BINMs are at the core of these breakthroughs. In several ways, these devices can improve drug delivery. They can increase the specificity of drug delivery, ensuring that the medications reach the cells or tissues that require them most. This can reduce the risk of negative effects while significantly increasing the drug’s effectiveness. Liposomes, for instance, nano-sized vesicles modeled after biological membranes, are frequently utilized as drug delivery systems. They can encapsulate both hydrophilic and hydrophobic medications, guard against deterioration, and release them gradually at the intended spot. Dendrimers, another category of BINMs, are spherical, highly-branching NPs. They are good candidates for targeted drug administration due to their well-defined structure, controllable size, and surface functional groups. Recently, scientists have started investigating the use of BINMs for targeted drug delivery that imitates the structure of cells, bacteria, and viruses. For instance, NPs with red blood cell (RBC) membrane coatings have shown promise in drug delivery while dodging the body’s immunological response. But creating these biologically inspired micro- and nanodevices is difficult. Addressing concerns with stability, biocompatibility, scalability, and reproducibility is necessary. Extensive testing and clinical trials are also necessary to determine the safety and effectiveness of these technologies for human usage.
Liposomes are widely recognized as a promising and versatile means of drug delivery. Liposomes present several advantages in comparison to traditional drug delivery systems. These advantages include targeted delivery to specific sites, controlled and sustained release of drugs, protection against degradation and clearance, enhanced therapeutic outcomes, and reduced toxic side effects. These beneficial characteristics have contributed to the effective authorization and medical utilization of numerous liposomal pharmaceutical products within recent decades [48][328]. Liposomes can be divided into several categories depending on their lamellarity and compartment structure. These categories include multivesicular liposomes (MVLs), unilamellar vesicles (ULVs), oligolamellar vesicles (OLVs), and multilamellar vesicles (MLVs). OLVs and MLVs both have an onion-like structure; however, MLVs have more than five lipid bilayers, while OLVs only have two to five concentric lipid bilayers. On the other hand, MVLs have several non-concentric aqueous chambers that are each surrounded by a single bilayer lipid membrane, giving them the appearance of a honeycomb. Small unilamellar vesicles (SUVs, 30–100 nm), large unilamellar vesicles (LUVs, >100 nm), and giant unilamellar vesicles (GUVs, >1000 nm) are subcategories of ULVs based on particle size. According to several research studies, ULVs come in various sizes, including SUVs that are less than 200 nm and LUVs that are between 200 and 500 nm in size. Numerous techniques are applied for the preparation of liposomes. The manufacturing methods that are frequently utilized encompass thin-film hydration, ethanol injection, and double-emulsion techniques. The conventional procedures in these processes encompass several steps. Firstly, multilamellar vesicles (MLVs) or unilamellar vesicles (ULVs) are prepared, depending on the chosen method. Secondly, the size of the vesicles may be reduced if deemed necessary. Thirdly, the drug solution(s) are prepared and loaded into the liposomes. In the case of passive drug loading, this step is combined with step one. Fourthly, buffer exchange and concentration are performed if required. Fifthly, sterile filtration or aseptic processing is carried out. Lastly, if deemed necessary, lyophilization is conducted, followed by packaging.
Dendrimers have emerged as crucial nanostructured carriers in nanomedicine for treating numerous diseases [49][334]. Thanks to their structural diversity, they can deliver medications and genes in various ways. For example, dendrimers with a hydrophobic center and a hydrophilic periphery can act like unimolecular micelles and successfully saturate hydrophobic medicines. The use of cationic dendrimers as non-viral gene carriers is widespread. Drugs and functional moieties can be attached to dendrimer surface groups to increase stability and solubility. Enhancing dendrimer compatibility and binding properties involves conjugating them with polymers like PEG or polysaccharides. Utilizing ligands like hyaluronic acid or mannose has improved tumor penetration and targeted distribution to specific cell types, such as macrophages. Compared to free medicines, dendrimer–drug conjugates have fewer systemic side effects and more localized efficacy. Dendrimer conjugation can lengthen the half-life of pharmaceuticals, improving medicinal efficacy and reducing administration frequency. Dendrimers increase the solubility of drugs, increasing their potency. When compared to timolol maleate, a study on a dendrimer–drug combination known as DenTimol demonstrated encouraging outcomes for the treatment of glaucoma. Various cleavable or stimuli-responsive linkages ensure that medications released from dendrimer–drug conjugates reach the intended area. For this aim, disulfide/thioketal linkers and pH-responsive linkers are frequently employed. Dendrimer–drug conjugates have promise as efficient drug delivery systems with controlled release mechanisms for better therapeutic results. Over free medicines, dendrimer–drug conjugates have several benefits, such as fewer systemic side effects and increased efficacy at the target site. They can lengthen a drug’s half-life and make it more soluble, enhancing patient compliance and therapeutic results. For instance, PAMAM dendrimers have been utilized successfully to deliver antiglaucoma medications, exhibiting better effects on decreasing intraocular pressure. Examining drug release from such conjugates is crucial since regulatory agencies’ classification of dendrimer–drug conjugates might be complicated. Drug release from dendrimers in tumor cells has been facilitated by cleavable linkers like disulfide and thioketal, increasing the effectiveness of cancer therapy. By serving as “unimolecular micelles” or “dendritic boxes,” dendrimers also provide drug encapsulation through their hydrophobic cavities, enhancing the solubility of hydrophobic medicines in water. PAMAM dendrimers have also been extensively used as gene transfection vectors because of their great biocompatibility and capability for nucleic acid loading. They can improve endosomal escape and cellular uptake, increasing transfection effectiveness. Dendrimers can overcome intracellular gene delivery hurdles when decorated with functional moieties like peptides. This results in successful gene delivery and tumor growth inhibition. Overall, dendrimer-based medication and gene delivery systems provide considerable promise for treating various disorders using nanomedicine [50][51][52][335,336,337].
Polymeric micelles (PMs) are nanostructures created through amphiphilic block copolymers (ABCs) self-assembled in an aqueous medium. These micelles possess a distinctive core-shell architecture. In conventional micelles, the hydrophobic segment of the polymer is oriented toward the interior, forming the core, whereas the hydrophilic segment is positioned on the outer surface. Reverse micelles exhibit an orientation that is opposite to that of regular micelles. Mixed micelles are generated by adding solubilizers to the existing surfactant micelle structure. The drug is expelled into the micelle core by the hydrophobic component of the copolymers, thereby enabling the solubilization of pharmaceuticals with low solubility. The intermolecular hydrophobic interactions between the drug and copolymers are important in modulating the drug release rate and enhancing its solubility. Numerous hydrophobic copolymers have undergone testing to solubilize drugs with low solubility efficiently. Polymeric micelles frequently contain a hydrophobic core enclosed by hydrophilic copolymers [53][338]. The hydrophilic portion of the polymer faces outward in normal micelles, while the lipophilic portion faces the core. The orientation of reverse micelles is the opposite. Solubilizers are included in the surfactant micelle to create mixed micelles. Pharmaceuticals that are difficult to dissolve are ejected into the micelle core by the copolymer’s hydrophobic component. Copolymers’ hydrophobic interactions with the medication are essential for reducing drug release and increasing solubility. It has been tested that different hydrophobic copolymers can successfully solubilize poorly soluble medicines. Drug leakage from polymeric micelles must be reduced during distribution, and drug release must be regulated to provide optimal therapeutic targeting. Either stable confinement of the drug payload within micellar cores or triggered release in response to internal or external stimuli are necessary for targeted drug delivery. While slowly released medications from capsules allow for pharmacological and toxicological effects, prolonged drug release from polymeric micelles in circulation assures congruent pharmacokinetics with the micelles. Stimuli-sensitive polymeric micelles use internal triggers, such as changes in pH, redox potential, temperature, enzyme profiles, and oxygen levels, to take advantage of disease-induced changes in target tissues. Outside stimuli, including heat, ultrasound, near-infrared light, or magnetic fields can also trigger drug release. These methods improve the adaptive drug carriers’ ability for precise drug delivery, especially in sick tissues like malignant ones. Micelles are distinguished by their core-shell structure. The corona shell shields the drug from the mononuclear phagocyte system, allowing for longer blood circulation and less toxicity. They make it possible for hydrophobic medicines to become stable and water-soluble, facilitating effective medication delivery. The ideal micelles for dispensing hydrophobic medications feature a hydrophilic corona to protect and stabilize the medication. Medicines with a high water solubility can have their intravenous administration of hydrophobic medicines made possible by polymeric micelles. Although polymeric micellar systems have several drawbacks, different methods have been created to overcome these obstacles. With the right approaches to drug loading issues, scale-up options, and thorough research into their behavior in biological systems, polymeric micelles can successfully find their place in the market for various biomedical uses. Polymeric micelles present promising opportunities in biomedical applications [54][55][339,340].
2.1.2. Nanotheranostic
For many illnesses, including AIDS, cancer, and microbial disorders, theranostic methods have been suggested. The medication is customized using this personalized treatment approach based on unique molecular profiles or the discovery of biomarkers. Nanotechnology advancements can now combine diagnostic and therapeutic approaches on a single platform. Nanomedicines increase the bioavailability of drugs, shield them from deterioration, and enable precise medication distribution within the body. Compared to conventional medicines, nanostages used in nanotheranostics provide simultaneous illness detection and treatment while improving medication penetration. This new field has the potential to significantly help the pharmaceutical and healthcare sectors by facilitating the creation of molecular sensors, imaging agents, and creative therapeutic agent carriers. Immunoassays and colorimetric tests, as well as gene therapy and targeted drug delivery, are nanotheranostic diagnostic and therapeutic tools that have the potential to transform the diagnosis and treatment of a wide range of illnesses, including cancer, AIDS, cardiovascular disease, infections, and burn wounds [56][341]. BINMs have significantly enhanced cancer diagnostics and treatments, largely due to their small size, ease of modification, high drug-loading capacity (thanks to their large surface-to-volume ratio), and efficient penetration and retention within targeted tissues. Furthermore, their superior biocompatibility, biodegradability, and multifaceted applications in bioimaging, bio-sensing, diagnostics, and therapeutics have escalated their potential in numerous biomedical fields [57][342]. Due to their potential to serve as alternative, biocompatible drug delivery systems in cancer theranostics, bio-inspired NPs that mimic natural body components have recently attracted a lot of attention. Unlike non-native drug delivery technologies, these NPs have the innate potential to change systemic bio-distribution, which is their main advantage. This research thoroughly explains numerous BINMs used in cancer theranostics, including liposomes, lipid NPs, bio-synthesized metal NPs, virus NPs, protein NPs, and others.
2.1.3. Gene Therapy
In several medical specialties, gene therapies are becoming more and more cutting-edge treatments. Gene therapy first proposed some 45 years ago as a viable treatment for hereditary monogenic illnesses, is currently being used to treat acquired conditions like cancer immunotherapy. The idea was that a single treatment could offer substantial, possibly curative advantages. For instance, gene-based therapies given to cells with a long lifespan may permit the continued production of crucial proteins. Hematopoietic stem cells (HSCs) that have undergone genetic engineering could provide long-lasting cell replacement, eliminating the requirement for ongoing enzyme administration or transfusion therapy. With initial clinical trials in the early 1990s producing poor findings, including little clinical benefit, unforeseen toxicities, and, in some cases, patient fatalities, converting gene therapy concepts into patient care has had difficulties. This caused a shift in attention to the fundamental science underlying gene therapy strategies. With a better understanding of viral vectors and target cells, a new wave of clinical trials in the late 1990s and early 2000s showed promise, but development was hampered by severe toxicities associated with high gene transfer efficiency. The discipline of gene therapy has made enormous strides over the past ten years, with improvements in safety, gene transfer effectiveness, and delivery spurring major clinical advancements. The FDA has approved several gene therapies, and other agencies worldwide have labeled others as “breakthrough therapies.” The science of gene therapy is about to undergo another revolutionary change because of recent advancements in targeted genome editing [58][343].
The utilization of BINMs has played a crucial role in advancing the field of micro/nanodevices for gene therapy. These nanomaterials are derived from biological systems and can imitate the structures and functions of biological molecules. As a result, they exhibit enhanced biocompatibility and functionality. Nanomaterials, including lipid-based NPs, protein-based NPs, and DNA/RNA-based nanostructures, function as carriers for gene delivery, offering improved stability, specificity, and efficiency. One illustration of this concept involves the utilization of lipid-based NPs to encapsulate nucleic acids, thereby enabling their efficient transport into cells.
Additionally, DNA nanostructures can be purposefully engineered to serve as carriers for therapeutic genes, allowing for direct delivery. Significantly, BINMs possess the capability to undergo modification or functionalization to augment their targetability and mitigate potential toxicity. By capitalizing on these benefits, micro/nanodevices employing BINMs have demonstrated gene therapy potential. Gene therapy aims to address diseases at the fundamental genetic level by mending, activating, or eliminating specific genes. The ongoing investigation and advancement in this particular domain are anticipated to result in the emergence of gene therapy approaches that are both more efficient and secure.
There is a growing interest in the supramolecular self-assembly of dendrons and dendrimers as a powerful and challenging method for generating advanced nanostructures that exhibit exceptional properties. Xu et al. proposed a novel approach involving supramolecular hybridization to fabricate a dendritic system inspired by biological systems [59][344]. This system demonstrated remarkable versatility and can be utilized as an efficient nanoplatform for various delivery applications. Multifunctional supramolecular hybrid dendrimers (SHDs) were formed by integrating dual-functionalized low-generation peptide dendrons (PDs) onto inorganic NPs, facilitated by an intelligent design. The structural composition of these superhydrophobic surfaces (SHDs) exhibited a highly organized nanoarchitecture, accompanied by a substantial presence of arginine peptides, and demonstrated the ability to emit fluorescence signals. As predicted, the utilization of a bio-inspired supramolecular hybrid strategy dramatically enhances the gene transfection efficacy of self-assembled hydrogel NPs (SHDs) by approximately 50,000 times when compared to standalone polymeric NPs (PDs) at equivalent ratios of polymer to DNA. The bio-inspired self-assembled hydrogel NPs (SHDs) demonstrate several advantageous characteristics in gene delivery. Firstly, they possess low cytotoxicity and are resistant to serum, which enhances their safety and efficacy. Secondly, these SHDs have inherent fluorescence, monitoring various intracellular processes, including cellular uptake, escape from endosomes, and gene release. Lastly, they can serve as a valuable reference for tracking the expression of desired proteins, providing an alternative method for assessing gene delivery efficiency. Significantly, it is important to note that in vivo animal trials have shown that self-healing hydrogels (SHDs) exhibit considerable effectiveness in gene transfection, specifically in muscle tissue and HepG2 tumor xenografts. These trials have also demonstrated the ability of SHDs to perform real-time bioimaging. The anticipated outcome of these supramolecular hybrid dendritic (SHD) structures is the stimulation of research inquiries to utilize bio-inspired dendritic systems for biomedical purposes, encompassing laboratory-based and live organism studies.
With the progress of molecular biology, pharmacogenomics, and proteomics, there is an opportunity to customize the development of bio-inspired nanosystems to cater to individual patients’ specific requirements. Bio-inspired nanomaterials provide numerous advantages, such as the ability to customize their surface, achieve targeted delivery, possess specific geometric properties, ensure biosafety, and facilitate proper disposition. Furthermore, the materials and procedures employed in fabricating these systems exhibit biocompatibility and environmental sustainability, as they necessitate limited processing compared to synthetic materials. Nevertheless, it is essential to acknowledge the potential apprehensions regarding residual solvents or reagents utilized during the synthesis process, which may harm biological systems. However, certain bio-inspired nanosystems exhibit enhanced pharmacological efficacy. There is expected to be a preference for gene carriers based on smart materials that are biocompatible, biodegradable, and safe in the future. These systems can detect the surrounding environment of the host following administration, enabling them to regulate the release of gene molecules that have been loaded within them at a particular target organ with accuracy and pre-determined control. This functionality serves to reduce the occurrence of undesired side effects. Therefore, utilizing bio-inspired gene delivery systems presents a distinct opportunity to develop anticipatory and individualized delivery systems for currently available medications, thereby holding great potential in shaping the future of the biomedical field [60][61][345,346].
2.1.4. Self-Propelling Active Nanovehicles
Chemical navigation is a crucial aspect of survival for a wide range of organisms, from bacteria to unicellular and multicellular organisms. The replication of these behaviors through artificial constructs is an emerging field of study, resulting in the development of active nanomaterials capable of converting external energy into mechanical work to achieve directed motion [62][347]. These nanomaterials can react to various stimuli, including chemical gradients, temperature changes, magnetic fields, and adhesion forces. Nevertheless, the development of self-propelling nano-constructs encounters various obstacles arising from physical limitations. For instance, water, which exhibits a high viscosity at the NP level, poses a significant challenge. Additionally, the randomizing effect of Brownian thermally driven fluctuations further complicates the control of the NPs’ directionality. Two strategies can be employed to address these limitations. The first strategy involves inducing non-reciprocal movements by altering the body shape. The second strategy consists in taking advantage of gradients that modify the local environment of the nanomaterials. Illustrations of these strategies being implemented encompass the utilization of artificial bacterial flagella, which can be effectively manipulated by applying rotating magnetic fields to generate propulsion. The deployment of “spermbots” has been observed, wherein these microscale robots can facilitate the transportation of sperm cells toward the oocyte. Certain NPs can generate gradients autonomously, resulting in self-phoresis or self-propelled movement. Various innovative applications have been documented, including the utilization of silicon nanowires that exhibit a responsive behavior to externally manipulated electrical fields, as well as the development of “microbullets” capable of vaporizing biocompatible fuel and effectively penetrating and altering the structure of tissues. The utilization of bio-inspired methodologies in the design of NPs exhibits considerable promise for their application in drug delivery, targeted therapy, and various other domains within the biomedical field [63][64][65][348,349,350].
2.1.5. Biohybrid Micro/Nanomotors
Throughout history, human ingenuity has frequently drawn inspiration from diverse natural biological systems, exemplified by the development of radar technology, which was influenced by bats’ utilization of ultrasonic waves. The advancement of autonomous artificial micro/nanomotors has been motivated by the existence of biological biomotors such as kinesins, dyneins, and sperm cells. Micro/nanomotors are devices capable of converting various types of energy into mechanical motion, enabling them to execute tasks that passive devices cannot accomplish [66][351]. Richard Feynman initially introduced the notion of these diminutive devices which has subsequently emerged as a prominent subject of scholarly investigation. Micro/nanomotors possess a diverse array of applications, particularly within biomedicine. These applications encompass drug delivery, biosensors, biological imaging, assisted fertilization, and microsurgery. Nevertheless, notable obstacles must be surmounted, with particular emphasis on the biocompatibility of said motors. To operate optimally, these entities must adapt effectively to the internal microenvironment of the organism, encompassing factors such as temperature, pH, and the immune system. Presently, the utilization of artificial motors is constrained by their inadequate biocompatibility, resulting in their susceptibility to immune system recognition upon introduction into the human body. To enhance biocompatibility, scholars are currently investigating the utilization of biocompatible and biodegradable substances such as polyethylene glycol (PEG) and magnesium. An emerging area of study pertains to biohybrid micro/nanomotors, wherein synthetic materials are integrated with biological constituents. Biohybrid motors exhibit enhanced biocompatibility, improved energy conversion efficiency, and the capacity to react to environmental stimuli intelligently. The cell is the building block of an organism and has receptors on its membrane that enable it to sense environmental inputs and modify its functions. Some cells have complex time-irreversible strokes and low-Reynolds-number autonomous motion processes. Researchers have used this autonomous mobility as motivation to build micro/nanomotors based on intact cells. High biocompatibility, adaptability to diverse internal conditions, and the ability to mix artificial micro/nanostructures with different cell properties like chemotaxis, magnetotaxis, and anaerobism to create multifunctional motors are all benefits of these biohybrid motors. A cell must be simple to grow and capable of large-scale, rapid multiplication to be a good candidate for biohybrid micro/nanomotors. There are several types of micro/nanomotors based on intact cells (sperm cells, bacteria, algae, blood cells, plant pollen, platelets, macrophages, etc.) and different biological components such as enzymes (catalase, urease, glucose oxidase, lipase, etc.) and cellular membrane (RBC, platelet, WBC, tumor cell, cancer cell, etc.) coating, operating in biomedical applications.
Sperm cells, also known as spermatozoa, are the male gametes that possess the ability to exhibit autonomous motility as a result of their flagellar structure. They possess the dual functionality of functioning as both a propulsion mechanism and a means of transporting goods, enabling autonomous movement and precise distribution capabilities. The micro-bio-robot design involved using sperm cells confined within a microtube, which demonstrated self-directed movement that was externally regulated through the implementation of a magnetic layer. Sperm cells possess considerable potential as vehicles for drug delivery, particularly in gynecologic ailments such as cervical cancer [67][352]. A micromotor propelled by sperm cells has been developed to facilitate the targeted release of drugs, demonstrating encouraging attributes for cancer treatment. Bacteria are abundant and come in various shapes, making them suitable candidates for biohybrid micro/nanomotors. Several bacteria, such as Magnetococcusmarinus, Escherichia coli, and others, have been utilized to fabricate biohybrid motors for biomedical applications. Magnetotactic bacteria can achieve self-propulsion using external magnetic fields, making them attractive for drug delivery and tumor targeting. Escherichia coli are frequently used due to their swimming ability, and they have been incorporated into micromotors for drug delivery and anti-tumor efficacy. Challenges include addressing safety concerns regarding pathogenic bacteria and ensuring the activity and fitness of bacteria on certain surfaces. Despite these challenges, bacterial biohybrid micro/nanomotors hold promise for advancing the field of micro/nanomotors.
Algae exhibit remarkable biological features despite lacking roots, stalks, and leaves. Spirulinaplatensis (Sp) is a suitable bio-template for biohybrid magnetic micromotors due to its naturally intact three-dimensional helical structure. Researchers used Sp to construct porous hollow micromotors to deliver medicinal and imaging chemicals in vivo. Sp-based biohybrid magnetic robots feature intrinsic fluorescence, MR signals, and low cytotoxicity, making them intriguing for blocking abnormal cell function, particularly malignant tumors, while retaining normal cell function. Sp-based magnet-powered microswimmers use ultrasonics to stimulate neural stem-like cell development. Ultrasound intensity can influence brain stem cell development, enabling minimally invasive neurodegenerative disease treatments. Algae, particularly Sp, have distinctive structures and intriguing biological features, making them promising in modern biotechnology. For biohybrid micro/nanomotors, different algae must be studied.
2.2. Bio-Inspired Nanobiosensors
Sensors are pivotal in many products, systems, and manufacturing processes, offering valuable feedback, monitoring capabilities, safety enhancements, and other advantageous features. When conventional sensor technology reaches a state of limited progress, exploring insights from non-engineering disciplines, such as biology, can foster innovative advancements. The field of biomimetic sensor technology is currently in its nascent stage, and it takes inspiration from the intricate sensory systems found in nature. These highly refined systems enable organisms to perform tasks such as navigation, spatial orientation, and prey detection with excellent efficiency. Engineers can construct various types of sensors by comprehending the fundamental principles of sensory physiology in biological systems. Biomimetic sensor designs can replicate biological systems directly or employ analogous principles. Both methodologies have demonstrated efficacy and yielded notable progress in sensor technology [68][353]. Biomimetic sensor designs offer distinct advantages in comparison to conventional sensor designs.
Retrieving archived sensor design information is a prevalent methodology in developing novel products that detect commonly encountered parameters. Nevertheless, employing unconventional approaches or drawing inspiration from diverse fields of study may be imperative in the context of atypical parameters. The field of sensor design has been influenced by nature, as it presents a wide range of sophisticated sensing and communication techniques observed in diverse organisms such as bacteria, plants, insects, mammals, and reptiles. The design of biomimetic sensors entails replicating various aspects of biological systems, including functional design, morphological design, principles, strategies, behaviors, and manufacturing techniques. The motivation behind these biomimetic devices is derived from rigorous methodologies, careful examination of natural phenomena, and the application of databases that document biological functionalities. Emulating the functionality, principles, morphology, or strategies observed in biological systems represents a form of biomimicry, which can be likened to the reverse-engineering process. In an alternative perspective, abstracting biological systems through analogical reasoning can be seen as an approach that aligns biology with engineering design principles. This approach involves seeking solutions to biological challenges by drawing inspiration from and imitating existing designs. Exploring natural phenomena to derive design inspiration or gain insights into the mechanisms by which biological systems process sensory information has resulted in notable advancements. The biological sensors found in nature have evolved over an extensive time spanning billions of years. These sensors provide enduring and efficient solutions that are well adapted to specific ecological niches. Frequently, these sensors demonstrate characteristics such as minimal energy consumption, heightened sensitivity, and redundancy. The redundancy concept serves as a valuable lesson derived from nature, as numerous biological systems exhibit multiple instances of redundancy to augment reliability and mitigate errors.
Chirality, also known as mirror dissymmetry, is an inherent characteristic observed in geometric entities, and it holds significant importance in biomolecules such as proteins and DNA. Circular dichroism spectroscopy is a technique used to evaluate the impact of a substance on biological, chemical, and physical characteristics. This method quantifies the disparity in the absorption of left and right circularly polarized light. Chirality is regarded as a principle inspired by biology in engineering. Chiral nanomaterials exhibit potential for various applications, such as sensing and catalysis, owing to their distinctive selectivity and specificity.
Nevertheless, there remains a lack of comprehensive understanding regarding the mechanisms that govern the transfer of chirality during the synthesis of inorganic nanomaterials possessing inherent chirality. Examining biological instances of chirality transfer can provide valuable insights for developing chiral inorganic nanomaterials across diverse applications. Chirality is a prevalent characteristic observed in biological entities, significantly influencing their geometries, properties, and behavior. Chiral objects, characterized by the absence of mirror symmetry, are widely observed in the natural world, and they have the potential to confer survival benefits to organisms. The phenomenon of chirality significantly influences the preferential incorporation of amino acids during the process of protein synthesis, thereby exerting a profound impact on the growth and behavior of both plants and animals. Organisms can utilize chiral structures to perceive polarized light and augment contrast within their surroundings. Inorganic materials also observe chirality due to molecular interactions and biological templates. Gaining insight into the processes by which chirality is transferred across various length scales is of utmost importance to effectively replicate and harness chiral nanostructures to design nanomaterials. The phenomenon of hierarchical chirality transfer, which occurs across multiple scales ranging from the molecular to the macroscopic level, has been documented in diverse biological systems. This observation has sparked interest and served as a source of inspiration for developing biomimetic materials and nanotechnologies [69][354]. Near-infrared (NIR) wavelengths are commonly favored in biomedical applications owing to their superior tissue penetration capabilities. The utilization of CdTe helices has been observed to effectively manipulate light within the near-infrared (NIR) wavelengths, rendering them valuable for various biomedicine and optical computing applications. Chiral molybdenum oxide NPs have the potential to be utilized in photothermal therapy, wherein they can selectively heat tumor tissue when exposed to circularly polarized light while minimizing damage to healthy tissue [70][355]. The bactericidal effects of gold nanobipyramids conjugated with D-Glu are enhanced, disrupting bacterial cell walls and facilitating the healing process in infected wounds when exposed to near-infrared (NIR) radiation [71][356]. The inherent structural chirality exhibited by gold nanomaterials can influence the immune system. Diverse immune responses are observed with left-handed and right-handed Au NPs, owing to their distinct interactions with specific receptors and subsequent activation of inflammasomes. The utilization of left-handed NPs as adjuvants in the influenza vaccine has been investigated, revealing a notable increase in antibody production and immune-related cell proliferation compared to right-handed NPs. These discoveries underscore the significance of nanoscale chirality within biological systems, alongside the molecular-scale chirality exhibited by L/D optical centers.
2.3. Bio-Inspired Organ-on-Chip (OOC)
Creating new medications is time-consuming and expensive, especially in the pre-clinical stage. Pre-clinical research has a history of using unethical animal experiments that do not always precisely anticipate how people will react to medications. Although they provide an alternative, two-dimensional cell culture models cannot match the intricacy of real tissues and organs. Three-dimensional cell culture models have been created to overcome these restrictions; however, they still lack some physiological elements. The development of organ-on-chip (OOC) systems, which are little devices that replicate the microenvironment of organs and tissues, has recently been made possible by microtechnology. OOCs can build human-based tissue-like structures, operate with minuscule drug concentrations for high-throughput screening, and add biosensors for real-time monitoring of cell survival and functionality, among other benefits. To replicate the response of different tissues to drug exposure methodically, several OOCs can be coupled. OOCs are a potential strategy for drug discovery because they combine the benefits of 2D and 3D cell culture models while offering a platform for drug testing and screening that is more physiologically appropriate [72][357].
2.3.1. Organ-on-Chip (OOC) Technology
The primary objective of OOC technology is to develop in vitro models that closely mimic the physiological conditions of human organs. This is achieved by integrating cell cultures within microfluidic channels and structures. These systems provide the benefits of a microenvironment that closely resembles physiological conditions and utilize human cell lines that have been extensively studied and characterized. Out-of-cell culture systems possess the inherent capability of parallelization and enhanced throughput, rendering them highly advantageous in drug screening. Nevertheless, constructing organic optoelectronic devices necessitates utilizing advanced manufacturing techniques and selecting meticulously chosen materials. Cell cultures are immobilized on substrates and structures in OOC devices. These chip systems’ cells can be divided into three major categories: primary, immortalized, and stem cells. Primary cells are taken straight from tissues or organs without being altered. They closely mirror their in vivo counterparts’ appearance and metabolism. Primary cells must be obtained, kept alive, and only cultivated temporarily.
Additionally, standardization might be challenging due to differences in cell populations and traits between extractions. Immortalized cells are standardized, easily accessible, and well-characterized cells. They can be made from clinical malignancies or by modifying original cells chemically or virologically so that continuous cell division lasts for a long time. However, relative to their initial in vivo state, the cells’ phenotype may change during immortalization. Because of their physiological properties and regulated differentiation potential, stem cells hold great promise. Induced pluripotent stem cells, produced by reprogramming adult tissues to produce pluripotent stem cells, are becoming increasingly popular due to ethical considerations and the restricted availability of embryonic stem cells. These cells can be differentiated into multiple cell types, facilitating research like personalized drug testing and autologous tissue engineering. In 2012, induced pluripotent stem cells’ discovery was given the Nobel Prize.
2.3.2. Organ Systems on Chips
Due to demographic shifts and the rising demand for new pharmaceuticals in pharmaceutical research, society is exposed to an increasing number of novel chemicals in today’s modern, globalized world. Reliable testing methods are required to ensure these substances are safe and effective. Animals were used mostly in the early toxicological, pharmacological, and environmental testing stages. The 3R approach (reduction, refinement, and replacement of animal experiments) has prompted a move toward alternative techniques. The development of alternative techniques has been encouraged by regulatory bodies like the European Parliament and the Council of the European Union, which has led to the EU’s prohibition on cosmetics containing chemicals that have undergone animal testing. According to industrial firms, alternative approaches offer the potential to advance fundamental research, medicine development, toxicity testing, and environmental studies. Excellent throughput screening, parallelization, excellent data quality, predictability in clinical trials, and cost savings are among the alternatives they are looking for to eliminate the usage of animals. Common in vitro systems, however, cannot fully satisfy all of these demands, which has increased demand for enhanced in vitro models and cutting-edge OOC technologies.
2.3.3. Two-Dimensional Cell Culture to OOC
Early in vitro cell culture models were two-dimensional (2D), but it soon became clear how important three dimensions were. Cell morphology and metabolism were improved by 3D cell culture employing extracellular matrix (ECM) components [73][358]. Predictability was improved by creating cell–cell interfaces by integrating various cell types onto the semiconductor. Microsystems, inspired by developments in the semiconductor industry, permitted controlled trials with smaller drug doses. They made it possible to imitate in vivo circumstances by precisely controlling the biological milieu and inducing physiological pressures or gradients. Surface alterations made possible by microtechnology also encourage cell self-organization. These developments boosted predictability and complexity without raising variability. For widespread usage in pre-clinical studies, OOC systems must accomplish simplicity, dependability, reproducibility, and ease of use.
2.3.4. Single OOC (SOOC)
Researchers have pursued the development of integrated body systems on a chip through a systematic approach, wherein the initial focus has been on creating individual organ chips that can subsequently be interconnected. The development of these SOOCs was facilitated through the utilization of microchip technology and the progress made in the semiconductor industry. The lung-on-chip was among the initial organ chips documented in Science magazine in 2010, garnering considerable interest [74][359]. Subsequently, many biomimetic organ systems on chips have been successfully developed. There are several SOOC systems, including liver-on-chip [75][360], kidney-on-chip [76][77][361,362], lung-on-chip [77][362], gut-on-chip [78][363], heart-on-chip [78][363], muscle-on-chip [79][364], blood–brain barrier-on-chip [80][365], splenon-on-chip [81][366], bone marrow-on-chip [82][367], etc.
2.3.5. Multi-OOC (MOOC)
The utilization of MOOCs represents an interim measure in investigating inter-organ interactions until a fully functional human organ system is realized on a chip. The integration of multiple organ chips enables the examination of intercellular communication and the assessment of different stages of drug metabolism. Two primary approaches exist to construct multi-organ chips: the linkage of pre-existing single-organ chips and the integration of multiple organs into a singular chip device. The latter methodology has been proposed by the TechnischeUniversität Berlin and TissUse GmbH, commencing with a biotechnological device that integrates two distinct compartments, namely the liver and skin [83][368]. The utilization of a chip system, comparable in size to a conventional microscope slide, facilitated enhanced spatial efficiency and ensured appropriate ratios of physiological fluid to tissue. Researchers have made progress in the step-by-step integration of supplementary organs, such as the small intestine, liver, renal secretion, and skin biopsies, thereby advancing the development of a comprehensive human-on-chip system [84][85][369,370].
2.3.6. Human-on-Chip (HOC)
The primary objective of OOC technology is to develop a human-on-chip (HOC) model that replicates the functionalities of several vital organs within a singular microfluidic platform. Numerous governmental initiatives, including those sponsored by the American Defense Advanced Research Projects Agency (DARPA) and the National Institutes of Health (NIH), provide financial backing for research endeavors in this particular domain. There exist two primary approaches in designing an HOC system: the first involves the interconnection of individual single-organ chips, while the second entails the integration of distinct organ compartments onto a single chip. It is imperative to surmount the obstacles encountered in engineering and implementation to attain precise emulation of physiological conditions and dependable predictions of drug effects [86][371]. One of the primary challenges in this context involves selecting appropriate cell types and mediums while also considering immune responses and the inherent variability in blood composition. Streamlining the culture conditions and chip construction is advisable to enhance the results’ clarity. Additionally, implementing a modular plug-and-play system could facilitate the interconnection of compatible chips in subsequent endeavors [87][372]. The active participation of the pharmaceutical industry and regulatory agencies is imperative to achieve successful development and validation of organ systems on chips.
2.3.7. Patient-on-Chip (POC)
Stem cells are a type of cellular entity characterized by their undifferentiated state and their capacity to differentiate into diverse specialized cell lineages. There are two primary classifications of stem cells: embryonic stem cells, which are obtained from embryos, and adult stem cells, which are sourced from adult tissues. The utilization of embryonic stem cells in research is hindered by ethical considerations, thus leading to the prevalent use of human-induced pluripotent stem cells (HiPSCs) as a viable substitute. HiPSCs are derived through reprogramming mature cells, acquiring characteristics akin to embryonic stem cells. This reprogramming enables HiPSCs to undergo differentiation into diverse cell lineages upon exposure to specific molecular cues. HiPSCs present a multitude of benefits in the realm of scientific investigation and medical interventions. One potential application of these technologies is the generation of patient-specific tissues for tissue engineering purposes and facilitating patient-specific drug testing. These advancements have the potential to enhance the field of personalized medicine. Incorporation of patient-derived HiPSCs into OOC systems presents a promising approach that synergistically harnesses the advantages of microfluidic technology and genetically compatible human cells. This methodology enables the replication of pathological conditions and genetic variations, enhancing the applicability and precision of drug testing and research endeavors. In addition, utilizing HiPSC technology holds promise in facilitating the advancement of a comprehensive HOC platform. Utilizing HiPSCs derived from a single donor to generate diverse organ tissues can potentially mitigate the occurrence of immune reactions. Furthermore, examining patient-specific variables, including genetic factors, age, gender, and ethnicity, can be readily conducted, thereby facilitating the development of more individualized therapeutic strategies in subsequent endeavors [88][373]. In general, HiPSCs exhibit considerable potential in facilitating the progression of scientific inquiry and pharmaceutical innovation, culminating in enhanced medical interventions that are more efficacious and tailored to individual patients [89][90][91][374,375,376].
2.3.8. Applications of OOC
Using OOCs exhibits significant potential as in vitro testing platforms for diverse applications. They possess utility in toxicity assessment for cosmetics and chemicals, rendering them indispensable in novel and generic drug advancement alongside specialized domains such as radiation examination. A substantial transformation in pre-clinical test practices toward enhanced efficiency and accuracy can only be achieved through collaborative endeavors. Once successfully designed and validated, OOC systems will substantially impact pharmaceutical research and development. These methods can lessen the need for animal testing and produce more trustworthy outcomes for biowaiver studies for generic formulations and medication development. This enhancement will result in more accurate clinical study forecasts and fewer late-stage failures. Additionally, OOC systems will create new possibilities for pharmacological R&D. They will make it possible to simulate sick creatures, giving researchers a controlled environment to investigate the causes of disease and potential cures. Additionally, custom chips can be produced to customize drug testing for specific patients, resulting in more efficient and individualized therapy. Organ systems on chips can potentially advance pharmaceutical research and significantly transform pre-clinical testing procedures.
OOC presents exciting possibilities for modeling diseases and developing new medications. Researchers have created disease chips to simulate specific disease states and analyze treatment reactions in a controlled setting. A lung-on-chip system was developed by Huh et al. to simulate pulmonary edema, a potentially fatal condition brought on by inflammation and fluid buildup in the lungs [92][377]. The chip enabled the testing of possible therapeutic substances, including angiopoietin-1 and GSK2193874, and faithfully replicated the effects of interleukin-2 (IL-2) therapy. Nesmith et al. created a bronchial smooth muscle tissue chip to research allergic asthma [93][378]. The IL-13 and acetylcholine exposure successfully caused the chip to mimic the hypercontraction observed in asthmatic patients. The RhoA inhibitor HA-1077 was put to the test by the researchers, and it showed promise as a possible therapeutic candidate for the treatment of allergic asthma. Tumor spheroids, hydrogels, and ECM proteins have all been used to create in vitro cancer models. Microfluidic systems are being investigated to model tumor formation, tumor–tissue interactions, and metastasis to increase physiological relevance. Researchers can lessen their reliance on animal models by using OOCs to test prospective medications and properly analyze cancer causes. These disease-specific OOCs have demonstrated encouraging outcomes when simulating illness states and assessing medication responses. They have the potential to transform pre-clinical testing, lessen the need for animal testing, and enhance therapy approaches for a range of disorders.
2.4. Cancer-on-Chip (COC)
The utilization of specialized multichannel systems in cancer-on-chip (COC) models has emerged as a potent approach for studying the tumor microenvironment (TME) and its involvement in metastasis. The utilization of microfluidic channels enables the replication of tumors’ biochemistry, geometry, and fluidic transport characteristics by these models, thereby facilitating the examination of intricate interactions associated with metastasis [94][379]. The functional COC platforms exhibit superior accuracy and capabilities to traditional models, enabling them to provide significant insights into the TME and cell interactions during metastasis. Invasion, intravasation, extravasation, and angiogenesis are all components of the intricate process known as metastasis. Micrometastases are formed when tumor cells extravasate to colonize other organs after invading the extracellular matrix or vascular endothelium and entering circulation. The endothelial blood vessel wall must be broken during the key phases of intravasation and extravasation. For early diagnosis, prognosis prediction, and treatment planning, microfluidic technology holds promise for isolating and counting circulating tumor cells (CTCs). Microfluidic-based COC models are crucial to examine the complex interactions between tumor cells and the TME during invasion, intravasation, and re-growth in secondary organs. These models offer insights that conventional approaches cannot. The primary TME, circulatory microenvironment, and secondary TME are three different tumor microenvironments that interact during metastasis. For cancer cells to pass the endothelium and enter the bloodstream, the extracellular matrix (ECM) must be broken down. Surviving circulating tumor cells (CTCs) multiply and create secondary cancers in distant organs by adjusting to the local microenvironment. The TME contains various stromal elements, including fibroblasts, immune cells, vessels, and ECM. Microfluidic models that continuously expose tumor cell development to biological fluids in a biologically appropriate microenvironment are used to research cancer. Cancer invasion, intravasation, extravasation, and the evaluation of anti-cancer medications can all be studied with the aid of these models.
COC and tumor-on-chip (TOC) are intricately interconnected and serve as mutually reinforcing methodologies within cancer investigation. The primary objective of TOC models is to gain insights into the behavior and characteristics of tumor cells within a precisely regulated microenvironment. These models frequently employ microfluidic channels and compartments to replicate the biochemical and biophysical stimuli that impact the development and advancement of tumors. TOC models have the potential to enhance their fidelity to the TME by including non-tumor cells, such as stromal components, immune cells, and endothelial cells, thereby increasing their complexity. The COC and TOC models employ microfluidic technology to facilitate the uninterrupted provision of essential nutrients, oxygen, and other factors crucial for cellular proliferation and intercellular communication. By establishing physiologically relevant conditions, these models offer more precise depictions of tumor behavior than traditional in vitro cell culture systems. Furthermore, both methodologies can be employed to screen anti-cancer pharmaceuticals and examine the effectiveness of prospective therapeutic interventions. Figure 2 depicts the basic components of a common TOC. The utilization of a tumor-on-chip system fabricated through 3D printing techniques facilitated the cultivation of cells for an extended duration, thereby replicating the intricate process of nutrient and anti-cancer drug transportation within authentic TMEs [95][380]. The analysis of convective and diffusive transport within the culture chamber was conducted by employing a fluorescent tracer. The GelMA/alginate microbeads were the most efficient in facilitating transport. The microbeads were utilized to cultivate Caco2 cells, and subsequent drug assays mimicking chemotherapy exhibited a rise in cell death and a decline in cellular metabolism. Hypoxic conditions were artificially created within the microspheres, emulating the oxygen-deprived environment typically found in avascular tumors observed in patients. The study showcased the capacity of TOC platforms created through 3D printing for drug testing and examining cancer biology. The increased dimensions of the chip facilitated a higher quantity of biological material that could be used for analysis. The transport characterization demonstrated efficient convective and thoroughly mixed conditions, rendering it a valuable instrument for replicating tumor scenarios and other tissue environments. Additional investigation is warranted to delve into the potential benefits of employing 3D-printed tumor-on-chip systems, specifically regarding their design flexibility and the feasibility of their fabrication and utilization.
Figure 2.
The basic components of TOC.
2.5. Bio-Inspired Wound Healing Dressing Mat
Bio-inspired wound healing dressing mats are a novel class of biomaterials specifically engineered to enhance the process of wound healing by leveraging inspiration from biological systems. These mats are designed to imitate the natural extracellular matrix, creating an environment that promotes cell growth, angiogenesis, and tissue regeneration. These environments provide advantageous conditions for wound healing, diminish the presence of microorganisms, and facilitate the regulated discharge of therapeutic substances such as growth factors and cytokines. Biomaterials derived from natural sources, such as silk proteins (fibroin and sericin), have demonstrated significant promise in wound dressings owing to their biocompatible nature and capacity to stimulate skin tissue regeneration. Integrating regenerative medicine and nanotechnologies offers a potentially effective strategy for tackling the complexities of wound management and promoting improving the healing process.
The integumentary system serves a vital role in numerous physiological processes. However, when the skin becomes compromised due to injuries, it can give rise to significant medical complications, such as heightened morbidity and mortality rates. Non-healing chronic wounds pose a significant challenge, particularly for individuals diagnosed with diabetes, as they may experience limb ulcers that can lead to severe consequences. The principal objective of wound management is to achieve expeditious healing while ensuring both functional and aesthetically satisfactory results. The wound healing process is intricate and encompasses various cellular interactions, secretion of factors, and interactions with the ECM. Comprehending these processes is imperative to formulate efficacious wound management strategies. The impact of diabetes on wound healing is detrimental, highlighting the need for a comprehensive comprehension of the wound environment and pathophysiology to develop more effective strategies for promoting wound healing. Utilizing biomaterials that can release signaling molecules, such as growth factors and cytokines, in a controlled manner has been shown to facilitate the process of angiogenesis and tissue regeneration. The development of effective biomaterials for tissue repair, including three-dimensional living tissues, has been facilitated by advancements in regenerative medicine, nanotechnologies, and bioengineering. Biomaterials derived from biological sources have demonstrated significant potential in treating tissue injuries and enhancing wound healing, owing to their biocompatible nature and capacity to stimulate skin tissue repair [96][381]. The investigation of biomaterials possessing wound-healing properties has been undertaken for diverse purposes in wound management. These biomaterials create advantageous microenvironments that promote cellular proliferation, inhibit microbial colonization, and facilitate the controlled release of therapeutic agents. The recent progress in wound healing approaches has created novel opportunities within the realm of regenerative medicine and tissue engineering.
As a promising biomaterial for tissue repair and regeneration, Bombyxmori’s silk fibroin has attracted much interest [97][382]. Numerous research teams have investigated the potential of silk fibroin to create cutting-edge methodologies for tissue engineering and wound healing applications, either alone or in combination with other materials and processed in various ways. As biomaterials for wound dressing, many forms of silk fibroin, such as hydrogels, sponges, films, and nanofibers, have been suggested. During various stages of wound healing, these materials maintain moist conditions, permit gas permeability, and improve cell responsiveness. Silkworm cocoon goods include fibroin hydrogel, electrospun fibroin, sponge, film, solution, and powder. These products have a variety of uses in bioengineering, particularly in the treatment of wounds.
2.6. Antimicrobial Surface
Using bio-inspired antimicrobial surfaces has generated considerable attention in diverse biomedical contexts owing to their efficacy in combating microbial hazards. These surfaces are influenced by natural defense mechanisms found in plants and microorganisms. They employ light-activated compounds or other biomimetic strategies to generate antimicrobial effects. Within medicine, these surfaces are utilized in various capacities, such as wound dressings, medical implants, and surgical instruments. Their primary function is to mitigate the risk of infections and expedite the healing process. In dentistry, dental implants and orthodontic devices are utilized to minimize bacterial colonization and the formation of biofilms, thereby improving oral health. In addition, implementing bio-inspired antimicrobial coatings on medical equipment and surfaces within hospitals enhances infection control measures and mitigates the potential for healthcare-associated infections. The potential of these bio-inspired antimicrobial surfaces, with their adaptability and inspiration drawn from biological systems, holds significant promise for enhancing healthcare and improving patient outcomes. This is achieved by offering robust protection against microbial pathogens in various biomedical environments.
A range of bio-inspired antimicrobial surfaces have been developed, each exhibiting unique mechanisms of action. Certain surfaces are designed to mimic the micro/nanostructures observed in natural entities such as cicada wings or lotus leaves. These surfaces possess rough and hydrophobic topographies, which effectively hinder the adhesion and colonization of bacteria. Consequently, these surfaces exhibit self-cleaning properties. Some researchers utilize synthetic antimicrobial peptides (AMPs), short chains of amino acids with a wide range of antimicrobial properties. These synthetic AMPs are employed to either disrupt the cell membranes of bacteria or hinder crucial cellular processes. Cationic polymers, which draw inspiration from the positive charge exhibited by antimicrobial peptides (AMPs), interact with bacterial cell membranes with a negative charge. These interactions ultimately disrupt the membrane structure, leading to the demise of the bacterial cells. Moreover, incorporating silver and other metal NPs into surfaces enables the gradual release of antimicrobial ions upon interaction with bacteria. This process disrupts bacterial metabolism and hinders DNA replication. Chitosan-based surfaces, derived from the exoskeletons of crustaceans, offer a biopolymer barrier of natural origin that effectively inhibits bacterial colonization. Mussel-inspired coatings, which utilize polymers functionalized with catechol groups, serve as effective platforms for integrating antimicrobial agents onto diverse surfaces. In addition, photodynamic antimicrobial therapy (PDT) involves the immobilization of light-sensitive agents on various surfaces, which, upon exposure to light, generates reactive oxygen species that effectively eliminate bacteria. In conclusion, using bio-inspired surfaces designed to release bacteriophages, viruses that selectively target and infect bacteria, results in the targeted eradication of bacterial pathogens. Utilizing a wide range of bio-inspired antimicrobial surfaces presents a potential avenue for improving healthcare outcomes and addressing the challenges posed by microbial infections.
2.6.1. Structure-Oriented Surface
Many plants and animals have evolved distinctive surface structures throughout millions of years of evolution, enabling them to endure external threats in difficult environmental circumstances. These organic and synthetic antimicrobial nanostructures, which are crucial to bioengineering, have piqued the curiosity of researchers. These surfaces’ superhydrophobicity and micro/nanotopographies are thought to be responsible for their antibiofouling qualities. Taro leaves, for example, have a unique uneven structural distribution that prevents Gram-negative bacteria from adhering even in humid situations. Staphylococcus aureus has been discovered to resist shark skin’s antibacterial properties. Studies using naturally occurring bactericidal surfaces, such as cicada wings with nanoneedle arrays, have demonstrated the ability to kill bacteria instantly upon direct contact in just 5 min. Dragonfly wings and gecko skin are examples of other species with mechanobactericidal surfaces. Although animal surfaces may have a lower water contact angle than plant surfaces, the antibacterial impact is similar, indicating that hydrophobicity is not the only factor affecting bactericidal effectiveness [98][383]. Creating next-generation bactericidal surfaces with physico-antimicrobial characteristics has drawn heavily on inspiration from nature. Research into naturally occurring nanostructures has sparked a number of ground-breaking innovations. Researchers fabricate artificial nanostructures on various substrates using bottom-up chemical synthesis and top-down multiway etching techniques. These nanostructures, like carbon nanotubes and ZnO nanorods, have physical and mechanical antibacterial properties that can damage bacterial cell membranes and prevent bacterial adherence. Hydrothermal synthesis and chemical deposition are two surface topography coatings and changes that improve the bactericidal effects. It is possible to replicate nanostructures seen in nature, such as those on cicada wings and pitcher plant surfaces, by combining several processes.
One or both probable manifestations of the antibacterial activity of naturally occurring nanostructured surfaces are the biocidal effect (total destruction of the cellular envelope) or the antibiofouling effect (inhibition of bacterial proliferation). The internalization or insertion of NPs that disrupt membrane function (nanotoxicological effects), physical puncturing, physical tearing, and chemical destructive extraction through oxidative stress are just a few of the factors contributing to the killing mechanism of physico-mechanical antibacterial materials. Mechanical antibacterial materials like carbon nanotubes and graphene impact bacterial adherence, internal cell architecture, and cell migration. These substances repeatedly rupture bacterial cells as part of a cumulative process. According to other studies, bacterial cells are prevented from approaching nanocolumn arrays by the height and spacing of the arrays, leading to a contacting physical puncturing mechanism. When the cells try to migrate on the nanocolumns’ surface, they are broken apart. Some scientists suggest that bacteria produce an extracellular polymeric substance (EPS) under external mechanical stress rather than being directly pierced, which causes bacterial membrane damage through strong attachment to nanocolumns. Since diverse materials and structural configurations may have unique antibacterial effects on different microbes, the precise mechanism is still unclear and up for debate. However, the success of physico-antimicrobial surfaces depends on their specific structures, which are required for their antibacterial capabilities. Compared to chemical mechanisms, the physical antibacterial mechanism is typically faster, and the material’s structure is key to generating an efficient antibacterial effect.
2.6.2. Peptide-Based Surface
Antimicrobial peptides (AMPs) exhibit considerable potential as viable therapeutic options for various diseases, particularly in combating multidrug-resistant bacteria. The global emergence of antibiotic resistance has garnered significant attention, prompting the exploration of alternative solutions, such as AMPs that possess wide-ranging antimicrobial properties. AMPs are synthesized by diverse organisms, identifying more than 5000 distinct AMPs to date [99][384]. These substances exhibit a specific mode of action by selectively interacting with microbial membranes, resulting in the formation of pores and ultimately leading to the demise of bacteria. Moreover, AMPs exhibit anti-inflammatory, regenerative, and anti-cancer characteristics.
Nevertheless, despite their considerable potential, AMPs encounter certain obstacles in their application. These challenges encompass the toxicity exhibited toward mammalian cells, vulnerability to proteases, and the high costs associated with their production methods. To tackle these concerns, there have been suggestions for using nanotechnology-based delivery methods to augment the stability and biological efficacy of AMPs. The development of bio-inspired NPs has been undertaken to preserve the activity of AMPs while mitigating any potential adverse effects. In addition, AMPs have been employed as surface coatings on implants to mitigate the risk of implant-related infections and promote bone regeneration. AMPs have demonstrated potential in cancer therapy due to their ability to specifically target malignant cells and facilitate the delivery of cancer medications or nucleic acids. AMP-based materials have demonstrated high efficacy in condensing and delivering nucleic acids, thereby protecting against degradation.
2.6.3. Metal/Metal Oxide NP-Based Surface
The wide range of features that metal and metal oxide NPs possess, such as non-toxicity, antibacterial activity, and anti-insecticidal activity, make them useful in the biomedical industry for identifying and treating serious illnesses [100][385]. Different bio-inspired metals and metal oxide NPs are essential for maintaining life processes, and deficiencies in these substances can cause diseases. For instance, Co NPs exhibit good magnetic, optical, and mechanical properties, making them useful for biomedical applications like magnetic resonance imaging (MRI) and drug delivery, while nanoceria, despite its lack of stability in living systems, shows promising applications in battling cancer and Alzheimer’s disease. Other NPs, such as those made of Au, Ag, Fe, MgO, Ni, Se, and ZnO, also exhibit distinctive properties that can be used in energy storage, biosensing, imaging, and therapies. Chemoresistive nanosensors are created using nanomaterials such as nanorods, nanotubes, and nanobelts, broadening the range of biomedical applications. Overall, diverse features of metal and metal oxide NPs hold considerable promise for increasing biomedical research and healthcare.
2.6.4. Chitosan-Based Surfaces
The antibacterial properties of chitosan have been thoroughly investigated for various uses in the biomedical, cosmetic, food, and agricultural industries. Researchers have studied its usage in self-preserving materials, which have created various goods with antibacterial qualities, including beads, films, fibers, membranes, and hydrogels. Studies on the antimicrobial effects of chitosan have changed over the past 20 years, moving from studies on foodborne and soilborne pathogenic fungi to studies on bacteria, with varied assays and methodologies revealing the underlying mechanisms and factors determining its efficiency. Chitosan’s potential as an antibacterial agent has been further boosted by the development of nanotechnology, which has made it possible to create materials with nanostructures that are more effective at the atomic level [101][386]. Although the precise mechanism underlying chitosan’s antibacterial activity is not entirely understood, many independent factors have an impact. According to the major hypothesized mechanism, Chitosan adhering to the bacterial cell wall causes cell disruption, changes in membrane permeability, and inhibition of DNA replication, which results in cell death. Chitosan’s polycationic structure, which interacts electrostatically with the anionic components of microbial surfaces, is essential to the substance’s antibacterial effect. Additionally, the shape and size of chitosan particles can affect how they interact with bacterial cell surfaces, with larger NPs behaving differently from smaller ones. Chitosan also has antifungal properties that limit spore germination and radial growth in fungi. Studies have demonstrated its effectiveness against several fungi linked to food and plant rotting. Additionally, chitosan can activate enzymes called chitinases in plant tissues, which act on various fungus species.
2.6.5. Mussel-Inspired Antimicrobial Coatings
Mussel-inspired coatings are developed from the adhesive properties of mussel foot proteins in marine mussels [102][387]. The proteins in question facilitate the strong attachment of mussels to diverse surfaces within moist and turbulent environments, such as coastal areas in the ocean. Researchers have successfully replicated the bioadhesive chemistry found in mussel foot proteins, resulting in coatings exhibiting strong surface adhesion and antimicrobial characteristics. The fundamental operational principle underlying mussel-inspired coatings centers on integrating catechol molecules. Catechol is a prevalent chemical functional group abundantly present in mussel foot proteins. This collective facilitates robust and enduring adherence to various surfaces, encompassing metals, polymers, ceramics, and even biological tissues. The coatings under consideration utilize catechol groups to establish a durable attachment mechanism by forming covalent and non-covalent bonds with the desired substrate. The antimicrobial properties of these coatings are derived from the distinctive amalgamation of adhesive chemistry and the intrinsic characteristics of catechol. The application of these coatings demonstrates a high level of efficacy in inhibiting bacterial colonization and the formation of biofilms. The presence of adhesive catechol groups results in the disruption of microbial cell membranes, causing destabilization of the membranes and subsequent leakage of vital cellular constituents. Furthermore, the surface roughness and hydrophobicity of the coatings also impede the attachment and proliferation of bacteria. Mussel-inspired antimicrobial coatings significantly advance healthcare outcomes and biomedicine by improving biocompatibility, preventing infections, and supporting tissue regeneration.
2.6.6. Bacteriophage-Based Antimicrobial Surface
Bacteriophages are widespread and are viruses that attack bacterial cells. When Ernest Hankin examined the waters of the Ganges and Jamuna Rivers in India in 1896, he discovered the initial signs of bacterial parasites in the environment. Hankin showed an unidentified material in the river water that has antibacterial capabilities against Vibrio cholerae, without specifically identifying phages. While working with Bacillus subtilis two years later, Russian bacteriologist Nikolay Gamaleya noticed a comparable incident [103][388]. The immobilization of bacteriophages plays a crucial role in advancing biotechnologies, presenting new prospects for detecting pathogenic microorganisms at minimal concentrations, developing materials possessing distinctive antimicrobial characteristics, and facilitating fundamental research on bacteriophages. Bacteriophages of indigenous origin exhibit a notable propensity for a particular bacterial species, and in some cases, a discernible subspecies, primarily due to the recognition of epitopes on the capsid proteins [104][389]. By means of chemical or genetic modifications, the binding specificity can be modified, thereby enabling redirection toward a diverse range of substrates and analytes beyond the scope of bacteria. Therefore, the attachment of bacteriophages to flat and particulate surfaces is a rapidly growing area of significant scientific fascination [105][390].
