Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Giovanna Liguori and Version 3 by Alfred Zheng.
The infiltration of primary tumors and metastasis formation at distant sites strongly impact the prognosis and the quality of life of cancer patients. Current therapies including surgery, radiotherapy, and chemotherapy are limited in targeting the complex cell migration mechanisms responsible for cancer cell invasiveness and metastasis. Extracellular vesicles (EVs) are lipid-enveloped particles involved in inter-tissue and inter-cell communication. Tumor-derived extracellular vesicles (TDEVs) impact cancer cell migration. They can not only be considered as a target for cancer therapy but can also be used for the development of anti-tumor therapeutic strategies.
- tumor-derived extracellular vesicles
- anti-cancer therapies
- metastasis
- cancer cell migration
- Cripto
- EV shuttle
- alternative signaling route
- cacner cell migration
1. Introduction
The degree of tumor infiltration and the formation of metastases are key aspects in the prognosis of patients with cancer diseases. Current treatment strategies such as surgery, radiotherapy, and chemotherapy, even in combination, have a limited effect on metastatic cancers; hence, cancer metastasis accounts for almost 90% of cancer deaths worldwide [1]. Therefore, there is an urgent need to develop more powerful therapeutic approaches targeting the complex mechanisms regulating cancer cell invasiveness and metastasis. Both these features rely on the ability of cancer cells to move and to migrate locally or at distant sites. Cancer cell migration is a central biological process in cancer pathogenesis, whose targeting represents an ambitious as well as desirable goal to prevent tumor spreading and metastasis. To manage this hallmark of cancer progression, a deep understanding of the mechanisms of cell migration and its regulatory signals is therefore fundamental.
Even though cell migration has been extensively studied and the main steps involved identified, many aspects still need to be unraveled. Cell migration depends on complex relationships with the surrounding cells and with the extracellular space. It can be single or collective and involves an orchestrated and finely tuned series of intracellular cytoskeletal as well as extracellular adhesion rearrangements [2][3]. The exchange of information among cells is attained through the release of specific soluble or immobilized signaling molecules and their interaction with corresponding receptors, or through direct cell-to-cell communication that includes gap junctions, cytonemes, tunneling nanotubes and extracellular vesicles (EVs) [4][5][6][7][8]. With cancer cell migration being based on and regulated by local and distant communication among cells, EVs, as important mediators of cell communication, play a strategic role in regulating the migration of cancer cells. Thanks to their ability to travel long distances, be transported into body fluids, and interact with specific target cells, EVs are deeply involved in cancerogenesis at multiple steps. EVs contribute to the regulation of cancer states during epithelial-mesenchymal transition (EMT) and its reverse, mesenchymal-epithelial transition (MET), as well as to cancer invasion, immune escape and metastasis at target sites [1][9][10][11][12]. Through EV production, transport and uptake, several proteins, lipids and nucleic acids with oncogenic properties can be transferred between cells. Therefore, EVs are considered new and promising sources of both diagnostic and prognostic biomarkers as well as therapeutic targets for a variety of cancer types, including pancreatic, ovarian, prostate, breast, colorectal cancer and glioblastoma multiforme (GBM) [13][14]. Moreover, due to their low immunogenicity, EVs may cross interspecies boundaries and are extremely suitable for the targeted delivery of anti-cancer drugs and the development of therapeutic applications to counteract cancer progression and tumor metastasis [15][16][17][18][19].
2. Exploitation of Tumor-Derived Extracellular Vesicles in Anti-Cancer Therapies
TDEVs can not only be considered as a target for cancer therapy but can also be used for the development of anti-tumor therapeutic strategies. First of all, due to the presence of tumor antigens on the membranes, TDEVs were used to develop cancer immunotherapies (Table 1) [20]. TDEVs were used to activate dendritic cells in vitro, which, when injected in vivo, were able to stimulate the host immune system by boosting T-cell expansion and function [21]. However, TDEV participation in almost all aspects of tumor progression and development hinders their direct use as safe cell-free cancer vaccines [20].
In recent years, the exploitation of TDEVs as drug-delivery systems has received considerable attention. In fact, TDEVs are easily taken up by cancer cells in vivo, especially by the ones resembling the tumor cells of origin, showing high tumor-targeting and permeability activity [22]. Actually, the beneficial use of TDEVs for anti-cancer drug delivery has been demonstrated in several studies. The encapsulation of the monomeric active ingredients of traditional Chinese medicine inside TDEVs was efficient in improving their therapeutic effect [23]. The loading of chemotherapeutic and other biological drugs into TDEVs was also shown to enhance their curative effect [24][25]. Purified EVs from MCF-7 breast cancer cells were loaded with small interfering RNAs, miRNA, and single-stranded DNA oligonucleotides, and they were able to induce anti-cancer features by silencing HER2 genes in recipient cells [26]. Some clinical trials using EVs from tumor cells for delivering drugs (e.g., NCT02657460 and NCT01854866) are ongoing [16], though their results are still not available. On the other hand, TDEVs might transport oncogenic cargo and eventually be able to promote tumor proliferation and metastasis formation. For this reason, the potential risks to human safety need to be carefully considered before starting clinical trials.
Noteworthily, TDEVs have been recently shown to have an antitumoral effect per se, paving the way for their possible use as natural anti-cancer therapeutics. In fact, EVs derived from NTERA2 teratocarcinoma cells are able to act on GBM cells, inducing a remarkable inhibitory effect on tumor cell migration, without inducing undesirable effects such as increased tumor cell proliferation or chemotherapy resistance [27]. Teratocarcinoma is a common type of testicular germ cell tumor mostly formed of embryonal carcinoma stem cells. After retinoic acid (RA) exposure, teratocarcinoma cells can differentiate into postmitotic neurons and glia, thus showing neural stem/progenitor cell properties [28][29]. NTERA2 cells or NTERA2-derived neural precursors partially differentiated with RA showed GBM tropism in vitro and in vivo, respectively, and were proposed as promising drug-delivery cellular systems for targeting GBM cells [30][31]. However, as teratocarcinoma is considered a highly aggressive cancer, the anti-migratory effect mediated by native NTERA2-derived EVs, without any drug loading, is quite unexpected. Even more unexpectedly, this effect seems to be mediated by the onco-developmental factor Cripto [27].
Cripto is a membrane-bound glycosylphosphatidyl inositol-anchored protein that can act as a cofactor for TGFβ and its family members, including Nodal and Activin, and it is also released from the plasma membrane as a soluble protein [32][33][34]. Cripto has a key role in early embryo development, for mesoderm formation and anterior-posterior patterning [35][36][37][38], and also in tumor progression. An antitumoral effect for Cripto was previously proposed by a study on colon tumor induction in mouse models, showing that Cripto haploinsufficiency increased colon tumorigenesis [39]. However, most studies pointed instead to an oncogenic role of Cripto, associated with increased cancer features, including metastasis induction, and a worse patient prognosis [32][40][41][42][43]. Cripto overexpression, and, more interestingly, the soluble Cripto form alone or the induction of Cripto shedding from the cell membrane, were found to stimulate the migration of different types of cells, including mammary epithelial cells, human umbilical vein endothelial cells, and GBM cells [44][45][46][47]. The migration impairment of GBM cells through Cripto-EVs, instead, may point to a high grade of complexity for the fine-tuning of Cripto localization, route, and consequent function [27].
Cell migration is an extremely finely regulated process. The same stimulus can have a different effect on cells depending on the cellular context (cell and cancer type) as well as the stimulus concentration. It has been clearly demonstrated that the key EMT inducer TGFβ is able to promote an incomplete or hybrid EMT phenotype in specific cell types, characterized by the acquisition of mesenchymal features, metastasis promotion and concomitant maintenance of cell–cell adhesion [48][49][50]. In specific contexts, TGFβ is also able to act in a very opposite way, behaving as a tumor suppressor [51][52]. Moreover, platelet-derived growth factors at low concentrations can promote cell migration, whereas at high concentrations, they may induce proliferation [53]. Both molecules have been found to be associated with EVs, in the soluble form as well as in the precursor form bound to the membrane [54][55], allowing us to hypothesize that encapsulation inside EVs and/or exposition on the EV surface might be an additional mechanism for regulating the spreading and activity of soluble and/or membrane-bound signaling molecules. In other words, EV sorting and delivery might be a physiological and/or pathological alternative route for key signaling molecules, modulating their final impact on cancer development and progression, as schematized in Figure 1.
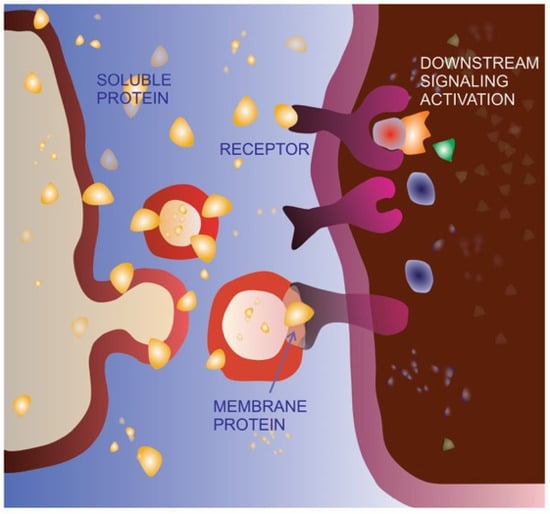
Figure 1. Extracellular vesicle-mediated delivery as an alternative signaling route. The same protein can be bound to the cell membrane, released in the extracellular space as a soluble molecule or delivered on the membrane of extracellular vesicles (EVs). As a soluble ligand, the protein can recognize, bind, and activate its receptor on the membrane of the target cell. The EV-delivered molecule is able to bind the receptor on the target cell but does not activate the same signaling pathway, possibly acting as a dominant-negative form.
The rationale for tumor cells producing EVs with anti-migratory effects might be to target circulating cancer cells and promote a mesenchymal-epithelial transition, with their exit from circulation and homing in the forming metastatic sites. Besides their intrinsic ability to target specific tumors and/or tumor microenvironments, unmodified TDEVs might concomitantly be able to deliver potential endogenous migration inhibitors and/or interfere with the migration signaling pathways of the target cell. The idea that, due to these features, specific subsets of EVs released by tumor cells might be enriched and used per se in anti-cancer therapeutic strategies, is extremely disruptive. Indeed, because of the low yield of commonly used loading procedures, the necessity of post-modification separation, and quality control, the introduction of exogenous material into the vesicles is definitely a bottleneck in the pipeline for the development of EV-based therapies. Finally, even in the case of the delivery of cancer therapeutic molecules, anti-migratory TDEVs might be the carriers of choice as they already have intrinsic anti-tumor properties, which could favor the action of the exogenous loaded molecule.
References
- Shahi, S.; Cianciarulo, C.; Nedeva, C.; Mathivanan, S. Extracellular Vesicles Regulate Cancer Metastasis. Subcell. Biochem. 2021, 97, 275–296.
- Merino-Casallo, F.; Gomez-Benito, M.J.; Hervas-Raluy, S.; Garcia-Aznar, J.M. Unravelling Cell Migration: Defining Movement from the Cell Surface. Cell Adhes. Migr. 2022, 16, 25–64.
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell Adhesion in Cancer: Beyond the Migration of Single Cells. J. Biol. Chem. 2020, 295, 2495–2505.
- Rak, J. Microparticles in Cancer. Semin. Thromb. Hemost. 2010, 36, 888–906.
- Veranič, P.; Lokar, M.; Schütz, G.J.; Weghuber, J.; Wieser, S.; Hägerstrand, H.; Kralj-Iglič, V.; Iglič, A. Different Types of Cell-to-Cell Connections Mediated by Nanotubular Structures. Biophys. J. 2008, 95, 4416–4425.
- Kralj-Iglic, V. Stability of Membranous Nanostructures: A Possible Key Mechanism in Cancer Progression. Int. J. Nanomed. 2012, 7, 3579–3596.
- Camussi, G.; Deregibus, M.-C.; Bruno2, S.; Grange1, C.; Fonsato, V.; Tetta, C. Exosome/Microvesicle-Mediated Epigenetic Reprogramming of Cells. Am. J. Cancer Res. 2011, 1, 98–110.
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444.
- Sung, B.H.; Parent, C.A.; Weaver, A.M. Extracellular Vesicles: Critical Players during Cell Migration. Dev. Cell 2021, 56, 1861–1874.
- Chang, W.H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. Methods Mol. Biol. 2021, 2174, 143–170.
- Mantile, F.; Franco, P.; Stoppelli, M.P.; Liguori, G.L. Biological Role and Clinical Relevance of Extracellular Vesicles as Key Mediators of Cell Communication in Cancer. In Biological Membrane Vesicles: Scientific, Biotechnological and Clinical Considerations. Advances in Biomembranes and Lipid Self-Assembly; Elsiever: Amsterdam, The Netherlands, 2020; Volume 33.
- Lin, L.; Zhou, Y.; Hu, K. Cell–Cell Communication and Extracellular Vesicles in Cancer. Cancers 2023, 15, 2419.
- Barnie, P.A.; Afrifa, J.; Gyamerah, E.O.; Amoani, B. Extracellular Vesicles as Biomarkers and Therapeutic Targets in Cancers; IntechOpen: London, UK, 2016; pp. 225–240.
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular Vesicles as Biomarkers and Therapeutic Targets for Cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39.
- Chen, J.; Tan, Q.; Yang, Z.; Jin, Y. Engineered Extracellular Vesicles: Potentials in Cancer Combination Therapy. J. Nanobiotechnol. 2022, 20, C29–C39.
- Ahmadi, M.; Hassanpour, M.; Rezaie, J. Engineered Extracellular Vesicles: A Novel Platform for Cancer Combination Therapy and Cancer Immunotherapy. Life Sci. 2022, 308, 120935.
- Ma, Y.; Dong, S.; Li, X.; Kim, B.Y.S.; Yang, Z.; Jiang, W. Extracellular Vesicles: An Emerging Nanoplatform for Cancer Therapy. Front. Oncol. 2021, 10, 606906.
- Wu, M.; Wang, M.; Jia, H.; Wu, P. Extracellular Vesicles: Emerging Anti-Cancer Drugs and Advanced Functionalization Platforms for Cancer Therapy. Drug Deliv. 2022, 29, 2513–2538.
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Dostdar, S.A.; Sokolov, A.V.; Brzecka, A.; Sukocheva, O.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; et al. Extracellular Vesicles in Cancer Nanomedicine. Semin. Cancer Biol. 2019, 69, 212–225.
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 33, 2005709.
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Théry, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-Derived Exosomes Are a Source of Shared Tumor Rejection Antigens for CTL Cross-Priming. Nat. Med. 2001, 7, 297–303.
- Xu, Y.; Feng, K.; Zhao, H.; Di, L.; Wang, L.; Wang, R. Tumor-Derived Extracellular Vesicles as Messengers of Natural Products in Cancer Treatment. Theranostics 2022, 12, 163–1714.
- Farooqi, A.A.; Desai, N.N.; Qureshi, M.Z.; Librelotto, D.R.N.; Gasparri, M.L.; Bishayee, A.; Nabavi, S.M.; Curti, V.; Daglia, M. Exosome Biogenesis, Bioactivities and Functions as New Delivery Systems of Natural Compounds. Biotechnol. Adv. 2018, 36, 328–334.
- Garofalo, M.; Saari, H.; Somersalo, P.; Crescenti, D.; Kuryk, L.; Aksela, L.; Capasso, C.; Madetoja, M.; Koskinen, K.; Oksanen, T.; et al. Antitumor Effect of Oncolytic Virus and Paclitaxel Encapsulated in Extracellular Vesicles for Lung Cancer Treatment. J. Control. Release 2018, 283, 223–234.
- Garofalo, M.; Villa, A.; Rizzi, N.; Kuryk, L.; Rinner, B.; Cerullo, V.; Yliperttula, M.; Mazzaferro, V.; Ciana, P. Extracellular Vesicles Enhance the Targeted Delivery of Immunogenic Oncolytic Adenovirus and Paclitaxel in Immunocompetent Mice. J. Control. Release 2019, 294, 165–175.
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng. 2016, 9, 315–324.
- Mantile, F.; Kisovec, M.; Adamo, G.; Romancino, D.P.; Hočevar, M.; Božič, D.; Bedina Zavec, A.; Podobnik, M.; Stoppelli, M.P.; Kisslinger, A.; et al. A Novel Localization in Human Large Extracellular Vesicles for the EGF-CFC Founder Member CRIPTO and Its Biological and Therapeutic Implications. Cancers 2022, 14, 3700.
- Andrews, P.W. Teratocarcinomas and Human Embryology: Pluripotent Human EC Cell Lines. Review Article. Apmis 1998, 106, 158–168.
- Pleasure, S.J.; Lee, V.M.-Y. NTera 2 Cells: A Human Cell Line Which Displays Characteristics Expected of a Human Committed Neuronal Progenitor Cell. J. Neurosci. Res. 1993, 35, 585–602.
- Attia, N.; Mashal, M.; Grijalvo, S.; Eritja, R.; Puras, G.; Pedraz, J.L. Cationic Niosome-Based HBMP7 Gene Transfection of Neuronal Precursor NT2 Cells to Reduce the Migration of Glioma Cells In Vitro. J. Drug Deliv. Sci. Technol. 2019, 53, 101219.
- Zhao, Y.; Wang, S. Human NT2 Neural Precursor-Derived Tumor-Infiltrating Cells as Delivery Vehicles for Treatment of Glioblastoma. Hum. Gene Ther. 2010, 21, 683–694.
- Persico, M.G.; Liguori, G.L.; Parisi, S.; D’Andrea, D.; Salomon, D.S.; Minchiotti, G. Cripto in Tumors and Embryo Development. Biochim. Biophys. Acta Rev. Cancer 2001, 1552, 87–94.
- Minchiotti, G.; Parisi, S.; Liguori, G.L.; D’Andrea, D.; Persico, M.G. Role of the EGF-CFC Gene Cripto in Cell Differentiation and Embryo Development. Gene 2002, 287, 33–37.
- Minchiotti, G.; Parisi, S.; Liguori, G.; Signore, M.; Lania, G.; Adamson, E.D.; Lago, C.T.; Persico, M.G. Membrane-Anchorage of Cripto Protein by Glycosylphosphatidylinositol and Its Distribution during Early Mouse Development. Mech. Dev. 2000, 90, 133–142.
- Xu, C.; Liguori, G.; Persico, M.G.; Adamson, E.D. Abrogation of the Cripto Gene in Mouse Leads to Failure of Postgastrulation Morphogenesis and Lack of Differentiation of Cardiomyocytes. Development 1999, 126, 483–494.
- Liguori, G.L.; Echevarría, D.; Improta, R.; Signore, M.; Adamson, E.; Martínez, S.; Persico, M.G. Anterior Neural Plate Regionalization in Cripto Null Mutant Mouse Embryos in the Absence of Node and Primitive Streak. Dev. Biol. 2003, 264, 537–549.
- Liguori, G.L.; Echevarria, D.; Bonilla, S.; D’Andrea, D.; Liguoro, A.; Persico, M.G.; Martinez, S. Characterization of the Functional Properties of the Neuroectoderm in Mo Use Cripto-/- Embryos Showing Severe Gastrulation Defects. Int. J. Dev. Biol. 2009, 53, 549–557.
- Ding, J.; Yang, L.; Yan, Y.T.; Chen, A.; Desai, N.; Wynshaw-Boris, A.; Shen, M.M. Cripto Is Required for Correct Orientation of the Anterior-Posterior Axis in the Mouse Embryo. Nature 1998, 395, 702–707.
- Giorgio, E.; Liguoro, A.; D’Orsi, L.; Mancinelli, S.; Barbieri, A.; Palma, G.; Arra, C.; Liguori, G.L. Cripto Haploinsufficiency Affects In Vivo Colon Tumor Development. Int. J. Oncol. 2014, 45, 31–40.
- de Castro, N.P.; Rangel, M.C.; Nagaoka, T.; Salomon, D.S.; Bianco, C. Cripto-1: An Embryonic Gene That Promoted Tumorigeneis. Future Oncol. 2010, 6, 1127–1142.
- Bianco, C.; Rangel, M.C.; Castro, N.P.; Nagaoka, T.; Rollman, K.; Gonzales, M.; Salomon, D.S. Role of Cripto-1 in Stem Cell Maintenance and Malignant Progression. Am. J. Pathol. 2010, 177, 532–540.
- Sousa, E.R.; Zoni, E.; Karkampouna, S.; La Manna, F.; Gray, P.C.; De Menna, M.; Julio, M.K. De A Multidisciplinary Review of the Roles of Cripto in the Scientific Literature through a Bibliometric Analysis of Its Biological Roles. Cancers 2020, 12, 1480.
- Freeman, D.W.; Sousa, E.R.; Karkampouna, S.; Zoni, E.; Gray, P.C.; Salomon, D.S.; De Julio, M.K.; Spike, B.T. Whence Cripto: The Reemergence of an Oncofetal Factor in ‘Wounds’ That Fail to Heal. Int. J. Mol. Sci. 2021, 22, 10164.
- Wechselberger, C.; Ebert, A.D.; Bianco, C.; Khan, N.I.; Sun, Y.; Wallace-Jones, B.; Montesano, R.; Salomon, D.S. Cripto-1 Enhances Migration and Branching Morphogenesis of Mouse Mammary Epithelial Cells. Exp. Cell Res. 2001, 266, 95–105.
- Bianco, C.; Adkins, H.B.; Wechselberger, C.; Seno, M.; Normanno, N.; De Luca, A.; Sun, Y.; Khan, N.; Kenney, N.; Ebert, A.; et al. Cripto-1 Activates Nodal- and ALK4-Dependent and -Independent Signaling Pathways in Mammary Epithelial Cells. Mol. Cell. Biol. 2002, 22, 2586–2597.
- Alowaidi, F.; Hashimi, S.; Alqurashi, N.; Wood, S.; Wei, M. Cripto-1 Overexpression in U87 Glioblastoma Cells Activates MAPK, Focal Adhesion and ErbB Pathways. Oncol. Lett. 2019, 18, 3399–3406.
- Alowaidi, F.; Hashimi, S.M.; Nguyen, M.; Meshram, M.; Alqurashi, N.; Cavanagh, B.L.; Bellette, B.; Ivanovski, S.; Meedenyia, A.; Wood, S.A. Investigating the Role of CRIPTO-1 (TDGF-1) in Glioblastoma Multiforme U87 Cell Line. J. Cell. Biochem. 2018, 120, 7412–7427.
- Li, W.; Zhang, X.; Wang, J.; Li, M.; Cao, C.; Tan, J.; Ma, D.; Gao, Q. TGFβ1 in Fibroblasts-Derived Exosomes Promotes Epithelialmesenchymal Transition of Ovarian Cancer Cells. Oncotarget 2017, 8, 96035–96047.
- Gao, J.; Zhu, Y.; Nilsson, M.; Sundfeldt, K. TGF-β Isoforms Induce EMT Independent Migration of Ovarian Cancer Cells. Cancer Cell Int. 2014, 14, 72.
- Jolly, M.K.; Somarelli, J.A.; Sheth, M.; Biddle, A.; Tripathi, S.C.; Armstrong, A.J.; Hanash, S.M.; Bapat, S.A.; Rangarajan, A.; Levine, H. Hybrid Epithelial/Mesenchymal Phenotypes Promote Metastasis and Therapy Resistance across Carcinomas. Pharmacol. Ther. 2019, 194, 161–184.
- Pardali, K.; Moustakas, A. Actions of TGF-β as Tumor Suppressor and pro-Metastatic Factor in Human Cancer. Biochim. Biophys. Acta Rev. Cancer 2007, 1775, 21–62.
- Seoane, J.; Gomis, R.R. TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277.
- Miaczynska, M. Effects of Membrane Trafficking on Signaling by Receptor Tyrosine Kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a009035.
- Rodrigues-Junior, D.M.; Tsirigoti, C.; Lim, S.K.; Heldin, C.-H.; Moustakas, A. Extracellular Vesicles and Transforming Growth Factor β Signaling in Cancer. Front. Cell Dev. Biol. 2022, 10, 1–20.
- Togliatto, G.; Dentelli, P.; Rosso, A.; Lombardo, G.; Gili, M.; Gallo, S.; Gai, C.; Solini, A.; Camussi, G.; Brizzi, M.F. PDGF-BB Carried by Endothelial Cell-Derived Extracellular Vesicles Reduces Vascular Smooth Muscle Cell Apoptosis in Diabetes. Diabetes 2018, 67, 704–716.
More
