Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Julie Jessop and Version 2 by Peter Tang.
Raman spectroscopy provides the flexibility and resolution needed for in-situ and real-time monitoring of a wide variety of photopolymerization systems, as well as characterization of polymers resulting from these inherently fast and energy-efficient reactions.
- Raman confocal microscopy
- free-radical photopolymerization
- cationic photopolymerization
1. Introduction
Photopolymerization systems provide compelling advantages for industrial applications due to their fast reaction kinetics, wide selection of monomers for physical property development, and energy-efficient initiation via illumination [1][2][1,2]. These same advantages can present challenges when attempting to monitor these reactions or characterize their resulting polymers; however, Raman spectroscopy can provide the flexibility and resolution needed. In particular, the commercial availability of fiber-optic-based Raman systems has opened the door for real-time and in situ monitoring of photopolymerizations for industrial and academic scientists and engineers alike.
Raman spectroscopy is based upon the rotational and vibrational transitions in molecules and is particularly well suited for the detection of chemical bond changes during polymerization [3][4][5][6][3,4,5,6]. The short intrinsic time scale of this method enables the monitoring of rapid reactions in real time. In addition, minimal sample preparation is required, and a variety of sample geometries may be analyzed (such as thin films or thick samples), as Raman spectroscopy is based on light scattering principles rather than absorption or transmission. Finally, improved instrumentation allows for enhanced signal collection. Modern detectors are significantly more sensitive, and lasers enable the isolation of one wavelength, which produces a clearer, stronger Raman spectrum.
Raman spectroscopy has proven valuable in polymer identification, reaction monitoring, and studies of polymer composites, blends, coatings, and orientated films [4][7][4,7]. However, with photopolymerization systems, the need for an illumination source to initiate the chain reaction adds complexity when attempting to apply an analytical technique to follow the reaction in real time. Adaptations to heat-based analytical techniques, such as differential scanning calorimetry [8], optical pyrometry [9], and thin-film calorimetry [10], are straightforward. In these techniques, an illumination source is mounted above the sample, and the heat flow from the exothermic reaction is measured and directly related to the rate of polymerization. Adaptations to spectroscopy-based analytical techniques, such as infrared [11], fluorescence [12], and Raman spectroscopy [6], require more finesse. In these techniques, the illumination source must be placed in such a way as to minimize or prevent its photons from impinging upon the sensitive detectors (e.g., perpendicular to the beam used to generate the spectroscopic signal). The resulting spectra must be analyzed to draw a direct relationship between spectral feature changes due to the reaction and the conversion from monomer to polymer.
Although the heat-based analytical techniques are relatively easy and inexpensive, spectroscopy-based techniques can provide much more information and resolution. For example, Raman spectroscopy can be used to calculate the conversion of the methacrylate (via free-radical photopolymerization) and of the epoxide (via cationic photopolymerization) during a hybrid photopolymerization since the peaks associated with each reactive group can be separately processed [13]. With photo-differential scanning calorimetry (PDSC), the exotherms from the simultaneous reactions overlap, making it impossible to determine the individual conversion of the two monomer types.
Vibrational spectroscopies, such as infrared (IR) and Raman spectroscopy, are especially useful analytical techniques for photopolymerization systems in that both qualitative (i.e., what is present?) and quantitative (i.e., how much is present?) information is provided. The two techniques rely on different mechanisms: Raman is a scattering technique based on the polarizability of molecular bonds, and IR is an absorption technique based on the dipole moment of molecular bonds [3]. Thus, the two are considered complementary techniques in that some spectral information is provided by both, while other information is only discernable through one or the other technique. For example, both techniques can be used to calculate the conversion of methacrylate monomers; however, Raman is a better choice for aqueous systems since water is Raman-inactive but can obscure important spectral details in IR measurements [14][15]. Because the Raman effect is comparatively small (only one in a billion photons results in the Raman scattering effect), it has often been overlooked as a practical analytical technique.
2. Raman Measurements in Photopolymerization Systems
2.1. Conversion Measurements
Conversion measurements are the most straightforward and common kinetic values obtained through Raman spectroscopy. The calculation requires identification of a reaction (rxn) peak, which is directly related to the reactive moiety on the monomer, and a reference (ref) peak, which is associated with molecular bonds not affected by the polymerization. Conversion is then calculated from the ratio of the peak intensity (or peak area) of these reaction and reference peaks: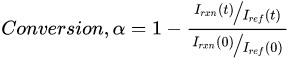 where I(t) denotes the peak intensity at time t, and I(0) represents the initial peak intensity before photopolymerization begins. This ratio method corrects for density changes and instrumental variations and is necessary when making conversion comparisons at different sampling times (e.g., in photopolymerized-methacrylate-based dental adhesive resins before and after water storage [15][18]).
Suitable reference and reaction peaks can be identified through Raman reference books [16][17][19,20], literature values, and/or experiments. The reaction peak is relatively standard for each type of reactive moiety (e.g., ~1640 cm−1 for C=C bonds in (meth)acrylates and ~790 cm−1 for cycloaliphatic epoxides); however, the reference peak differs depending on the structure of the monomer. For example, 3-ethyl-3-phenoxymethyl oxetane (POX) includes a phenyl group that provides a strong reference peak at 980 cm−1, while 3-ethyl-3-[(2-ethylhexyloxy)methyl] oxetane (EHOX) has aliphatic groups that present a reference peak at 1450 cm−1 [18][21]. Both monomers contain the oxetane moiety, which provides the reaction peak at 1150 cm−1. Ideally, the reference and reaction peaks should be close to one another so that they are affected similarly by any spectral perturbations (fluorescence, changes in density and refractive index during polymerization, etc.). Thus, they both are typically selected from the fingerprint region (i.e., 200–1800 cm−1). In addition, the reference peak and reaction peaks should be selected from bonds that are present on the same molecule to avoid issues due to concentration gradients or inhomogeneities in the sample.
To verify suitability of reaction and reference peak selections, a conversion calibration curve relating the monomer mass fraction to the Raman intensity ratio (in Equation (1)) can be constructed by dissolving various amounts of polymer in its monomer (e.g., polyhydroxyethylmethacrylate (PHEMA) in HEMA [15][18]). However, most systems do not have commercially available polymers, and higher mass fractions of polymers take a long time to dissolve in the monomer. Thus, real-time Raman spectroscopy of the photopolymerization provides a more feasible confirmation that the choice of reference and reaction peaks is appropriate. Visual inspection of a 3-D waterfall plot is a facile way to verify that the reaction peak decreases with illumination time, while the reference peak remains constant. This confirmation is important since Raman peaks attributed to non-reactive bonds adjacent to the reactive moiety may change during the reaction. For example, the (meth)acrylate carbonyl peak at 1720 cm−1 decreases with reaction of the C=C; thus, it is a poor reference peak but can be effectively used as an apparent reaction peak [19][25]. If conversion measurements are calculated from real-time Raman spectra with a stable baseline, then Equation (1) can be simplified as follows since Iref(t)/Iref(0) ≈ 1 [20][26]:
where I(t) denotes the peak intensity at time t, and I(0) represents the initial peak intensity before photopolymerization begins. This ratio method corrects for density changes and instrumental variations and is necessary when making conversion comparisons at different sampling times (e.g., in photopolymerized-methacrylate-based dental adhesive resins before and after water storage [15][18]).
Suitable reference and reaction peaks can be identified through Raman reference books [16][17][19,20], literature values, and/or experiments. The reaction peak is relatively standard for each type of reactive moiety (e.g., ~1640 cm−1 for C=C bonds in (meth)acrylates and ~790 cm−1 for cycloaliphatic epoxides); however, the reference peak differs depending on the structure of the monomer. For example, 3-ethyl-3-phenoxymethyl oxetane (POX) includes a phenyl group that provides a strong reference peak at 980 cm−1, while 3-ethyl-3-[(2-ethylhexyloxy)methyl] oxetane (EHOX) has aliphatic groups that present a reference peak at 1450 cm−1 [18][21]. Both monomers contain the oxetane moiety, which provides the reaction peak at 1150 cm−1. Ideally, the reference and reaction peaks should be close to one another so that they are affected similarly by any spectral perturbations (fluorescence, changes in density and refractive index during polymerization, etc.). Thus, they both are typically selected from the fingerprint region (i.e., 200–1800 cm−1). In addition, the reference peak and reaction peaks should be selected from bonds that are present on the same molecule to avoid issues due to concentration gradients or inhomogeneities in the sample.
To verify suitability of reaction and reference peak selections, a conversion calibration curve relating the monomer mass fraction to the Raman intensity ratio (in Equation (1)) can be constructed by dissolving various amounts of polymer in its monomer (e.g., polyhydroxyethylmethacrylate (PHEMA) in HEMA [15][18]). However, most systems do not have commercially available polymers, and higher mass fractions of polymers take a long time to dissolve in the monomer. Thus, real-time Raman spectroscopy of the photopolymerization provides a more feasible confirmation that the choice of reference and reaction peaks is appropriate. Visual inspection of a 3-D waterfall plot is a facile way to verify that the reaction peak decreases with illumination time, while the reference peak remains constant. This confirmation is important since Raman peaks attributed to non-reactive bonds adjacent to the reactive moiety may change during the reaction. For example, the (meth)acrylate carbonyl peak at 1720 cm−1 decreases with reaction of the C=C; thus, it is a poor reference peak but can be effectively used as an apparent reaction peak [19][25]. If conversion measurements are calculated from real-time Raman spectra with a stable baseline, then Equation (1) can be simplified as follows since Iref(t)/Iref(0) ≈ 1 [20][26]:
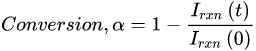 However, if the baseline changes during the photopolymerization or if conversion measurements are calculated at discrete points in time (e.g., Raman spectra are collected at regular intervals after shuttering the light source to determine dark cure in epoxides [21][14]), the reference peak values must be included in the conversion equation.
Once Raman spectra have been collected, the data must be transformed to obtain conversion measurements. Software packages included with the Raman instrument are often helpful with this process. For each spectrum, a spectral range can be selected for each peak, and either the peak intensity or the peak area can be automatically calculated. In some software packages, these peak measurements can be mathematically manipulated to calculate conversion using Equation (1). Otherwise, the peak measurements can be exported to a spreadsheet (e.g., Excel) for the conversion calculation. If the reaction or reference peak is not well resolved or on a similar magnitude as the noise in the spectrum, then a more rigorous data processing method may be required, such as taking the second derivative of the spectrum [22][27]. For optimal results, the pre-polymerization ratio, Irxn(0)/Iref(0), must be reliable; therefore, extra care must be taken in collecting these data. Longer exposure times and multiple accumulations should provide an average spectrum with an appropriately high signal-to-noise ratio (S/N). When processing real-time data, some smoothing of the final profiles may be necessary (e.g., a five-point moving average) since lower concentrations have small peak intensities, which introduce noise in the conversion data [20][26].
Conversion data can be used directly to study the effect of formulation choices and processing variables on kinetic outcomes. Because individual reactive moieties can often be resolved, Raman monitoring is useful beyond homopolymerizations for more complicated systems involving copolymerization or hybrid polymerization. For example, improved kinetic outcomes were realized through comonomer systems of 3,4-epoxycyclohexylmethyl-3′,4′-epoxycyclohexanecarboxylate (EEC) and epoxidized polybutadiene oligomer (EPOH) [23] and of EEC and oxetanes [24]. In addition, the synergy between free-radical and cationic-ring-opening photopolymerizations has been characterized for epoxide–acrylate hybrid monomers [13][25][13,28] and systems [20][26], as well as oxetane–acrylate hybrid systems [26][29]. Likewise, the impact of other formulation components such as photoinitiators, impurities, and additives can be explored. For example, in cationic photopolymerizations, photoinitiator concentration [13], photoinitiator structure [27][30], water concentration [25][28], and alcohol size and functionality [28][31] play large roles in the extent and rate of polymerization. In free-radical photopolymerizations, examples include demonstrating the effect of crosslinker concentration on conversion for droplet polymerization in a microfluidic device [29][32] and the impact of TiO2 nanoparticle loading on photopolymerization outcomes for acrylate materials intended for micro-optical devices [30][33]. Processing variables that affect conversion results include reaction temperature, spectral output of light source, effective irradiance, illumination time, annealing conditions, etc. For example, a central composite design used conversion as a response to model the effects of effective irradiance, exposure time, and sample depth in shadow cure of EEC [21][14]. Annealing time and temperature have also been optimized for ECC and another diepoxide based on conversion outcomes [31][34].
However, if the baseline changes during the photopolymerization or if conversion measurements are calculated at discrete points in time (e.g., Raman spectra are collected at regular intervals after shuttering the light source to determine dark cure in epoxides [21][14]), the reference peak values must be included in the conversion equation.
Once Raman spectra have been collected, the data must be transformed to obtain conversion measurements. Software packages included with the Raman instrument are often helpful with this process. For each spectrum, a spectral range can be selected for each peak, and either the peak intensity or the peak area can be automatically calculated. In some software packages, these peak measurements can be mathematically manipulated to calculate conversion using Equation (1). Otherwise, the peak measurements can be exported to a spreadsheet (e.g., Excel) for the conversion calculation. If the reaction or reference peak is not well resolved or on a similar magnitude as the noise in the spectrum, then a more rigorous data processing method may be required, such as taking the second derivative of the spectrum [22][27]. For optimal results, the pre-polymerization ratio, Irxn(0)/Iref(0), must be reliable; therefore, extra care must be taken in collecting these data. Longer exposure times and multiple accumulations should provide an average spectrum with an appropriately high signal-to-noise ratio (S/N). When processing real-time data, some smoothing of the final profiles may be necessary (e.g., a five-point moving average) since lower concentrations have small peak intensities, which introduce noise in the conversion data [20][26].
Conversion data can be used directly to study the effect of formulation choices and processing variables on kinetic outcomes. Because individual reactive moieties can often be resolved, Raman monitoring is useful beyond homopolymerizations for more complicated systems involving copolymerization or hybrid polymerization. For example, improved kinetic outcomes were realized through comonomer systems of 3,4-epoxycyclohexylmethyl-3′,4′-epoxycyclohexanecarboxylate (EEC) and epoxidized polybutadiene oligomer (EPOH) [23] and of EEC and oxetanes [24]. In addition, the synergy between free-radical and cationic-ring-opening photopolymerizations has been characterized for epoxide–acrylate hybrid monomers [13][25][13,28] and systems [20][26], as well as oxetane–acrylate hybrid systems [26][29]. Likewise, the impact of other formulation components such as photoinitiators, impurities, and additives can be explored. For example, in cationic photopolymerizations, photoinitiator concentration [13], photoinitiator structure [27][30], water concentration [25][28], and alcohol size and functionality [28][31] play large roles in the extent and rate of polymerization. In free-radical photopolymerizations, examples include demonstrating the effect of crosslinker concentration on conversion for droplet polymerization in a microfluidic device [29][32] and the impact of TiO2 nanoparticle loading on photopolymerization outcomes for acrylate materials intended for micro-optical devices [30][33]. Processing variables that affect conversion results include reaction temperature, spectral output of light source, effective irradiance, illumination time, annealing conditions, etc. For example, a central composite design used conversion as a response to model the effects of effective irradiance, exposure time, and sample depth in shadow cure of EEC [21][14]. Annealing time and temperature have also been optimized for ECC and another diepoxide based on conversion outcomes [31][34].
2.2. Other Kinetic Data
Conversion profiles can be further manipulated to obtain additional kinetic information. The rate of polymerization (Rp) is calculated from the first derivative of the conversion profile. Because experimental data are often noisy, the derivative of the best fit trendline is used rather than individual data points. For example, a triple exponential expression was used for 3,6,9,12-tetraoxatetradeca-1,13-diene (DVE-3) [6], while a four-parameter logistic function was used for EEC [28][31]. From the Rp profiles, the propagation rate constant (kp) can be calculated by rearranging the propagation mechanistic equation: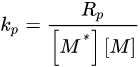 The monomer concentration, [M], is known, and the active center concentration, [M*], can be estimated [6][32][6,36]. In addition, the first-order kinetic rate constant for termination/trapping of active centers (kt/t) can be found by dividing Rp/[M] into its first derivative [32][36]:
The monomer concentration, [M], is known, and the active center concentration, [M*], can be estimated [6][32][6,36]. In addition, the first-order kinetic rate constant for termination/trapping of active centers (kt/t) can be found by dividing Rp/[M] into its first derivative [32][36]:
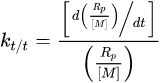 Moreover, real-time Raman conversion data can be used to calculate reactivity ratios (ri) for photopolymerizations of comonomer formulations [18][21]. These conversion data enable calculation of instantaneous mole fractions of the monomer in the feed (fi) and in the copolymer (Fi), from which the reactivity ratios are estimated:
Moreover, real-time Raman conversion data can be used to calculate reactivity ratios (ri) for photopolymerizations of comonomer formulations [18][21]. These conversion data enable calculation of instantaneous mole fractions of the monomer in the feed (fi) and in the copolymer (Fi), from which the reactivity ratios are estimated:
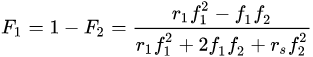 where the subscripts refer to a specific monomer (i.e., monomer 1 and monomer 2) in the formulation.
where the subscripts refer to a specific monomer (i.e., monomer 1 and monomer 2) in the formulation.
