Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Alice Vilela.
The natural biopolymer chitin and its deacetylated derivative chitosan are abundant in nature. They are obtained from different sources, including the crustacean shells and the cell walls of fungi. Chitin and chitosan have various applications in the beverage industry, such as a flocculent to improve the clarification process, reduce metals and contaminates, and extend shelf-life. They are also used as material for immobilizing microorganisms and enzymes, allowing bioprocesses to develop that preserve the quality of alcoholic and non-alcoholic beverages.
- clarification agent
- contaminants reduction
- antioxidant activity
- antimicrobial activity
- immobilization
1. Introduction
Chitosan (deacetylated chitin) has been gaining increasing attention due to its biodegradability, renewability, nontoxicity, and non-allergenic properties in the case of fungal chitosan [1]. A vast quantity of living organisms synthesizes chitin and occurs as a building material in the cell walls of fungi and yeast or the exoskeleton of arthropods, crustaceans (shrimp and crabs), insects (beetles, ants, brachiopods, scorpions, and cockroaches) as well as in algae (green algae and brown algae) [2,3][2][3]. Chitosan is generally considered safe (GRAS) by the Food and Drug Administration (FDA). Chitosan has been described as having several applications in the beverage industry (alcoholic and non-alcoholic beverages), such as a chelating agent for metal ions, an antioxidant, antimicrobial, flocculant, and a clarifying agent [4,5,6][4][5][6]. Chitosan and chitin have been applied as flocculants [7]. They can be used for clarification of non-alcoholic and alcoholic beverages, such as fruit juices [8,9,10,11][8][9][10][11] and beer [4], since these increase the suspended particles sedimentation rate and remove particles that could, for example, impact in color and limpidity. Only fungal chitosan is authorized by the European authorities and by the International Organisation of Vine and Wine to be used in wines as a fining agent for clarification, reduction of heavy metals, prevention of iron and copper haze, reduction of the contaminant contents (particularly ochratoxin A), and for antimicrobial action (namely, Brettanomyces spp.) [12]. Several authors have studied the application of chitosan in wine, for example, to wine protein stabilization [13,14,15,16][13][14][15][16], for avoiding wine oxidation [17[17][18][19][20],18,19,20], as an antimicrobial agent [21[21][22][23][24][25],22,23,24,25], and for removing volatile phenolic compounds from red wine [26,27,28][26][27][28]. Recently, Castro-Marín et al. [29] published a review article concerning the different applications of chitosan in winemaking and summarizing the chemical mechanisms underlying its action.
The amino groups of chitosan are protonated in acidic environments, and chitosan is expected to show a performance characteristic of a polyelectrolyte [4]. Electrostatic interaction between chitosan (positively charged) and the acidic protein pepsin (enzyme) is dependent on pH values [30]. Moreover, the interaction between chitosan and pectin has been studied by Marudova et al. [31], describing the action of chitosan as an effective crosslinker at pH 5.6 and exhibiting gel behavior dependent on the pectin esterification degree. It has also been shown that the complexation of chitosan with alginate, pectin, or carrageenan produces coagulating agents with enhanced protein adsorption and a more significant limpidity increase than only chitosan application [32].
2. Chitin and Chitosan Structural Properties
Chitosan is a natural polysaccharide containing glucosamine and N-acetylglucosamine monomeric units. Chitin is produced by chemical treatments involving the extraction by acid treatment to dissolve the calcium carbonate, named demineralization, followed by the alkaline solution to dissolve proteins, called deproteinization [2,33][2][33]. The chitin extraction could also be performed by biological treatments using enzymes and microorganisms [34], namely proteolytic enzymes to digest the proteins, or a fermentation process using microorganisms, which permits the digestion of proteins and minerals [35,36][35][36]. Decolorization is also frequently performed to eliminate pigments to obtain colorless pure chitin [33]. This process should be adjusted according to the chitin source due to the diversity in the ultrastructure of the chitin material obtained from the different sources [33]. Chitosan can then be obtained by partial deacetylation of chitin through hydrolysis by a chitin deacetylase [37,38,39][37][38][39] or by a chemical procedure [40]. From a chemical point of view, both acids and alkalis can be used to deacetylate chitin; nevertheless, glycosidic bonds are highly vulnerable to an acid; thus, alkali deacetylation is more frequently used [41]. At an industrial scale, deacetylation is usually a nonenzymatic process whereby chitosan is obtained by removing R-NHCOCH3 residue by treating it with solid alkali (sodium hydroxide solution 40–50%) at high temperatures (100 °C) [40]. Deacetylation describes a reaction that removes an acetyl functional group. When deacetylation (expressed as a molar percentage) is higher than 50 mol%, the biopolymer becomes soluble in acidic aqueous solutions. It is called chitosan and behaves as a cationic polyelectrolyte due to the protonation of amine groups in the presence of H+ ions [42]. Therefore, chitosan—poly-β-(1,4)-d-glucosamine is a deacetylated cationic linear biopolymer of chitin—poly-β-(1,4)-N-acetyl-d-glucosamine (Figure 1), one of the most abundant natural polysaccharides found in the composition of fungi cell walls [43,44,45][43][44][45] and exoskeletons of insects [46,47[46][47][48][49],48,49], cephalopods, and crustaceans [49,50,51,52,53,54][49][50][51][52][53][54]. Chitosan is a linear polysaccharide composed of randomly distributed β-(1–4)-linked d-glucosamine and N-acetyl-d-glucosamine units. The ratio between these two monomeric units, expressed as the degree of acetylation or deacetylation, ranges from 40 to 99% (degree of deacetylation), and the molar weight ranges from 2 to more than 200 kDa [55]. The chitosan functional proprieties depend on structural characteristics, for instance, degree of deacetylation, molecular weight, and purity. Various chitosans are available commercially, which differ primarily in the degree of deacetylation and molecular weight.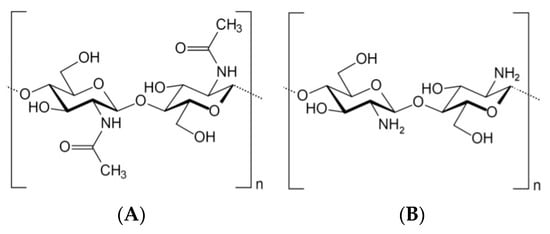
Figure 1.
Chitin (
A
) and chitosan (
B
) chemical structure.
3. Chitosan Applications in Beverages
3.1. Clarification Agent, Metals and Contaminants Reduction
In beverages like fruit juice, clarification is an essential step in the production process, mainly to remove pectins and other compounds in the fluid. Juice limpidity and homogeneity are the two significant characteristics of clarified juices and are achieved by eliminating all suspended solids [58]. Chitosan coagulates the anionic suspended particles such as pectin and protein, and consequently, their separation from beverages is fast, and their turbidity decreases [6,10,59,60][6][10][59][60]. This behavior is related to the chitosan physicochemical properties associated with the presence of amine functions [31]. According to Rizzo et al. [61], the concentration of chitosan used in the clarification process and pH, in addition to the initial juice turbidity to be treated, are essential variables for the coagulation process using chitosan and, consequently, to reach the desired limpidity. Domingues et al. [62] also showed that besides the chitosan concentration, the pH value is an essential factor for the juice turbidity to decrease, as all samples of passion fruit juice at pH 6 presented higher turbidity removal after the treatment with chitosan. Rao et al. [6] also showed that the optimal pH for green tea clarification using chitosan was at pH 5.5. It was observed to be concentration-dependent, leading to higher turbidity reduction in juices when chitosan content was increased [60,62][60][62]. It was also shown that chitosan with a higher deacetylation degree is more effective in protein flocculating; this could be related to the augmented charge density from the free amino groups [63].3.2. Extending the Shelf-Life
There is an increase in consumer demand for safe and healthier products; in this way, the consumption of freshly processed products has been observed, and the beverage industries need to search for compounds to extend the shelf life of these products by applying natural products. The antimicrobial and antioxidant activity of chitosan permits its application to open the beverage shelf life, as shelf life is constrained by microbial spoilage and oxidation [57,82,83,84][57][64][65][66]. The antioxidant activity of chitosan is related to the scavenging effect on free radicals [83,85,86][65][67][68]. Chitosan deacetylation degree influenced its antioxidant activity, and this activity rises with unsubstituted amino groups [57]. Therefore, the antioxidant activities of chitosan increased with an increase in deacetylation degree [87][69]. The molecular weight of chitosan has also been described to have a primary effect on its antioxidant activities; with low molecular weight (16 to 190, 127 kDa), chitosans have more marked scavenging effects on superoxide and hydroxyl radicals than those with high molecular weight (>300 kDa), probably related to the compact structure of the chitosan with a high molecular weight that limited the antiradical activity of the hydroxyl and amino groups [88,89][70][71]. For antimicrobial activity, the most important factors are the type of microorganism [84[66][72][73][74],90,91,92], the chitosan charge density, concentration, molecular weight [84[66][74],92], hydrophilic/hydrophobic characteristics, chelating capacity, and the degree of deacetylation [84][66]. It has been suggested that the polycationic nature of chitosan that forms from acidic solutions below pH 6.5 is a crucial factor. A higher positive charge density leads to electrostatic solid interaction. Therein, the positive charge is associated with the deacetylation degree of chitosan [93][75]. The chitosan antifungal and antimicrobial activity against different fungi, Gram-positive and Gram-negative bacteria, is related to the chitosan cationic properties in an acidic media at pH values below chitosan pKa. As protonated amino groups bind to the negatively charged carboxyl groups, such as bacterial cell wall surface peptidoglycans, altering their barrier properties, leading to permeabilization and destruction of external membranes [94][76]. Therefore, Chitosan is most active at the fungi or bacteria cell surface, leading to permeabilization [90,92,95,96,97], which results in intracellular material leakage and consequently cell death [96,98,99][77][78][79].4. Chitosan Immobilization
Chitosan, like alginate, forms a gel by ionotropic gelation (or coacervation) and is a polymer with numerous applications in immobilization technology of enzymes and microorganisms due to its nontoxic, biocompatible, biodegradable, and antimicrobial properties [111][80]. Chitosan-bearing protonated amino groups can interact with a wide variety of natural or synthetic anionic species, such as negatively charged proteins, DNA [112[81][82],113], and synthetic basic polymers, such as sodium tripolyphosphate (TPP) [114,115][83][84] to form ionic complexes. Ionotropic gelation has been used in the production of polymeric (micro and nan) particles for many applications, namely in biomedicine [116][85] and the pharmaceutical industry, including interferon [117,118,119][86][87][88] and antioxidant administration [120][89]. This technique is versatile and relatively simple. It is possible to produce particles in a wide range of sizes [121][90]. This ionic gelation method to prepare Chitosan/TPP nanoparticles presents the advantages of simple operation, low equipment requirements, low cost, good repeatability, environmentally friendly, and easy large-scale preparation [122,123][91][92], Figure 2.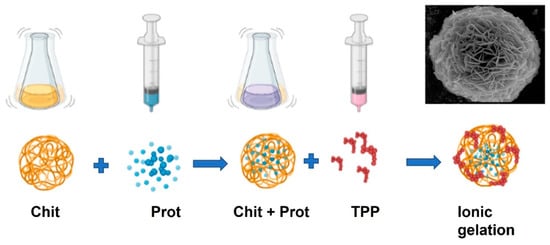
Figure 2. Schematic illustration of the preparation procedure for protein chitosan immobilization. An ionic gelation technique can prepare protein-loaded chitosan beads. Chitosan (Chit) is first mixed with protein (Prot) to provide a homogeneous solution (Chit + Prot). Then, it interacts with sodium tripolyphosphate (TPP) solution to encapsulate the protein during the ionic gelation process.
4.1. Chitosan Microorganism’s Immobilization
Microorganisms’ immobilization consists of the physical confinement of intact cells to a region of space with conservation of biological activity. The use of these methodologies for alcoholic fermentation offers many advantages over the use of the conventional free yeast cell method. The most studied methods for yeast immobilization include using organic supports, mainly alginate. Some benefits of the yeast-immobilization systems include high cell densities, product yield improvement, lowered risk of microbial contamination, better control, and reproducibility of the processes, as well as reuse of the immobilization system for batch fermentation and continuous fermentation technologies [130,131][93][94].
According to Martin and Etievant [132][95], for alcoholic beverage production, the cell carrier or immobilization matrix must fulfill specific requirements: (i) Large surface, with chemical groups favoring cells to adhere; (ii) Easy to handle and regenerate; (iii) High and retained cell viability and operational stability; (iv) Does not affect catalytic activity; (v) Uniform and controllable porosity to allow the free exchange of substrates, products, cofactors, and gases; (vi) Mechanical, chemical, thermal, and biological stability; (vii) Easy to handle, cost-effective, and amenable to scale-up immobilization technique; (viii) Does not affect product quality.
4.2. Chitosan Enzyme Immobilization
Enzymes are functional biological macromolecules that catalyze chemical reactions at very high rates and with high molecular precision. Many winemakers use enzymes to improve extraction, enhance aromas, and block malolactic fermentation (MLF). For instance, in white wines, monoterpenes are the most important compounds responsible for the smell; most of these compounds are present in grapes as nonvolatile, odorless glycoconjugates. Therefore, enzymatic hydrolysis has long been proposed as an alternative for efficiently releasing aromatic compounds at mild winemaking conditions [140][96]. Commercial preparations of soluble enzymes from Aspergillus niger with α-l-arabinofuranosidase, α-l-mannosidase, and βG (β-d-glucosidase) activities have been used in alcoholic fermentation, usually added at the end of the fermentation stage [141][97]. These glycosidases are quickly inactivated in winemaking conditions media, making them less efficient. Moreover, catalyst residues remain in the final wine, thus requiring the addition of a fining agent to stop the reaction [142][98]. Due to these disadvantages, the co-immobilization of glycosidases has been proposed, increasing the stability of the enzymes and recovering the biocatalyst from the medium [143][99].References
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50.
- Mathur, N.K.; Narang, C.K. Chitin and chitosan, versatile polysaccharides from marine animals. J. Chem. Educ. 1990, 67, 938–942.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Gassara, F.; Antzak, C.; Ajila, C.M.; Sarma, S.J.; Brar, S.K.; Verma, M. Chitin and chitosan as natural flocculants for beer clarification. J. Food Eng. 2015, 166, 80–85.
- Ghorbel-Bellaaj, O.; Jridi, M.; Khaled, H.; Jellouli, K.; Nasri, M. Bioconversion of shrimp shell waste for the production of antioxidant and chitosan used as fruit juice clarifier. Int. J. Food Sci. Technol. 2012, 47, 1835–1841.
- Rao, L.; Hayat, K.; Lv, Y.; Karangwa, E.; Xia, S.; Jia, C.; Zhong, F.; Zhang, X. Effect of ultrafiltration and fining adsorbents on the clarification of green tea. J. Food Eng. 2011, 102, 321–326.
- Roberts, G.A.F. Chitin Chemistry; McMillan Press, Ltd.: London, UK, 1992; pp. 85–91.
- Soto-Peralta, N.V.; Muller, H.; Knorr, D. Effects of chitosan treatments on the clarity and color of apple juice. J. Food Sci. 1989, 54, 495–496.
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-Chin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol. 2017, 104, 953–962.
- Tastan, O.; Baysal, T. Clarification of pomegranate juice with chitosan: Changes on quality characteristics during storage. Food Chem. 2015, 180, 211–218.
- Tastan, Ö.; Baysal, T. Chitosan as a novel clarifying agent on clear apple juice production: Optimization of process conditions and changes on quality characteristics. Food Chem. 2017, 237, 818–824.
- OIV. International Code of Oenological Practices. International Organisation of Vine and Wine. 2021. Available online: http://www.oiv.int/fr/normes-et-documents-techniques (accessed on 30 October 2021).
- Vincenzi, S.; Polesani, M.; Curioni, A. Removal of specific protein components by chitin enhances protein stability in a white wine. Am. J. Enol. Vitic. 2005, 56, 246–254.
- Chagas, R.; Monteiro, S.; Ferreira, R.B. Assessment of potential side effects of common fining agents used for white wine protein stabilization. Am. J. Enol. Vitic. 2012, 63, 574.
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309.
- Arenas, I.; Ribeiro, M.; Filipe-Ribeiro, L.; Vilamarim, R.; Costa, E.; Siopa, J.; Cosme, F.; Nunes, F.M. Effect of winemaking technology on protein stability, macro-molecular, and phenolic composition of Albariño white wines: Comparative efficiency of chitosan, k-carrageenan and bentonite as heat stabilisers. Foods 2021, 10, 608.
- Nunes, C.; Maricato, É.; Cunha, Â.; Rocha, M.A.M.; Santos, S.; Ferreira, P.; Silva, M.A.; Rodrigues, A.; Amado, O.; Coimbra, J.; et al. Chitosan–genipin film, a sustainable methodology for wine preservation. Green Chem. 2016, 18, 5331–5341.
- Spagna, G.; Pifferi, P.G.; Rangoni, C.; Mattivi, F.; Nicolini, G.; Palmonari, R. The stabilization of white wines by adsorption of phenolic compounds on chitin and chitosan. Food Res. Int. 1996, 29, 241–248.
- Spagna, G.; Barbagallo, R.N.; Pifferi, P.G. Fining treatments of white wines by means of polymeric adjuvants for their stabilization against browning. J. Agric. Food Chem. 2000, 48, 4619–4627.
- Chinnici, F.; Natali, N.; Riponi, C. Efficacy of chitosan in inhibiting the oxidation of (+)-catechin in white wine model solutions. J. Agric. Food Chem. 2014, 62, 9868–9875.
- Ferreira, D.; Moreira, D.; Costa, E.M.; Silva, S.; Pintado, M.M.; Couto, J.A. The antimicrobial action of chitosan against the wine spoilage yeast Brettanomyces/Dekkera. J. Chitin Chitosan Sci. 2013, 1, 240–245.
- Nardi, T.; Vagnoli, P.; Minacci, A.; Gautier, S.; Sieczkowski, N. Evaluating the impact of a fungal-origin chitosan preparation on Brettanomyces bruxellensis in the context of wine aging. Wine Stud. 2014, 3, 4574.
- Elmaci, S.B.; Gülgör, G.; Tokatli, M.; Erten, H.; İşci, A.; Özçelik, F. Effectiveness of chitosan against wine-related microorganisms. Antonie Van Leeuwenhoek 2015, 107, 675–686.
- Petrova, B.; Cartwright, Z.M.; Edwards, C.G. Effectiveness of chitosan preparations against Brettanomyces bruxellensis grown in culture media and red wines. OENO One 2016, 50, 49–56.
- Valera, M.J.; Sainz, F.; Mas, A.; Torija, M.J. Effect of chitosan and SO2 on viability of Acetobacter strains in wine. Int. J. Food Microbiol. 2017, 246, 1–4.
- Milheiro, J.; Ribeiro, L.F.; Cosme, F.; Nunes, F.M. A simple, cheap and reliable method for control of 4-ethylphenol and 4-ethylguaiacol in red wines. Screening of fining agents for reducing volatile phenols levels in red wines. J. Chromatogr. B 2017, 1041–1042, 183–190.
- Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Reducing the negative sensory impact of volatile phenols in red wine with different chitosans: Effect of structure on efficiency. Food Chem. 2018, 242, 591–600.
- Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Data on changes in red wine phenolic compounds, headspace aroma compounds and sensory profile after treatment of red wines with chitosans with different structures. Data Brief. 2018, 17, 1201–1217.
- Castro-Marín, A.; Colangelo, D.; Lambri, M.; Riponi, C.; Chinnici, F. Relevance and perspectives of the use of chitosan in winemaking: A review. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–15.
- Boeris, V.; Micheletto, Y.; Lionzo, M.; da Silveira, N.P.; Pico, G. Interaction behavior between chitosan and pepsin. Carbohydr. Polym. 2011, 84, 459–464.
- Marudova, M.; MacDougall, A.J.; Ring, S.-G. Pectin–chitosan interactions and gel ormation. Carbohydr. Res. 2004, 339, 1933–1939.
- Savant, V.D.; Torres, J.A. Fourier transform infrared analysis of chitosan based coagulating agents for treatment of surimi waste water. J. Food Technol. 2003, 1, 23–28. Available online: https://medwelljournals.com/abstract/?doi=jftech.2003.23.28 (accessed on 24 November 2021).
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133.
- Khanafari, A.; Marandi, R.; Sanatei, S. Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. Iran. J. Environ. Health Sci. Eng. 2008, 5, 1–24.
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25.
- Gortari, M.C.; Hours, R.A. Biotechnological processes for chitin recovery out of crustacean waste: A mini-review. Electron. J. Biotechnol. 2013, 16, 1–14.
- Kafetzopoulos, D.; Martinou, A.; Bouriotis, V. Bioconversion of chitin to chitosan: Purification and characterization of chitin deacetylase from Mucor rouxii. Proc. Nat. Acad. Sci. USA 1993, 90, 2564–2568.
- Il’ina, A.V.; Tkacheva, Y.V.; Varlamov, V.P. Depolymerization of High-Molecular-Weight Chitosan by the Enzyme Preparation Celloviridine G20x. Appl. Biochem. Microbiol. 2002, 38, 112–115.
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421.
- No, H.K.; Meyers, S.P. Preparation and Characterization of Chitin and Chitosan—A review. J. Aquatic Food Prod. Technol. 1995, 4, 27–52.
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and Chitosan Preparation from Shrimp Shells Using Optimized Enzymatic Deproteinization. Process Biochem. 2012, 47, 2032–2039.
- Younes, I.; Ghorbel-Bellaaj, O.; Chaabouni, M.; Rinaudo, M.; Souard, F.; Vanhaverbeke, C.; Jellouli, K.; Nasri, M. Use of a fractional factorial design to study the effects of experimental factors on the chitin deacetylation. Int. J. Biol. Macromol. 2014, 70, 385–390.
- Pochanavanich, P.; Suntornsuk, W. Fungal chitosan production and its characterization. Lett. Appl. Microbiol. 2002, 35, 17–21.
- Zamani, A.; Edebo, L.; Sjostrom, B.; Taherzadeh, M.J. Extraction and precipitation of chitosan from cell wall of zygomycetes fungi by dilute sulfuric acid. Biomacromolecules 2007, 8, 3786–3790.
- Streit, F.; Koch, F.; Laranjeira, M.C.M.; Ninow, J.L. Production of fungal chitosan in liquid cultivation using apple pomace as substrate. Braz. J. Microbiol. 2009, 40, 20–25.
- Ai, H.; Wang, F.; Yang, Q.; Zhu, F.; Lei, C. Preparation and biological activities of chitosan from the larvae of housefly, Musca domestica. Carbohydr. Polym. 2008, 72, 419–423.
- Nemtsev, S.V.; Zueva, O.Y.; Khismatullin, M.R.; Albulov, A.I.; Varlamov, V.P. Isolation of chitin and chitosan from honeybees. Appl. Biochem. Microbiol. 2004, 40, 39–43.
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354.
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibeka, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795.
- Kandra, P.; Challa, M.M.; Jyothi, H.K. Efficient use of shrimp waste: Present and future trends. Appl. Microbiol. Biotechnol. 2012, 93, 17–29.
- Pachapur, V.L.; Guemiza, K.; Rouissi, T.; Sarma, S.J.; Brar, S.K. Novel biological and chemical methods of chitin extraction from crustacean waste using saline water. J. Chem. Technol. Biotechnol. 2016, 91, 2331–2339.
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2013, 33, 379–403.
- Kaya, M.; Baran, T.; Erdogan, S.; Mentes, A.; Ozusaglam, M.A.; Cakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. 2014, 45, 72–81.
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083.
- Mati-Baouche, N.; Elchinger, P.-H.; De Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212.
- Abdou, E.S.; Elkholy, S.S.; Elsabee, M.Z.; Mohamed, E. Improved Antimicrobial Activity of Polypropylene Films by Plasma Surface treatment and Modification with Chitosan. J. Appl. Polym. Sci. 2008, 108, 2290–2296.
- Rocha, M.A.M.; Coimbra, M.A.; Nunes, C. Applications of chitosan and their derivatives in beverages: A critical review. Curr. Opin. Food Sci. 2017, 15, 61–69.
- Close, H.N.; Sin, S.; Yusof, N.S.A.; Hamid, R.A. Rahman Optimization of enzymatic clarification of sapodilla juice using response surface methodology. J. Food Eng. 2006, 73, 313–319.
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Clarification of fruit juice with chitosan. Process Biochem. 2004, 39, 2229–2232.
- Rungsardthong, V.; Wongvuttanakul, N.; Kongpien, N.; Chotiwaranon, P. Application of fungal chitosan for clarification of apple juice. Process Biochem. 2006, 41, 589–593.
- Rizzo, L.; Di Gennaro, A.; Gallo, M.; Belgiorno, V. Coagulation/chlorination of surface water: A comparison between chitosan and metal salts. Sep. Purif. Technol. 2008, 62, 79–85.
- Domingues, R.C.C.; Faria, S.B., Jr.; Silva, R.B.; Cardoso, V.L.; Reis, M.H.M. Clarification of passion fruit juice with chitosan: Effects of coagulation process variables and comparison with centrifugation and enzymatic treatments. Process Biochem. 2012, 47, 467–471.
- Ariffin, A.; Shatat, R.S.; Norulaini, A.N.; Omar, A.M. Synthetic polyelectrolytes of varying charge densities but similar molar mass based on acrylamide and their applications on palm oil mill effluent treatment. Desalination 2005, 173, 201–208.
- Gutiérrez, T.J. Chitosan applications for the food industry. In Chitosan: Derivatives, Composites and Applications; Wiley: Hoboken, NJ, USA, 2017; Chapter 8; pp. 183–232.
- Friedman, M.; Juneja, V.K. Review of Antimicrobial and Antioxidative Activities of Chitosans. Food J. Food Prot. 2010, 73, 1737–1761.
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63.
- Castro, A.; Culcasi, M.; Cassien, M.; Stocker, P.; Thetiot-Laurent, S.; Robillard, B.; Chinnici, F.; Pietri, S. Chitosan as an antioxidant alternative to sulphites in oenology: EPR investigation of inhibitory mechanisms. Food Chem. 2019, 285, 67–76.
- Chien, P.; Li, C.; Lee, C.; Chen, H. Influence of micronized chitosan on antioxidative activities in grape juice. Food Nutr. Sci. 2013, 4, 224–228.
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844.
- Xing, R.; Liu, S.; Guo, Z.; Yu, H.; Wang, P.; Li, C.; Li, Z.; Li, P. Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorgan. Med. Chem. 2005, 13, 1573.
- Chien, P.-J.; Sheu, F.; Huang, W.-T.; Su, M.-S. Effect of molecular weight of chitosans on their antioxidative activities in apple juice. Food Chem. 2007, 102, 1192–1198.
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Xavier Malcata, F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134.
- Palma-Guerrero, J.; Lopez-Jimenez, J.A.; Perez-Berna, A.J.; Huang, I.C.; Jansson, H.B.; Salinas, J.; Villalaín, J.; Read, N.D.; Lopez-Llorca, L.V. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol. Microbiol. 2010, 75, 1021–1032.
- Park, S.-C.; Nah, J.-W.; Park, Y. pH-dependent mode of antibacterial actions of low molecular weight water-soluble chitosan (LMWSC) against various pathogens. Macromol. Res. 2011, 19, 853–860.
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem. Eng. 2008, 40, 485–491.
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283.
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155.
- Fernandez-Saiz, P.M.J.; Ocio Lagaron, J.M. The use of chitosan in antimicrobial films for food protection. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2010, 5, 1–11.
- Ganan, M.; Carrascosa Martinez-Rodriguez, A.J. Antimicrobial activity of chitosan against Campylobacter spp. and other microorganism and its mechanism of action. J. Food Prot. 2009, 72, 1735–1738.
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981.
- Strand, S.P.; Lelu, S.; Reitan, N.K.; de Lange Davies, C.; Artursson, P.; Vårum, K.M. Molecular design of chitosan gene delivery systems with an optimized balance between polyplex stability and polyplex unpacking. Biomaterials 2010, 31, 975–987.
- Gao, P.; Xia, G.; Bao, Z.; Feng, C.; Cheng, X.; Kong, M.; Liu, Y.; Chen, X. Chitosan based nanoparticles as protein carriers for efficient oral antigen delivery. Int. J. Biol. Macromol. 2016, 91, 716–723.
- Cho, Y.; Shi, R.; Borgens, R.B.J. Chitosan nanoparticle-based neuronal membrane sealing and neuroprotection following acrolein-induced cell injury. Biol. Eng. 2010, 4, 2.
- Nguyen, T.V.; Nguyen, T.T.H.; Wang, S.-L.; Vo, T.P.K.; Nguyen, A.D. Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res. Chem. Intermed. 2016, 43, 3527–3537.
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447.
- Canepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a drug delivery system based on chitosan nanoparticles for oral administration of interferon-α. Biomacromolecules 2017, 18, 3302–3309.
- Fernández-Quiroz, D.; Loya-Duarte, J.; Silva-Campa, E.; Arguelles-Monal, W.; Sarabia-Sainz, A.; Lucero-Acuña, A.; del Castillo-Castro, T.; San Román, J.; Lizardi-Mendoza, J.; Burgara-Estrella, A.J.; et al. Temperature stimuli-responsive nanoparticles from chitosan-graft-poly (N-vinylcaprolactam) as a drugdeliverysystem. J. Appl. Polym. Sci. 2019, 136, 47831.
- Pedroso-Santana, S.; LamazaresArcia, E.; Fleitas-Salazar, N.; Gancino Guevara, M.; Mansilla, R.; Gomez-Gaete, C.; Altamirano, C.; Fernández, K.; Ruiz, A.; Toledo Alonso, J.R. Polymeric nanoencapsulation of alpha interferon increases drug bioavailability and induces a sustained antiviral response in vivo. Mater. Sci. Eng. C 2020, 116, 111260.
- Othman, N.; Masarudin, M.J.; Kuen, C.Y.; Dasuan, N.A.; Abdullah, L.C.; Jamil, S.N. Synthesis and Optimization of Chitosan Nanoparticles Loaded with l-Ascorbic Acid and Thymoquinone. Nanomaterials 2018, 8, 920.
- Sacco, P.; Pedroso-Santana, S.; Kumar, Y.; Joly, N.; Martin, P.; Bocchetta, P. Ionotropic Gelation of Chitosan Flat Structures and Potential Applications. Molecules 2021, 26, 660.
- Jain, A.; Thakur, K.; Sharma, G.; Kush, P.; Jain, U.K. Fabrication, characterization, and cytotoxicity studies of ionically cross-linked docetaxel loaded chitosan nanoparticles. Carbohydr. Polym. 2016, 137, 65–74.
- Hashad, R.A.; Ishak, R.A.; Fahmy, S.; Mansour, S.; Geneidi, A.S. Chitosan-tripolyphosphate nanoparticles: Optimization of formulation parameters for improving process yield at a novel pH using artificial neural networks. Int. J. Biol. Macromol. 2016, 86, 50–58.
- Nedović, V.A.; Manojlovic, V.; Bugarski, B.; Willaert, R. State of the art in immobilized/encapsulated cell technology in fermentation processes. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Nedović, V.A., Zuidam, N.J., Eds.; Springer: London, UK, 2010; pp. 119–146.
- Moreno-García, J.; García-Martínez, T.; Mauricio, J.C.; Moreno, J. Yeast Immobilization Systems for Alcoholic Wine Fermentations: Actual Trends and Future Perspectives. Front. Microbiol. 2018, 9, 241.
- Martin, B.; Etievant, P.X. Quantitative determination of solerone and sotolona in flor sherries by two dimensional-capillary GC. J. High Resol. Chromat. Chromat. Commun. 1991, 14, 133–135.
- Günata, Y.Z.; Bayonove, C.L.; Tapiero, C.; Cordonnier, R.E. Hydrolysis of grape monoterpenyl β-d-glucosides by various β-glucosidases. J. Agric. Food Chem. 1990, 38, 1232–1236.
- Claus, H.; Mojsov, K. Enzymes for wine fermentation: Current and perspective applications. Fermentation 2018, 4, 52.
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463.
- Spagna, G.; Barbagallo, R.N.; Greco, E.; Manenti, I.; Pifferi, P.G. A mixture of purified glycosidases from Aspergillus niger for oenological application immobilised by inclusion in chitosan gels. Enzym. Microb. Technol. 2002, 30, 80–89.
More
