Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 6 by Jessie Wu and Version 10 by Jessie Wu.
Squaraine dye is a popular class of contrast near-infrared (NIR) dyes. Squaraine dyes have shown the ability to be modified with various heterocycles. The indole moiety is the most notable heterocycle incorporated in squaraine dyes.
- squaraine dye
- synthesis
- optical properties
- near-infrared region
- indole
- quinoline
1. Introduction
Small molecular organic dyes are a research hotspot. There have been various classes of dyes reported over the years. Squaraine dyes are one of the popular classes of near-infrared dyes that have been described. The unique characteristic that differentiates them from other dyes is the central linking unit; this unit is known a squaraine, where the name for these dyes is obtained. The core unit is composed of an unsaturated, four-membered ring [1][2][3]. This unique linking core unit provides the properties associated with squaraine dyes. Some of their other characteristics are sharp absorption bands, a high molar extinction coefficient, and photoconductivity abilities [4][5][6].
The squaraine core is composed of a four-membered ring that is electron-deficient when incorporated into the dye [7]. The electrons in the squaraine core are delocalized, allowing them to encompass the whole conjugated system. This squaraine core is capped with donor units, forming a donor-acceptor-donor system, allowing the dye to be stabilized by a π-conjugated system [1][7]. The squaraine core scaffold is zwitterionic and has multiple resonances, due to the delocalized electrons [8][9][10]. The zwitterionic nature is an essential part of the dye, as it facilitates electron movement through the scaffold [2][3]. The targeting specificity of the squaraine dyes can be improved through conjugation by synthesizing them with various possible functional groups. The overall optical characteristics of the compound may be affected by the addition of heterocycles and functional groups. Regardless of the varying functional groups and heterocycles, squaraine dyes benefit from excellent chemical and photophysical properties [2][3].
The use of near-infrared squaraine dyes aims to achieve absorbance and fluorescence in the near-infrared region (NIR), and this region ranges from 650 to 1700 nm [11][12]. This region is optimal for biomedical [11][12] and solar energy applications [13]. In terms of biomedical applications, the region enables a higher signal-to-noise ratio for bioimaging. This is due to the limited autofluorescence of biological molecules, resulting in reduced light scattering in this region [14]. Red shifting into the NIR range allows greater penetration of light into tissues, allowing for better spatial visualization [14][15]. Solar radiation is composed of about 50% near-infrared light [16]. To create an efficient solar cell, the light-absorbing materials need to absorb light in this region [17]. In addition, the lower-energy photons of NIR result in a higher short-circuit current density [18].
In recent years, the indole heterocycle has been a popular donor unit for squaraine dyes. There have been numerous applications in various fields reported for this class [4][19][20][21]. In comparison, when looking at other heterocycles, such as quinoline- and perimidine-based squaraine dyes, there are only a few scattered reported dyes and associated applications. This is the case even though they have exciting and notable optical properties that differ from indole-based squaraine dyes.
2. Synthesis
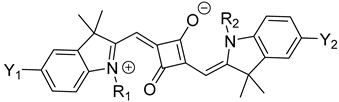
There are two different classes of squaraine dyes: symmetrical and unsymmetrical. The symmetrical squaraine dyes contain identical donor units on each side, while the unsymmetrical, as the name suggests, contain two different donor groups. Typically, the donor units are bound to the first and third positions of the squaraine unit, due to their desirable, red-shifted optical properties compared to the 1,2 regioisomer [22]. For symmetrical squaraine dyes, the synthesis involves the condensation reaction in a single-pot reaction mixture [23], as utilized in Equation (1).

(1)
Equation (1): general synthesis of symmetrical squaraine dyes.
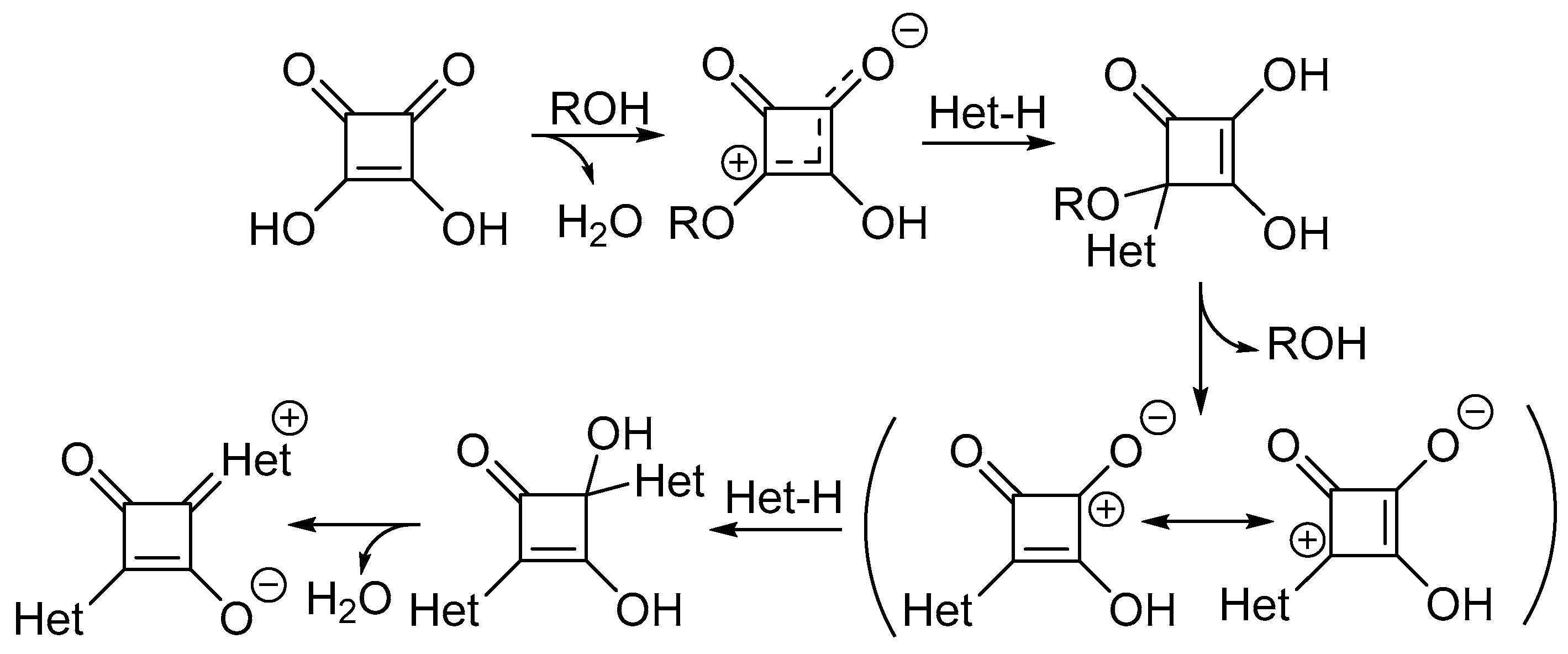
Scheme 1. Proposed mechanism for synthesis of squaraine dyes [27].
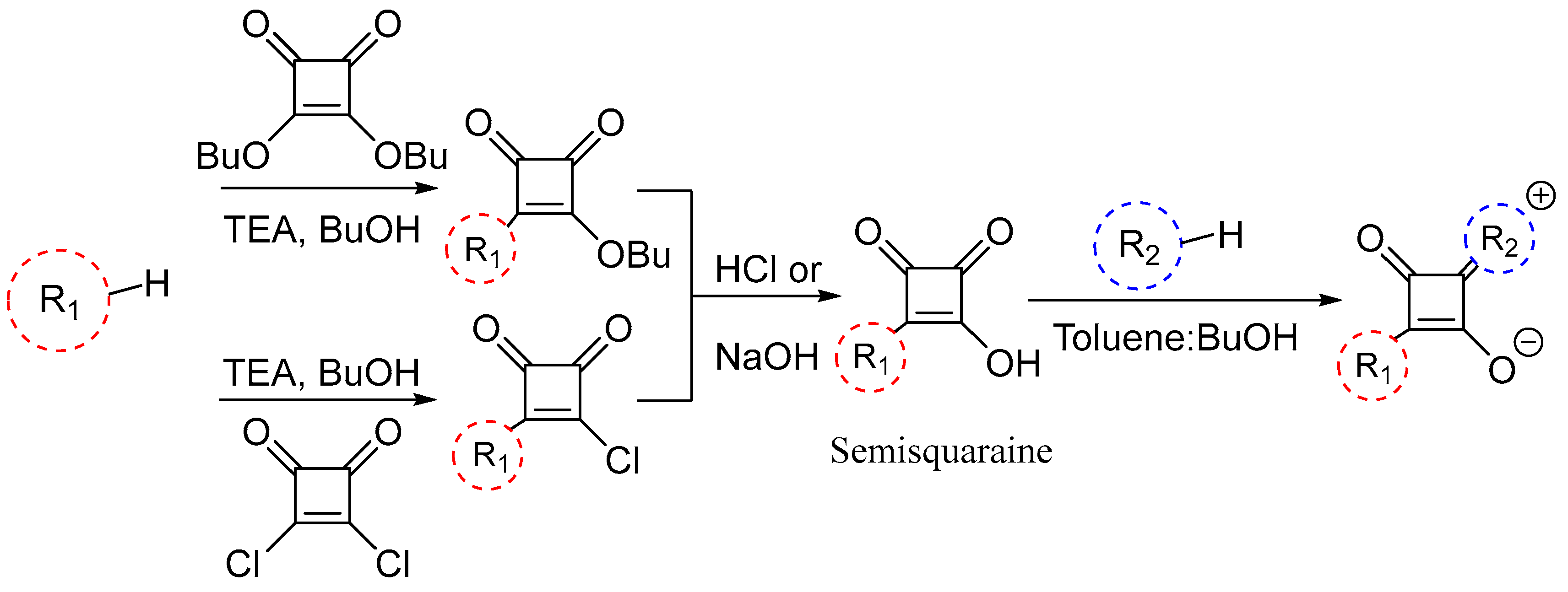
Scheme 2. General synthesis of unsymmetrical squaraine dyes.
Table 1. Comparison of the reaction time and yield between traditional method and microwave method. T he reaction time accounts for the complete synthesis of symmetrical dyes, whereas the reaction time for unsymmetrical dye is from the semisquaraine group containing ethyl ether(-OEt) [28].
References
- Bacher, E.P. Shedding New Light on Squaraines: Utilizing Squaraine Dyes as Effective Tools in Organic Synthesis; University of Notre Dame: Notre Dame, IN, USA, 2020.
- Sreejith, S.; Carol, P.; Chithra, P.; Ajayaghosh, A. Squaraine dyes: A mine of molecular materials. J. Mater. Chem. 2008, 18, 264–274.
- Ajayaghosh, A. Chemistry of squaraine-derived materials: Near-IR dyes, low band gap systems, and cation sensors. Acc. Chem. Res. 2005, 38, 449–459.
- Yadav, Y.; Owens, E.; Nomura, S.; Fukuda, T.; Baek, Y.; Kashiwagi, S.; Choi, H.S.; Henary, M. Ultrabright and Serum-Stable Squaraine Dyes. J. Med. Chem. 2020, 63, 9436–9445.
- Devi, D.G.; Cibin, T.; Ramaiah, D.; Abraham, A. Bis (3, 5-diiodo-2, 4, 6-trihydroxyphenyl) squaraine: A novel candidate in photodynamic therapy for skin cancer models in vivo. J. Photochem. Photobiol. B Biol. 2008, 92, 153–159.
- Park, J.; Barolo, C.; Sauvage, F.; Barbero, N.; Benzi, C.; Quagliotto, P.; Coluccia, S.; Di Censo, D.; Grätzel, M.; Nazeeruddin, M.K. Symmetric vs. asymmetric squaraines as photosensitisers in mesoscopic injection solar cells: A structure–property relationship study. Chem. Commun. 2012, 48, 2782–2784.
- Fan, J.; Wang, Z.; Zhu, H.; Fu, N. A fast response squaraine-based colorimetric probe for detection of thiols in physiological conditions. Sens. Actuators B Chem. 2013, 188, 886–893.
- Wang, Z.; Pradhan, A.; Kamarudin, M.A.; Pandey, M.; Pandey, S.S.; Zhang, P.; Ng, C.H.; Tripathi, A.S.; Ma, T.; Hayase, S. Passivation of grain boundary by squaraine zwitterions for defect passivation and efficient perovskite solar cells. ACS Appl. Mater. Interfaces 2019, 11, 10012–10020.
- Friães, S.; Lima, E.; Boto, R.E.; Ferreira, D.; Fernandes, J.R.; Ferreira, L.F.; Silva, A.M.; Reis, L.V. Photophysicochemical properties and in vitro phototherapeutic effects of iodoquinoline-and benzothiazole-derived unsymmetrical squaraine cyanine dyes. Appl. Sci. 2019, 9, 5414.
- Xiao, X.; Cheng, X.F.; Hou, X.; He, J.H.; Xu, Q.F.; Li, H.; Li, N.J.; Chen, D.Y.; Lu, J.M. Ion-in-conjugation: Squaraine as an ultrasensitive ammonia sensor material. Small 2017, 13, 1602190.
- Zhang, F.; Tang, B.Z. Near-infrared luminescent probes for bioimaging and biosensing. Chem. Sci. 2021, 12, 3377–3378.
- Shou, K.; Qu, C.; Sun, Y.; Chen, H.; Chen, S.; Zhang, L.; Xu, H.; Hong, X.; Yu, A.; Cheng, Z. Multifunctional biomedical imaging in physiological and pathological conditions using a NIR-II probe. Adv. Funct. Mater. 2017, 27, 1700995.
- Yan, Z.; Guang, S.; Su, X.; Xu, H. Near-infrared absorbing squaraine dyes for solar cells: Relationship between architecture and performance. J. Phys. Chem. C 2012, 116, 8894–8900.
- Escobedo, J.O.; Rusin, O.; Lim, S.; Strongin, R.M. NIR dyes for bioimaging applications. Curr. Opin. Chem. Biol. 2010, 14, 64–70.
- Wainwright, M. Therapeutic applications of near-infrared dyes. Color. Technol. 2010, 126, 115–126.
- Barolet, D.; Christiaens, F.; Hamblin, M.R. Infrared and skin: Friend or foe. J. Photochem. Photobiol. B Biol. 2016, 155, 78–85.
- Qin, C.; Wong, W.Y.; Han, L. Squaraine Dyes for Dye-Sensitized Solar Cells: Recent Advances and Future Challenges. Chem. –Asian J. 2013, 8, 1706–1719.
- Meng, D.; Zheng, R.; Zhao, Y.; Zhang, E.; Dou, L.; Yang, Y. Near-Infrared Materials: The Turning Point of Organic Photovoltaics. Adv. Mater. 2022, 34, 2107330.
- He, Y.; Mei, J.; Zhou, M.; Zhang, Y.; Liang, Q.; Xu, S.; Li, Z. Colorimetric and fluorescent probe for highly selective and sensitive recognition of Cu2+ and Fe3+ based on asymmetric squaraine dye. Inorg. Chem. Commun. 2022, 142, 109592.
- Jo, M.; Choi, S.; Jo, J.H.; Kim, S.-Y.; Kim, P.S.; Kim, C.H.; Son, H.-J.; Pac, C.; Kang, S.O. Utility of Squaraine Dyes for Dye-Sensitized Photocatalysis on Water or Carbon Dioxide Reduction. ACS Omega 2019, 4, 14272–14283.
- Beverina, L.; Salice, P. Squaraine compounds: Tailored design and synthesis towards a variety of material science applications. Eur. J. Org. Chem. 2010, 2010, 1207–1225.
- Lima, E.; Reis, L.V. ‘Lights, squaraines, action!’–the role of squaraine dyes in photodynamic therapy. Future Med. Chem. 2022, 14, 1375–1402.
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine dyes: Molecular design for different applications and remaining challenges. Bioconjugate Chem. 2019, 31, 194–213.
- Ronchi, E.; Ruffo, R.; Rizzato, S.; Albinati, A.; Beverina, L.; Pagani, G.A. Regioselective Synthesis of 1,2- vs 1,3-Squaraines. Org. Lett. 2011, 13, 3166–3169.
- Sprenger, H.-E.; Ziegenbein, W. Condensation Products of Squaric Acid and Tertiary Aromatic Amines. Angew. Chem. Int. Ed. Engl. 1966, 5, 894.
- Treibs, A.; Jacob, K. Cyclotrimethine Dyes Derived from Squaric Acid. Angew. Chem. Int. Ed. Engl. 1965, 4, 694.
- Law, K.-Y.; Bailey, F.C.; Bluett, L.J. Squaraine chemistry. On the anomalous mass spectra of bis(4-dimethylaminophenyl)squaraine and its derivatives. Can. J. Chem. 1986, 64, 1607–1619.
- Barbero, N.; Magistris, C.; Park, J.; Saccone, D.; Quagliotto, P.; Buscaino, R.; Medana, C.; Barolo, C.; Viscardi, G. Microwave-Assisted Synthesis of Near-Infrared Fluorescent Indole-Based Squaraines. Org. Lett. 2015, 17, 3306–3309.
- Sprenger, H.-E.; Ziegenbein, W. Cyclobutenediylium Dyes. Angew. Chem. Int. Ed. Engl. 1968, 7, 530–535.
- Treibs, A.; Jacob, K. Cyclobutenderivate der Pyrrolreihe, II1) Über Vierring-trimethin-Farbstoffe. Justus Liebigs Ann. Der Chem. 1968, 712, 123–137.
- Law, K.-Y.; Bailey, F.C. Squaraine chemistry. A new approach to symmetrical and unsymmetrical photoconductive squaraines. Characterization and solid state properties of these materials. Can. J. Chem. 1993, 71, 494–505.
- Keil, D.; Hartmann, H. Synthesis and characterization of a new class of unsymmetrical squaraine dyes. Dyes Pigment. 2001, 49, 161–179.
- Terpetschnig, E.; Lakowicz, J.R. Synthesis and characterization of unsymmetrical squaraines: A new class of cyanine dyes. Dyes Pigment. 1993, 21, 227–234.
- Cohen, S.; Cohen, S.G. Preparation and Reactions of Derivatives of Squaric Acid. Alkoxy-, Hydroxy-, and Aminocyclobutenediones1. J. Am. Chem. Soc. 1966, 88, 1533–1536.
- Tatarets, A.L.; Fedyunyaeva, I.A.; Terpetschnig, E.; Patsenker, L.D. Synthesis of novel squaraine dyes and their intermediates. Dyes Pigment. 2005, 64, 125–134.
More