You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Deok-Chun Yang and Version 3 by Lindsay Dong.
The use of in vitro tissue culture for herbal medicines has been recognized as a valuable source of botanical secondary metabolites. The tissue culture of ginseng species is used in the production of bioactive compounds such as phenolics, polysaccharides, and especially ginsenosides, which are utilized in the food, cosmetics, and pharmaceutical industries.
- P. ginseng
- in vitro tissue culture
- ginseng breeding
1. Introduction
Panax species, commonly referred as ginseng, which belong to the Araliaceae family, are slow-growing perennial herbal medicines with adaptive properties [1]. The word ‘Panax’ comes from the Greek word ‘pan’ (all) and ‘zxos’ (treatment of medicine), which means cure-all [2]. There are three commonly used commercial ginseng species, including Panax. ginseng, P. quinquefolius, and P. notoginseng [3][5]. Most of the secondary compounds, especially ginsenosides, have been recorded in the roots. They act as tonic agents and stimulants that have been used in Asian countries for thousands of years, and they are becoming increasingly popular all over the world [4][6]. Pharmacological studies have demonstrated that ginseng species are rich in bioactive compounds such as ginsenosides, polysaccharides, flavonoids, phenolics, and volatile oils [5][7]. Among them, ginsenosides are known as the main bioactive ingredients responsible for the pharmaceutical efficacy of ginseng species [6][8], such as their anti-cancer [7][9], anti-fatigue [8][10], anti-inflammatory [9][11] activity and their prevention of cardiovascular disease [10][12], obesity [11][13], and cerebrovascular diseases [12][14], etc.
However, the prolonged cultivation period, susceptibility to pathogens and replant diseases, limited availability of arable land, and labor-intensive cultivation practices have impeded farmers from meeting the growing market demand [13][15]. Moreover, the use of pesticides and the fluctuating environmental conditions resulting from global warming have compelled researchers and plant scientists to explore alternative methods to meet the demands of a rapidly increasing population [14][17]. Traditionally, there are two sources for obtaining ginseng species, one of which involves harvesting wild ginseng species. However, due to the over-exploitation of wild ginseng species and the destruction of arable land for growing ginseng species, the amount of wild ginseng is decreasing [15][18]. Another origin of ginseng species supply is to grow it in fields or forests, which is a time-consuming and labor-intensive process [16][19]. Furthermore, replanting disease will also result from intensive replanting, where replanting a second time in the same place will often lead to failure [17][20]. For these reasons, ginseng is becoming increasingly difficult to obtain and more expensive.
To address the above problems, tissue culture approaches have developed rapidly in recent years to produce bioactive compounds with high content and activities that not only have health-promoting properties but also significantly alter natural sources. The first attempt at plant cell cultivation was by the Austrian botanist Haberlandt in 1902, who isolated plant cells and cultivated them outside the whole plant [18][21]. The successful development of a nutrient medium by Murashige and Skoog in 1962, commonly known as MS medium, has remained in use, with minor adjustments [19][22]. The introduction of this specific nutrient medium, along with a range of plant growth regulators (PGRs), has significantly revolutionized the field of plant tissue culture research, leading to its successful integration as a viable commercial venture offering numerous advantages and possibilities. Multiple investigations have subsequently demonstrated that undifferentiated plant cells, such as calluses and cell suspensions, can be a valuable resource for producing identical secondary metabolites found in naturally occurring plants. It represents a significant advancement in plant research, over a century after Haberlandt’s initial attempts in the field [20][23]. Plant tissue culture technology is helpful for plant transformation, clonal propagation, breeding, and protection of pharmaceutical plants and crops. Figure 1 describes the history and establishment of ginseng species’ in vitro plant tissue culture [21][22][23][24][25][26][27][24,25,26,27,28,29,30].
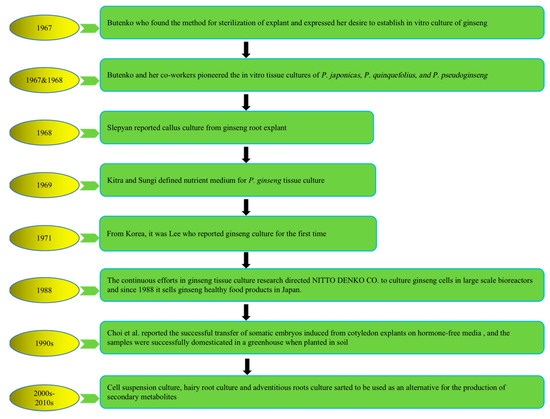

Figure 1. History of in vitro plant tissue culture of ginseng species. Note: This figure shows the in vitro cultivation of ginseng species from 1967 to present.
2. In Vitro Culture of P. ginseng Technologies
Tissue culture is classified based on the purpose of the culture and the source of materials. Several processes were established in P. ginseng based on the organization of the cells and organs (Figure 23), producing a consistent quality of P. ginseng and promoting the sustainable application of the species. In addition, under controlled culture conditions, numerous factors influence the quantity and quality of ginsenosides, such as medium constituents, pH, light conditions, culture temperature, explants, and abiotic factors.
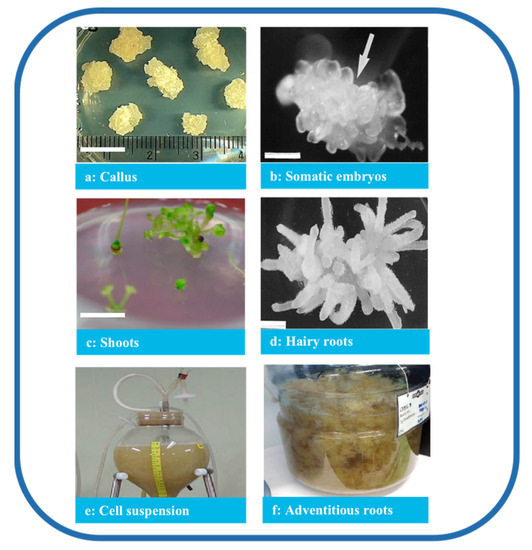

Figure 23. Types of P. ginseng tissue culture. Note: (a) Callus (bar 1 cm) [28][32], (b) somatic embryos (bar 1 mm) [29][33], (c) shoots (bar 1 cm) [28][32], (d) hairy roots (bar 820 μm) [30][34], (e) cell suspension [31][35], and (f) adventitious roots [32][36].
2.1. Direct Organogenesis of P. ginseng
Direct organogenesis is the induction of roots and shoots directly from explants without forming a callus. Shoot culture demonstrated genetic stability and the potential to produce secondary metabolites. However, the research on the direct organogenesis of ginsenoside production is limited. Among the limited research available, it is vital to discuss the work of Hee-Young Lee et al., who studied the regeneration of P. ginseng from embryos obtained from the cultures of anthers. The results from the study indicated the optimum conditions required for the regeneration of P. ginseng—for example, cold treatment matters. The highest callus induction rate was achieved when the explants were cultured post-pretreatment at 4 °C.
On the other hand, the findings also report that the application of PGRs also affects shoot and root production. The shoots and roots can be induced on a medium supplemented with Gibberellin A3 (GA3) and 3-Indolebutyric acid (IBA) at the concentration of 28.9 μM and 14.7 μM, respectively [28][32]. Another study suggested that supplementing naphthaleneacetic acid (NAA) and IBA enhances the organogenic potential. Though IBA attained the highest shoot and root production rates, the roots induced by NAA showed better growth and were thicker than those of IBA. In addition, the roots induced by NAA also attained the highest ginsenosides production rates [33][37].
2.2. Indirect Organogenesis of P. ginseng
Indirect organogenesis, called callogenesis, is regenerating plantlets from the callus. The morphology and characteristics of calluses also influence organogenesis and biomass production. Friable and compact calluses are the two types of callus used in suspension culture and regeneration research, respectively [34][38].
2.2.1. Callus Culture
To date, explants, such as roots, stems, seeds, leaves, buds, petioles, anthers, and hypocotyls, have been used to induce ginseng callus. Among them, the leaves and roots are the most common ones. Typical callus induction and culture are carried out using Murashige and Skoog’s (MS) basic medium or Gamborg medium (B5) with 3% sucrose and various PGRs at different concentrations. Researchers have investigated the effects of PGRs, among which 2,4-Dichlorophenoxyacetic acid (2,4-D) is the most potent one for the induction of the callus of many plant species. A summary of callus cultures is given in Table 12. Generally, the ginseng explants are cultured in the dark at 23 ± 2 °C. Wang et al. successfully induced callus from P. ginseng roots using MS medium supplemented with 2 mg/L of 2,4-D and 0.5 mg/L of Kinetin (KT) [35][39]. Similarly, Chang et al. [36][40] induced callus from ginseng roots using MS medium enriched with 1 mg/L of 2,4-D. However, the growth of the callus was initially slow, with only 1 cm of elongation in diameter after ten weeks. Nevertheless, it grew vigorously when the callus was subcultured on a new medium at 6–8-week intervals. In another study, Liu et al. used 3-year-old fresh ginseng roots as explants to induce callus on a modified MS medium enriched with 2 ppm of 2,4-D, 0.5 ppm of thidiazuron (TDZ), and 1 g/L of peptone [37][41]. They also induced another callus from 2-year-old ginseng roots on MS medium supplemented with 1 mg/L of 2,4-D and 0.1 mg/L of KT, sub-culturing every 15 days. As a result, after six months, they obtained three types of calluses [38][42].
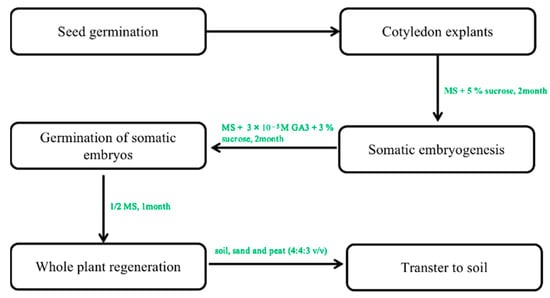
Table 12.
Callus induction and culture of
P. ginseng
.
| Explants | Medium | PGRs | Other Factors | Ref. | |
|---|---|---|---|---|---|
| 2,4-D | KT | ||||
| roots | MS | 2 mg/L | 0.5 mg/L | [35][39] | |
| roots | MS | 1 mg/L | [36][40] | ||
| roots | MS | 2 mg/L | 1 g/Lpeptone, 0.5 mg/L TDZ | [37][41] | |
| roots | MS | 1 mg/L | 0.1 mg/L | [38][42] | |
2.2.2. Somatic Embryogenesis of P. ginseng
Using somatic embryogenesis for propagation allows a quick propagation of the superior ginseng lines while decreasing the variability commonly associated with seed propagation. The first and most crucial step in this process is the transition of somatic cells to embryonic cells. Somatic embryogenesis involves de-differentiating somatic cells into totipotent embryonic stem cells, which can produce embryos under appropriate in vitro conditions, ultimately developing into a whole plant [39][40][43,44]. Several studies have been conducted in ginseng to explore and optimize the conditions necessary for successful somatic embryogenesis [41][45]. In the next stage, they will develop into a whole plant after somatic embryogenesis. In P. ginseng, the first observation of somatic embryogenesis was reported in the callus derived from the roots by Butenko [22][25]. Since then, the regeneration of plants has been achieved through somatic embryogenesis using ginseng calluses derived from roots [36][42][40,46], zygotic embryos [43][47], somatic embryos [44][48], and protoplasts [45][49] isolated from somatic embryos (Table 23). The basic medium provides the nutritional composition and necessary elements for the growth and development of the explants. Most studies have employed MS medium for callus formation, proliferation, and somatic embryogenesis. In addition, Schenk and Hildebrandt medium (SH) and B5 have seldom been used for shoot regeneration and embryoid formation [46][47][50,51]. The in vitro propagation of somatic embryos in P. ginseng has been achieved using 2,4-D, KT, and NAA, but their concentrations and combinations vary depending on the type of explants. Somatic embryo germination requires either chilling treatment for 8 weeks or GA3 hormone treatment at concentrations over 1.0 mg/L. Ultrastructural observation of cotyledon cells showed that without the treatment of chilling or GA3, somatic embryos contained large amounts of lipid reserves, dense cytoplasm, proplastids, and inactive mitochondria. Conversely, after chilling or GA3 treatment, the well-developed chloroplasts and functioning mitochondria with multiple cristae were seen in somatic embryos, indicating they may enter dormancy after maturation, similar to zygotic embryos. Recent studies have reported over 80% plant survival in hybrid ginseng, achieved by culturing embryos on GA3-supplemented medium, transferring them to hormone-free 1/2 SH medium, treating developed taproots with GA3 to break shoot dormancy, and transferring them to the soil. Therefore, GA3 pretreatment is crucial for successful transplantation [48][52]. Other factors, such as the salt content of the medium, also play a crucial role in somatic embryo induction. Choi et al. studied the effects of macrosalt stress on the embryogenesis of P. ginseng. The results showed that the highest frequency of somatic embryogenesis was observed on a medium containing 61.8 mM NH4NO3 with a ratio of NH4+:NO3− at 21:39. Among the test media, including MS, B5, and SH, the maximum formation rate of the somatic embryo was observed when cotyledon explants were cultured on 1% agar MS medium with the supplementation of sucrose at 5% [26][49][29,53]. Different attempts have been made to regenerate ginseng through tissue culture using somatic embryogenesis techniques [50][51][54,55]. However, most regenerated plants cannot survive when transferred to soil. Shoot or multiple shoot formation has been successful from somatic embryos. However, taproots cannot be obtained, as the reproductive capacity of the multi-shoot complex gradually decreases and eventually disappears during long-term subculture (over 12–18 months). This phenomenon was observed in ginseng, where a single somatic embryo can regenerate into a plant with well-developed roots and shoots. In contrast, multiple fused somatic embryos result in multiple shoots [46][50]. Only the study by Choi et al. [26][27][29,30] reported the successful transfer of somatic embryos induced from cotyledon explants on hormone-free media at 12–66% frequency in the regenerated plant. In his study, ginseng plants with well-developed shoots and roots regenerated from single embryos were successfully domesticated in a greenhouse when planted in soil (Figure 34) [52][56]. This regeneration protocol is very effective in the induction of whole plants.
Figure 24. The protocol of somatic embryogenesis from callus to whole plants. Note: This figure shows the indirect organogenesis. First, the optimal callus was selected to form the embryogenic callus. In the next step of somatic embryogenesis, a regenerated whole plant can be obtained under optimal culture conditions.
Furthermore, optimal physical conditions, including light, temperature, and relative humidity, were identified for the in vitro propagation of ginseng species. A photoperiod of 14–16 h per day, with a cold white fluorescent lamp providing a light intensity of 24–80 μmol m−2 s−1, and a temperature of 23 ± 2 °C were found to be appropriate for the incubation and maintenance of cultures [29][49][33,53].
In addition, there is a desperate need for a reliable and fast method to propagate the superior chemotype of ginseng species. For this purpose, it is crucial to establish a fully controlled in vitro micro-saline environment using shakers, temporary soaking, or bioreactors, which will enable the production of healthy and uniform seedlings. Additionally, molecular-marker-assisted protocols are highly recommended for verifying clonal fidelity and ensuring the production of identical clones. A well-known barrier to the effectiveness of plant production is the deterioration of culture vigor and regenerability over time. Various phenotypes, such as changes in plant height, biomass, grain yield, resistance to disease and pests, acid and salt tolerance, and agronomic performance, have all been linked to somaclonal variation. Over the 20 years of ginseng cell subculture, ginsenosides comprised just around 0.024% of the dry weight [53][57]. Raul Sanchez-Muñoz et al. indicated that the fundamental obstacle in creating commercially viable plant biofactories appears to be the alterations in methylation patterns, the primary mechanism predicted to be implicated in yield loss over time [54][58]. Therefore, clone maintenance should be investigated further for stable biomass and secondary metabolites production.
Table 23.
List of the common conditions for somatic embryogenesis of ginseng.
| Explants | Medium | PGRs | Embryogenesis Rate | Other Factors | Ref. |
|---|---|---|---|---|---|
| seeds | MS | 2,4-D+ kinetin/ hormone free |
45%/32.5% | Most of the single embryos were formed on a hormone-free medium, but multiple embryos were formed on a hormone-containing medium. | [46][50] |
| cotyledons | MS | 2,4D+BA+ lactalbumin hydrolysate |
87% | The use of glucose can enhance somatic embryo formation. | [55][59] |
| cotyledon | MS | 61.8 mM of NH4NO3 |
56.3% | The highest frequency of somatic embryo formation occurred in the following order: NH4NO3 > KNO3 > KH2PO4 > MgSO4 > CaCl2. | [26][29] |
| zygotic embryos | MS | 2,4-D+ kinetin | NM | NM | [56][60] |
NM: not mentioned.
