1. Introduction
A thorough understanding of the techniques and design of any process for removal of azo dyes is essential. There are multiple techniques applied for contaminant removal from water/wastewater. These techniques cover all stages of the water/wastewater treatment plant, including initial, primary, secondary, and tertiary treatment
[1][33]. The development of each stage is influenced by the increase in demand for high-quality water. To illustrate, some urban cities and industries treat water to the secondary or even more advanced stage. According to Rajasulochana and Preethy, the development of water treatment techniques is affected by different factors, such as the cost of the process, types of contaminants and their concentration in water, the heterogeneity of discharged pollutants, and the required level of purity
[2][34]. Hence, it is impossible to treat most of the contaminants in a single stage
[2][34]. In general, this overview covers the techniques used in the advanced stages to remove dyes from wastewater. The data obtained from the sources of dyes help to categorize the contaminates associated with water pollution, hence using suitable techniques in the removal process
[1][33]. The water treatment technologies used in dye removal are classified into two types: physicochemical and biological treatment processes
[3][4][5][6][1,5,6,35]. The physicochemical processes involve technologies such as membrane filtration (separation), ion exchange, reverse osmosis, chemical precipitation, coagulation and flocculation, electrochemical treatment, photocatalytic, and adsorption
[7][36]. The treatment techniques can be combined to form hybrid water treatment techniques. Usually, hybrid techniques have more removal efficiency and can solve environmental problems handled by using conventional techniques.
2. Coagulation and Flocculation
Many water treatment plants use coagulation and flocculation as water clarification techniques. Historically, coagulation and flocculation have been used to supply clean water (sweet water)
[8][9][10][37,38,39]. However, coagulation is used for water clarification and not for water purification due to the lack of knowledge about germs and waterborne diseases
[8][9][10][37,38,39]. Coagulation and flocculation must be distinguished from the precipitation process. Basically, the coagulation process is the removal of non-settleable minute particles from an aqueous solution by neutralizing the solution
[10][39]. Usually, coagulation is used to remove colloidal impurities. The addition of coagulants such as alum neutralizes the solution, and then particles flocculate together, forming micro flocs
[10][39]. Moreover, chemicals such as polymers are added to enhance the flocculation and form larger particles known as macro flocs
[9][10][38,39]. In simple words, the basic principle behind coagulation and flocculation is the accumulation of microparticles to form settleable or floatable macro particles. The agglomerated particles are removed from water via sedimentation and filtration.
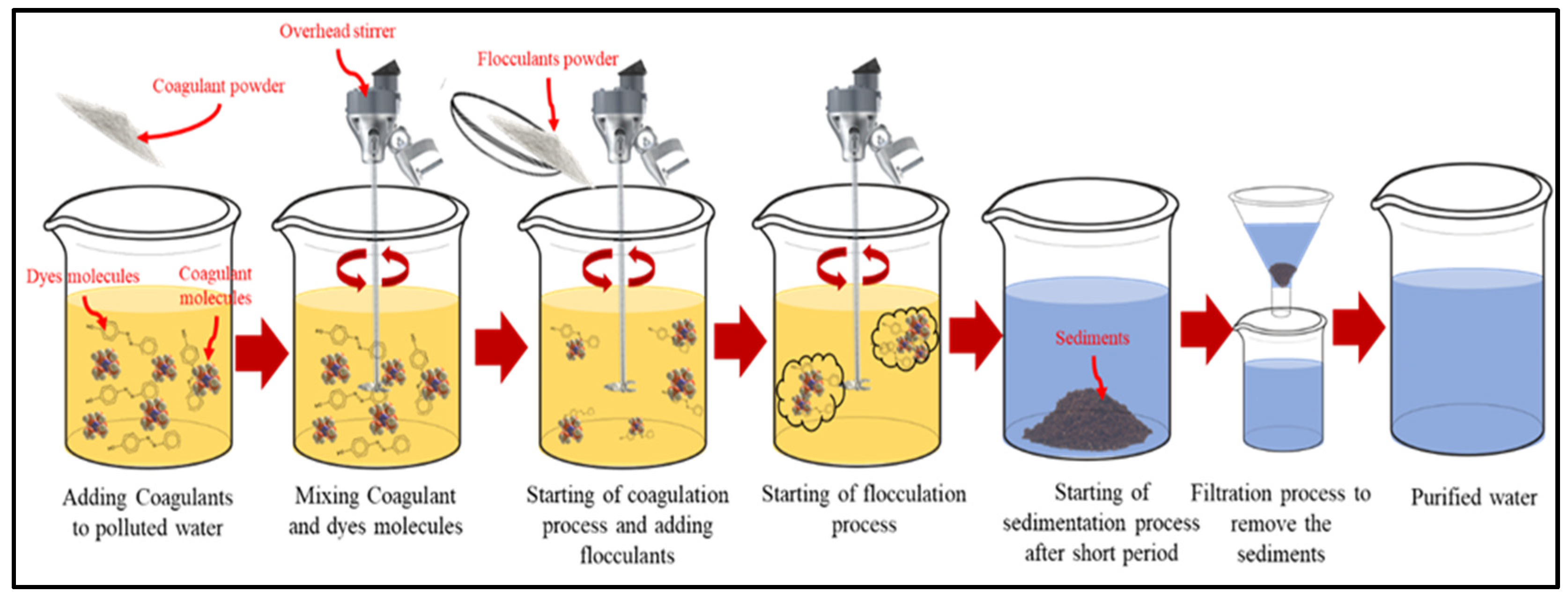
Figure 1 illustrates the steps of the coagulation and flocculation process.
Figure 1. Schematic of Coagulation–Flocculation for textile dye wastewater treatment.
Although coagulation and flocculation are the most used treatment methods, the removal performance of the methods is low compared to other techniques
[10][39]. The efficiency of coagulation is influenced by different factors such as the type and dosage of coagulants/flocculants, stirring speed and time, temperature and settling time, retention time (RT), and pH level of the solution
[10][11][12][39,40,41]. The properties of applied coagulants are the vital factor in determining the coagulation efficiency
[10][12][39,41]. Some of the coagulants used in the water treatment field are magnesium, iron derivatives, lime, and aluminum salt. According to Mathuram et al., ferrous sulphate, ferric chloride, and ferric chloride sulphate showed high efficiency as coagulants in removing several types of dye
[13][42]. Typically, coagulation is mixed with other water treatment techniques such as electrochemical processes and adsorption to enhance the treatment efficiency
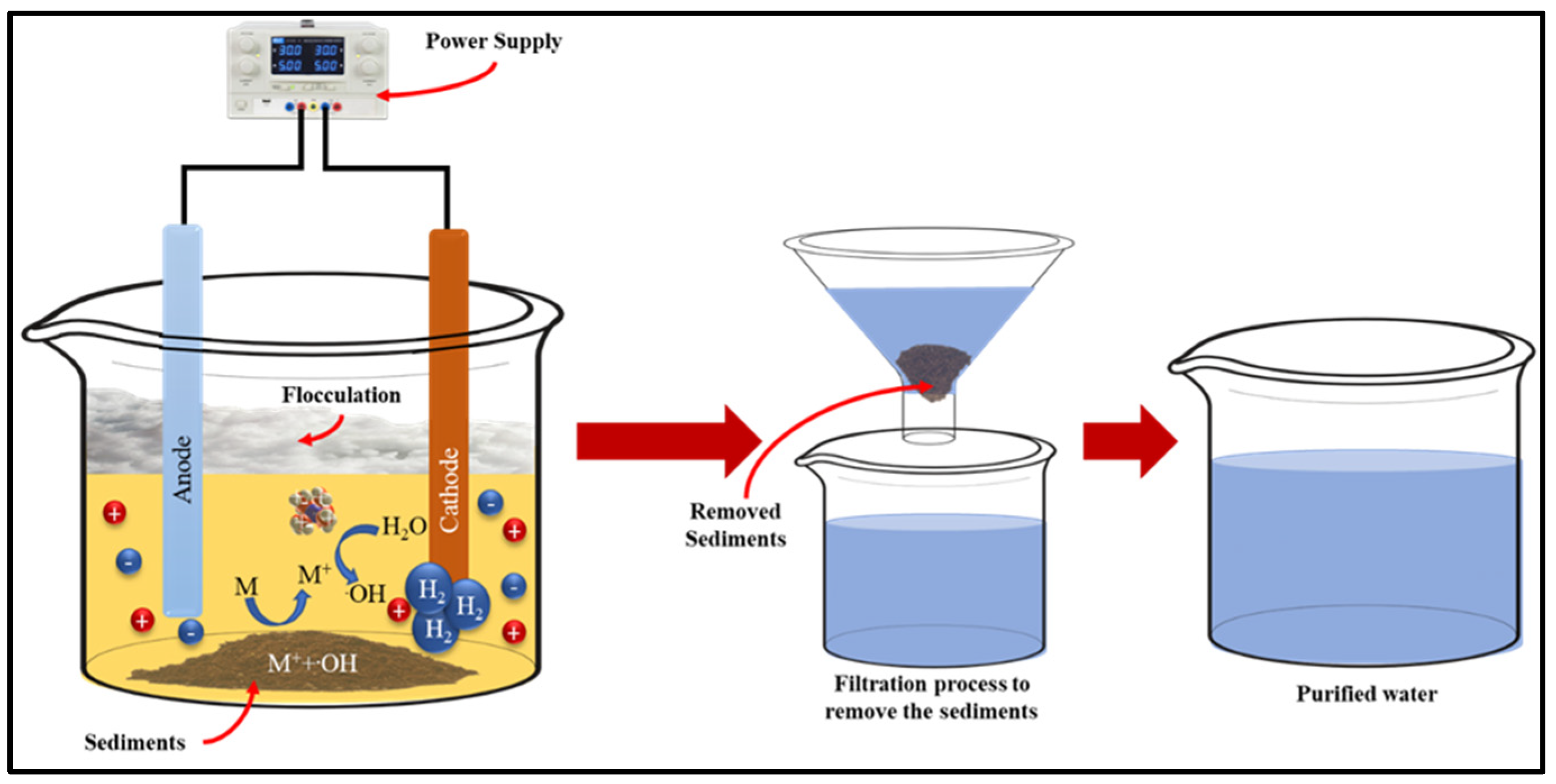
[14][43]. Electrocoagulation involves the use of metal electrodes submerged in polluted water. The electrodes dissolve metal ions which adsorb the dye molecules and precipitate to form sediments (
Figure 2)
[15][16][44,45]. Furthermore, magnesium carbonate and hydrated lime are utilized to adsorb azo dyes and form coagulants
[17][18][46,47].
Figure 2. Schematic of electrocoagulation for textile dye wastewater.
Wang et al., published an interesting study on the application of the coagulation process for removing different types of azo dyes from water
[19][48]. They used cucurbituril for coagulation and targeted three different azo dyes, Congo Red, acid red 1, and orange II
[20][8]. The obtained results demonstrated the tendency of the coagulants to remove Congo Red and acid red 1. The reported removal rate for these dyes was above 95% under pH 6. Furthermore, the result showed that the removal percentage is influenced by pH, contact time, and the structure of the dyes
[19][48].
Even though chemical coagulants have several advantages in terms of cost and simplicity, they produce a significant amount of toxic contaminants and impurities. Moreover, the process can be costly in the case of adopting electrocoagulation, as it consumes high electricity
[15][44]. Thus, there is a crucial need to develop non-toxic, highly efficient coagulants to eliminate the presence of toxic substances in water.
3. Photocatalytic Degradation
Photocatalysis is an advanced abiotic oxidation method used to remove contaminants from wastewater. Indeed, the photocatalysis process is preferred over other techniques due to its unique advantages, such as eliminating contaminants under harsh conditions in a short time
[21][49]. Studies have revealed that photocatalysis is highly efficient for removing endocrine-disrupting chemicals (EDCs) from water
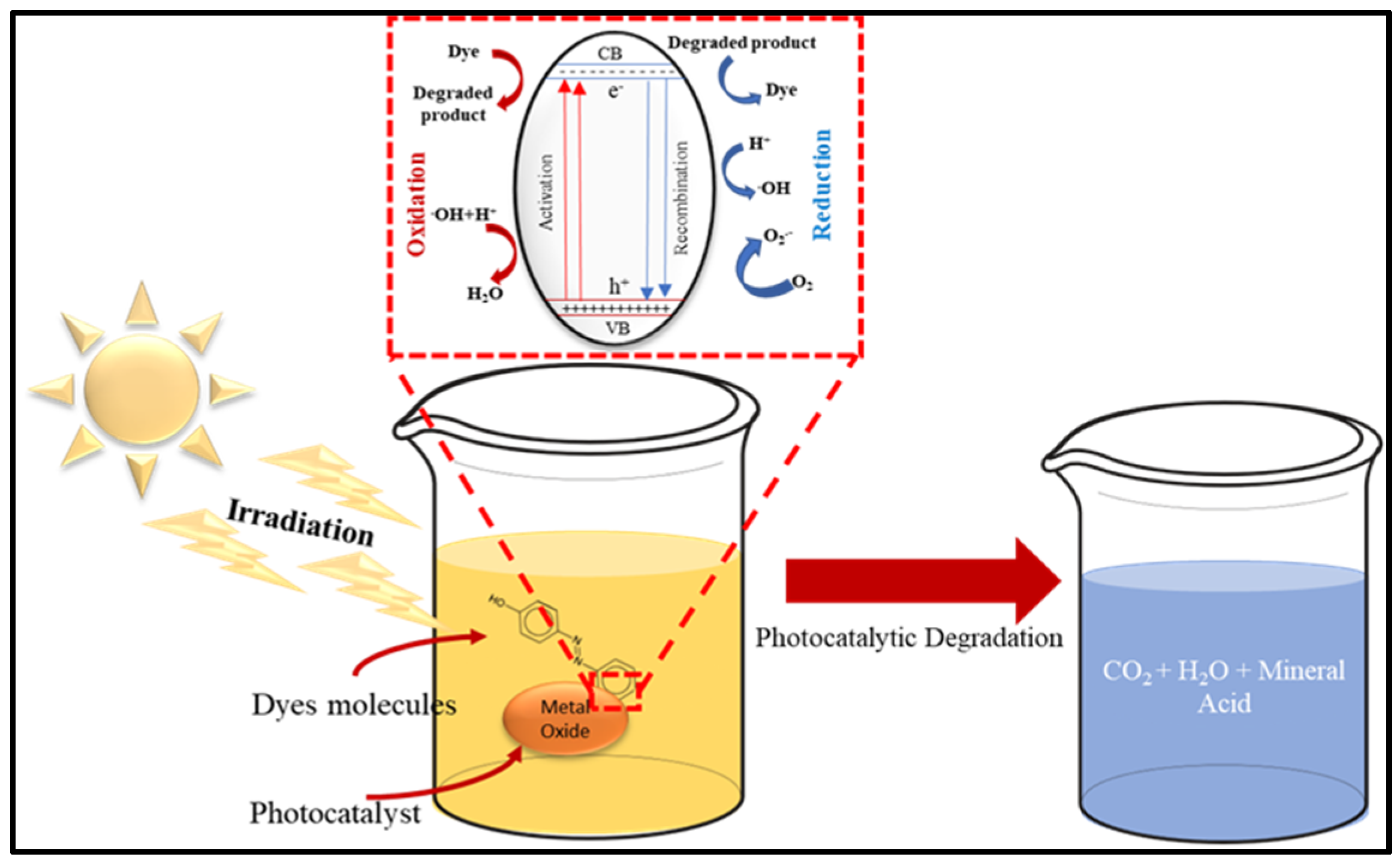
[22][12]. The process is conducted under light radiation where photocatalyst and electron–holes pairs form. The photocatalyst formation and the electron–holes pairs are associated with the degradation of pollutants in water
[22][12] (
Figure 3).
Figure 3. Schematic of Photocatalytic Degradation of toxic dye.
There are several types of photocatalyst used in water treatment, such as titanium dioxide (TiO2), iron (Fe), zinc oxide (ZnO), and copper oxide (CuO). TiO2 is highly popular among other photocatalysts as it has high efficiency in degrading different organic contaminants. In addition, TiO2 is inexpensive and environmentally friendly. Nonetheless, photocatalytic degradation has multiple disadvantages that limit the scope of its applications. For instance, the photocatalytic process is considered costly for large-scale applications. Moreover, the operation of the photocatalytic process is extremely difficult as the process is pH dependent. Photocatalysts have the tendency to form an aggregation of nanomaterials; therefore, it is usually combined with other chemicals and techniques. Although the process is considered eco-friendly to some extent, the process produces highly toxic byproducts.
Mabuea et al., performed research on photocatalyst applications for azo dye degradation
[23][50]. The study covered the use of the transition metal carbides molybdenum and tungsten, along with a multiwalled carbon nanotube (MWCNT) for the photocatalytic degradation process. Molybdenum and tungsten were coated with transition metals like iron (Fe), cobalt (Co), and nickel (Ni), which enhances the decomposition performance. The transition metal carbides were used for Congo Red azo dye degradation under sunlight irradiation
[23][50]. The process was repeated in the dark where no photocatalytic reaction is initiated, and under the light but in the absence of a photocatalyst. The results revealed that the MWCNT doped with Ni and Co had the highest decomposition efficiency, which was 97.1%. The results demonstrated that the performance of the photocatalytic process is better compared to photolysis for decomposing Congo Red azo dye
[23][50].
4. Ion Exchange
Ion exchange is a form of sorption process used widely for water treatment to reduce the level of ionic chemical species and hardness of water. Basically, ion exchange is a process in which two ions with similar charges are interchanged between two electrolytes or electrolyte particles and complex
[24][51]. The ion exchange process is influenced by the coulombic attraction
[24][51]. In the water treatment field, the ion exchange process is used for decontamination, separation, and purification
[24][25][51,52]. Typically, the ion exchange technique is combined with other techniques such as membrane separation, coagulation and flocculation, and adsorption to improve the removal efficiency. The synthetic compounds used as ion exchangers are known as resins. These compounds are solid, insoluble, and contain weakly bonded ions on the surface. When ions in the solution pass through the exchange resin, the ion exchange process occurs between ions on the resin surface and ions in the solution
[24][26][51,53]. The process continues until the ion exchange reaches equilibrium. However, when equilibrium is established, and resins are saturated, backwashing is applied to regenerate the resins and remove accumulated ions and contaminants
[26][53]. The ion exchange process can be explained using the following formula:
where A
+ is the exchangeable ion on the ion exchanger, and X
+ and Y
− are ions in the aqueous solution. Ion exchange resins are classified into two types: gel and porous resins. Gel resins have the cross-linked structure of a polymer. The polymer is connected in the form of a matrix and has different functional groups uniformly distributed among the structure
[24][25][26][51,52,53]. The unique structure of the polymer helps in increasing the volume of ions attached to the functional groups. These functional groups work as ion active sites where the exchange process occurs. The increased volume of the diffused ions means more ions in the solution will attach with the large number of active sites on the resin. Porous resins have pore surfaces where ions can attach easily. The porosity of porous resin varies according to the size of the structure. Porous resins can be microporous, mesoporous, and macroporous. The size of the resin affects the surface area, hence affecting the ion exchange capacity of the resin. A higher surface area means more ions diffuse and are attached to the resin.
Different cationic and anionic resins are used to remove ionic dyes, such as methylene blue, methyl orange, and malachite green
[27][54]. However, the removal efficiency of the ion exchangers relies on several factors, such as the concentration of the dyes in the targeted aqueous solution, and the amount of the ion exchangers. Basically, the removal percentage of ionic dyes from contaminated water increases with decreased concentration of dye and increased amount of resin. Recently, anionic ion exchangers have been used widely to remove azo dyes from wastewater
[27][54]. Although the ion exchange process has shown great performance in eliminating different types of pollutants from water, it is considered a very expensive process. Moreover, the ion exchange process produces highly toxic sludges that need to be carefully discharged. Additionally, ion exchange has time-consuming and costly operational processes. Thus, the adsorption process and membrane filtration are combined with ion exchange to eliminate some of the limitations of the technique and to increase the removal performance.
5. Electrochemical Technique
The electrochemical method has shown promising results in many fields and applications, such as batteries, sensors, soil treatment, and fuel cell technology. In general, electrochemical techniques have been used in water and wastewater treatment to eliminate water contaminants
[28][55]. The concept of using electrochemical techniques for water remediation revolves around applying an electrical current to start a chemical reaction to generate new substances. Usually, electrochemical techniques are used in combination with other water treatment techniques, such as coagulation, flotation, adsorption, and membrane filtration. Some of the most popular techniques used for dye removal are electrocoagulation, electrochemical reduction, indirect electro-oxidation, photo-assisted electrochemical, and electrodialysis
[1][26][28][29][30][7,33,53,55,56]. As a result of the variety of electrochemical methods available today, researchers can change the controlling parameters independently to improve the effectiveness of treatment. In other words, electrochemical treatment of dye-contaminated water needs a minimum amount of energy.
Generally, electrochemical oxidation of dye-contaminated water is conducted via either direct anodic oxidation, or chemical reaction. In direct anodic oxidation, the reaction occurs during the oxidation and reduction processes. Indeed, direct anodic oxidation has inefficient decontamination performance. On the other hand, a chemical reaction occurs during the generation of electro-species
[28][55]. Typically, during the chemical reaction, some of the organic dyes transform into biodegradable composites such as carboxylic acids, or totally oxidize into CO
2 and inorganic ions
[28][55]. Although electrochemical techniques have high removal efficiency for dye particles, they are considered extremely expensive techniques and usually require pre-treatment of the contaminated effluents.
6. Membrane Filtration
A wide variety of membrane technologies have been used extensively in the field of water treatment. It is true that membrane technologies have demonstrated significant growth in the field of water treatment compared to other methods of treatment. The attention drawn toward membrane technologies is driven by the fact that it requires low energy, low-to-no chemical usage, and inexpensive operational cost
[31][32][57,58]. Therefore, it is considered environmentally friendly, with some limitations. The limitations of membrane technologies are caused by membrane fouling with toxic sludge production.
Generally, based on chemical structure, membrane technologies can be classified into two types: organic and inorganic membranes. Furthermore, the membrane can also be classified as isotropic and anisotropic membranes. Primarily, isotropic membranes are uniformly composed membranes with poor porous structure. Whereas an anisotropic membrane is composed of a non-uniformed structure with multiple layers
[31][33][57,59]. Organic membranes consist mainly of organic polymers and can have different pore sizes. On the other hand, inorganic membranes are synthesized from materials such as metals, silica, and ceramics. Membranes can be further classified based on the size of the pores into macro membranes, micromembranes, and nanomembranes, which all contribute to multiple processes such as microfiltration, ultrafiltration, nanofiltration, and reverse osmosis.
For dye removal, nanofiltration and reverse osmosis are considered the most efficient types of membrane techniques. This is due to the proper nano size of the pores in the membrane, which catch the azo dye particles more efficiently compared to ultra- or microfiltration techniques. It has been found that a membrane with a weight cut-off lower than 10,000 Daltons is suitable for eliminating dye molecules from water
[11][22][12,40]. Although membrane filtration is considered a cost-efficient technique for removing contaminants from water, there are some limitations that restrict its application up to a certain extent. For instance, the membrane process is affected by the flow rate of the medium and requires scheduled cleaning to remove the concentrated waste. Moreover, membrane filtration has poor contaminant removal efficiency compared to other techniques
[31][32][57,58]. Nonetheless, membrane filtration techniques are usually used with other methods such as electrochemical, adsorption, or ion exchange as hybrid techniques. Membrane hybrid techniques are considered more reliable and efficient as they reduce the limitations of the water treatment methods.
7. Electrodialysis Process
Electrodialysis is an advanced hybrid treatment method used in several wastewater treatment plants around the world. In fact, electrodialysis is one of the great examples of hybrid treatment methods in the water treatment field. Basically, electrodialysis is a combination of electrochemical and ion exchange methods
[30][56]. Primarily, the electrodialysis method is used for the desalination of brackish water to remove salt. Additionally, it has been used as a removal process for heavy metal ions and ionic dyes
[28][30][55,56]. In electrodialysis, the electric potential generated via the electrochemical process is used to drive electrolyte ions through a series of selective ion exchange membranes. Hence, the electric potential is considered as a driving force of the process
[28][55]. The selectivity and the chemical structure of the ion exchange membranes in the electrodialysis method are like the ion exchange resins
[28][30][55,56]. However, the difference can be observed in the mechanical requirement of the membrane process. The simple structure of the electrodialysis cell consists of two conductive electrodes separated via two ion exchange membranes. The separation of the membranes divides the electrodialysis cell into three compartments where chemical reactions occur. When a direct current (DC) is applied to the electrodialysis cell, cations and anions in the solution start to move towards their perspective electrodes (cathode and anode). During the transportation of the cations and anions, they pass through ion exchange membranes that help in ion removal and separate the compartment of the electrodialysis cell by ions volume
[30][56]. The concentration of ionic contaminants decreased in the middle compartment. However, the concentration is higher close to the anode and cathode sides. During the process, cations can only pass through a cation exchange membrane, while anions pass thought an anion exchange membrane. Usually, the electrodialysis method involves a formation of acid and alkaline layers near the anode and cathode, respectively. As a result, additional hydrogen and hydroxide ions move to the middle compartment. In the middle compartment, hydrogen and hydroxide ions neutralize and form water. Selective ion exchange membranes are used to reduce the negative effect of the movement of hydrogen and hydroxide ions and hence increase the current efficiency.
8. Biodegradation Techniques
In the last few decades, the adoptability of biodegradation techniques for water treatment has increased dramatically. The rapid development in biodegradation techniques is mainly driven by the special characteristics of the techniques, such as the low operational cost, high removal efficiency, and the generation of a reduced amount of toxic byproduct
[34][60]. Biodegradation techniques are used for the degradation or elimination of chemicals such as heavy metals, azo dyes, microplastics, and organic toxicants. Moreover, in some contaminated areas, microorganisms are found to be living on digesting dyes as a source of carbon and nitrogen
[22][12]. Nevertheless, the consumption of discharged dyes by microbial communities in the affected areas has a negative impact on the environment. The uncontrolled consumption of dyes by microbial communities can affect the ecosystem via increasing the population of the microbes which will eventually destroy the ecosystem
[35][61]. For instance, Krishnamoorthy et al. conducted a study on the impact of discharged azo dyes on the abundance of
Saccharibacteria [36][2]. The study was conducted on the Noyyal River in Tamil Nadu, India. Typically, textile industries around the Noyyal river discharge a huge amount of contaminated textile wastewater that contains a high concentration of azo dyes. The presence of azo dyes in the river resulted in a higher population of Saccharibacteria and Proteobacteria. Consequently, the ecosystem of the river is becoming unstable. Moreover, Saccharibacteria has a negative impact on human health as it is associated with oral mucosal infectious diseases
[37][28].
Generally, biodegradation for dye remediation can be carried out using fungi, bacteria, algae and plants, and yeasts. The efficiency of the microorganism is determined by its natural activity and availability
[38][62]. The biodegradation of the azo dyes is mainly influenced by the used cell or enzymes. However, the degradation process can also involve the use of microbial biomass, which is more efficient in eliminating the toxic dyes
[38][62]. The biodegradation process decomposes the dye particles completely. However, it is a time-consuming process and is often not efficient for a large volume of contaminated water.
9. Adsorption
The importance of the adsorption method in the water treatment field is crucial. IN particular, the adsorption method is involved with other treatment methods such as precipitation, coagulation, and ion exchange to treat industrial wastewater and groundwater. Basically, adsorption in water treatment is a process used to remove dissolved contaminants via adhering them to a surface of solid particles
[39][63]. In other words, adsorption is a process of diffusing and adhering atoms, molecules, or ions onto the surface of solid particles. The adhesion of the particles occurs due to the existence of imbalanced forces between the solid surface and the contaminated particles
[39][63]. The solid surface that attracts molecules is known as an absorbent, and the particles which are adsorbed are known as adsorbates. The adsorption process can be illustrated by the following formula, where A is adsorbate, B is adsorbent, and AB is the output when A is adsorbed by B:
The adsorption method is preferable due to its simplicity and flexibility. In fact, operations using the adsorption process are very simple compared to other treatment methods. Adsorbents are added directly to the water sources or by mixing basins. In addition, the adsorption treatment process is safe, cheap, and can be used to remove various types of contaminants (organic, inorganic, and biological)
[1][29][39][7,33,63]. The special characteristics of the adsorption method are affected by several factors such as the aqueous solution properties, type of adsorbate, adsorbent type, structure of the pollutant, operating conditions, contaminant disposal, and particle regeneration
[39][40][63,64].
In general, there are two types of adsorption: chemical adsorption—chemisorption—and physical adsorption—physisorption
[39][41][63,65]. Basically, chemical adsorption is defined as the adhesion of particles to the solid surface via chemical bonds such as ionic, covalent, or metallic bonds
[39][63]. In contrast, physical adsorption involves the adhesion of particles to a solid surface via physical bonds such as the van der Waals force
[39][63]. Physical adsorption differs significantly from chemical adsorption in terms of their bond strength.
In order to develop the optimum adsorbent, a full understanding of the method and the behavior of the interaction between adsorbents and adsorbates is mandatory. Lately, attention has been drawn toward using activated carbon to treat textile dye wastewater. Activated carbon has various advantages and is considered in multiple applications due to its unique features.
10. Application of Activated Carbon for Dye Removal from Wastewater
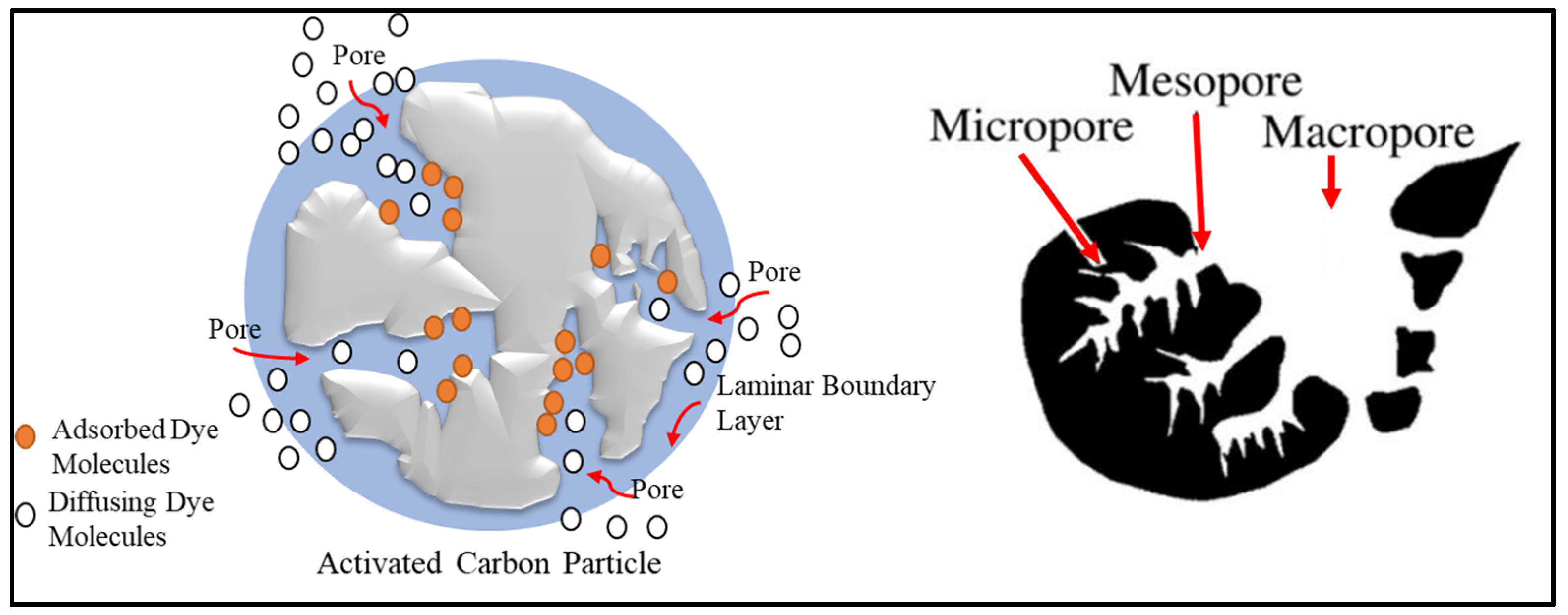
Activated carbon, also known as activated charcoal, is a carbonaceous material composed of many open pores on the surface (
Figure 4). Activated carbon is used in many applications, like the automotive field, to filter the interior air of the vehicle, in gasoline tanks to eliminate the amount of discharged toxic substances, and as electrodes for the batteries and electrochemical capacitors
[42][66]. Activated carbon is part of the graphite family, and therefore it is amorphous in nature. Activated carbon has remarkable properties, such as a unique pore structure, high surface area, and chemical polarity. These properties depend on the type of the precursor and the preparation process
[43][44][45][67,68,69]. The enlarged surface area and porous texture are formed via exposing turbostratic carbon to different chemical reactions (
Figure 4)
[45][69]. The chemical reaction interacts with the surface molecules of activated carbon and produces gases. Consequently, gases escape from the surface and create pores. The chemical reaction involved in activated carbon preparation is known as activation.
Figure 4. Pore structure and distribution on activated carbon.
In recent decades, commercial activated carbon has been used for multiple applications. However, commercial activated carbon is not cost-effective because it is produced from non-renewable sources such as coal and petroleum. Thus, efforts are geared towards using eco-friendly, renewable, and, most importantly, inexpensive sources of precursors for producing activated carbon. Biomass precursors have been introduced as a highly effective alternative. As a result, biomass based on agricultural wastes is used to synthesize new forms of activated carbons
[45][69]. There are many types of agricultural wastes that can be used as precursors for activated carbons. For instance, palm kernel shells, waste coffee beans, cassava peel waste, rice husk, sugarcane bagasse, olive husk, hazelnut shells, almond shells, walnut shells, and many more
[42][43][45][66,67,69].
The type of the precursor influences the properties of the produced activated carbon, such as the surface area, porosity, and particle size
[45][69]. Activated carbon is classified according to particle size into three types, Powdered Activated Carbon (PAC), Granular Activated Carbon (GAC), and Activated Carbon Fibers (ACF)
[42][66]. Activated carbon is categorized by particle size because it is difficult to distinguish its physical properties and surface characteristics
[42][66]. PAC has a particle size between 0.015–0.025 mm, whereas GAC and ACF have a particle size between 0.6–3 mm and 10–20 µm, respectively
[42][66]. PACs and GACs are usually used in the water treatment field, whereas ACFs are used for gas adsorption due to their special microporous structure.
Generally, PAC is best for batch adsorption, whereas GAC is best for the column adsorption process used industrially. Nonetheless, PAC is typically used to establish the process kinetics and isotherms initially, then based on the harder nature of the starting biomass containing more lignin, the industry prepares GAC particles. Because softer, cellulosic biomass such as leaves, and grass are not utilized to produce granular activated carbon. ACF fibers are generally great for small-scale applications as they will have more weight loss, like PAC.
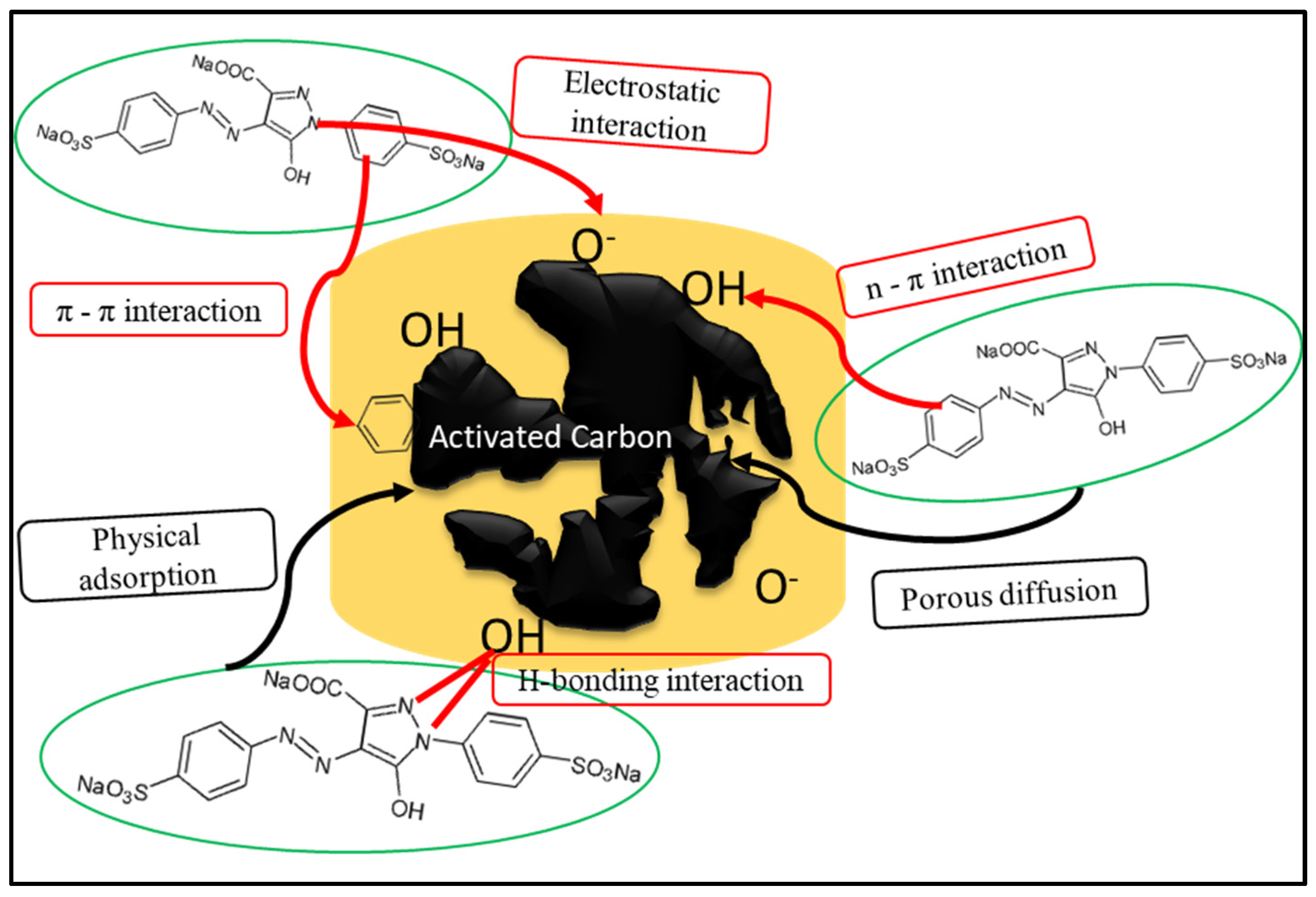
The interaction between the particles of toxic dye as an adsorbate and activated carbon as an adsorbent is mainly influenced by several possible adsorption mechanisms. Initially, the interaction between the adsorbent and adsorbate begins with the surface and intra-particle diffusion processes. Then, several adsorbing mechanisms may occur at the surface of the adsorbent, such as π–π interactions, hydrogen interactions, acid–base reactions, electrostatic interactions, hydrophobic interactions, and van der Waals forces
[45][69]. Typically, electrostatic attraction and ion exchange are the most common adsorption mechanisms of dyes using activated carbon
[45][69]. The adsorption mechanisms are mainly influenced by the functional groups on the surface of the activated carbon and ions in the dye solution. Thus, modification of the activated carbon surface will enhance the reactivity and promote adsorption of more dye particles. Usually, multiple adsorption mechanisms occur simultaneously during the adsorption process.
Figure 5 shows an example of possible mechanisms of dye adsorption.
Figure 5. Possible mechanisms for adsorbing dyes onto activated carbon.
Synthesis of activated carbons involves two stages, thermochemical carbonization, and activation
[45][69]. These two stages can occur simultaneously, one-step process, or sequentially, two-steps process. The thermochemical carbonization stage is conducted either via hydrothermal process, pyrolysis, torrefaction, or combustion
[45][69]. However, the activation stage involves physical, chemical, or physiochemical activation
[42][66]. Generally, the carbonization process occurs in an inert atmosphere where oxygen is absent to prevent biomass burning. Nevertheless, combustion occurs with oxygen to reduce energy content in biomass
[42][43][66,67]. The temperature required for the carbonization process is in the range of 200–400 °C, while for pyrolysis and hydrothermal it is between 400–850 °C and 200–350 °C, respectively
[42][45][66,69].
The product obtained from the thermochemical carbonization processes is known as char. Indeed, char is considered an adsorbent, but it has poor adsorptive capacity. Thus, different chemical reagents, such as acids or bases, are used to activate the surface of the char and produce activated carbon. This process is known as chemical activation. Usually, acids such as phosphoric, sulfuric, and hydrochloric acids are used in the activation process
[45][69]. The main goal of using chemical activation is to enhance the number and size of pores on the surface of the carbon. Hence, it can catch more of the dye particles. The adsorption capacity varies with the type of activation reagent used. Physical and chemical activations are the most used methods in preparing activated carbon
[45][69]. While the physiochemical activation method combines both physical and chemical activation, it is often used to enhance the adsorption performance for specific applications. Generally, activation processes are used to improve the porosity of the activated carbon particles. The generated pores are classified into macropores (>50 nm), mesopores (2–50 nm), and micropores (<2 nm)
[42][66].
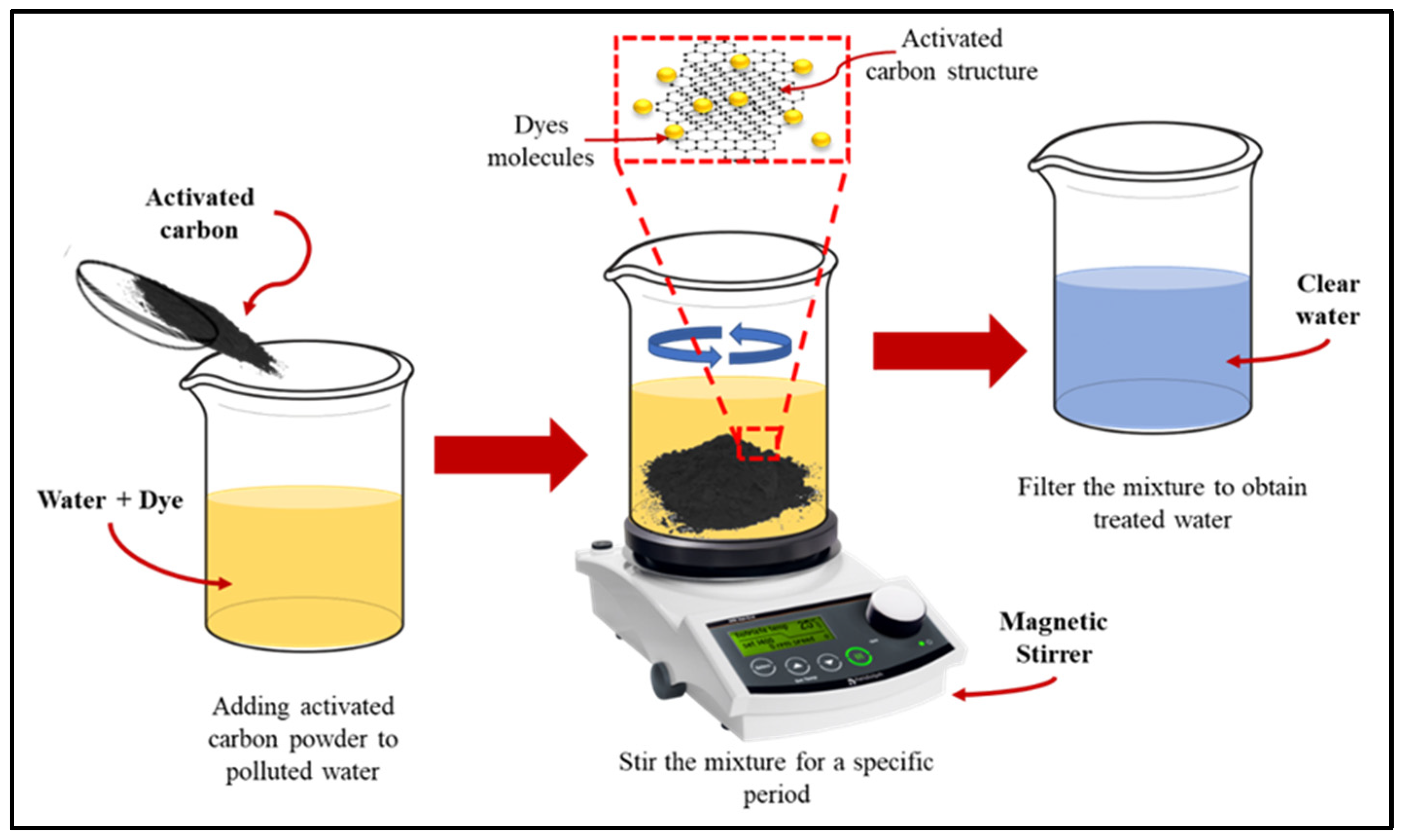
Figure 6 shows the adsorption process of dyes molecules using activated carbon.
Figure 6. Adsorption mechanism of activated carbon for dyes particles.