Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Alice Vilela.
Red fruits are not only a source of vitamins but are also a rich source of minerals (phosphorus, calcium, iron, potassium, magnesium, manganese, sodium, and copper). Shows sweet cherry, blackberry, blueberry, raspberry, and strawberry mineral composition.
- antioxidant activity
- phenolic compounds
- volatile compounds
- vitamins
- minerals
- fatty acids
- fibers
- consumer perception
- health benefits
1. Vitamins and Minerals
Red fruit berries are the best dietary sources of bioactive compounds, namely vitamins and minerals with antioxidant properties [1,2][1][2]. Since red fruits do not usually undergo any processing to be consumed, their antioxidant properties are not reduced. According to Nile and Park [17][3], 100 g of the edible portion of raspberries, blackberries, or blueberries could provide more than 50% of the Recommended Dietary Allowance for manganese, vitamin C (ascorbic acid), and vitamin B9 (folic acid).
1.1. Vitamins
Vitamins are organic substances with solid antioxidant potential that our bodies cannot synthesize sufficiently yet are essential for their sound development, even in trace amounts. According to Rodriguez-Amaya [18][4], there are 14 known vitamins grouped into two large groups of molecules: fat-soluble (A, D, E, and K) and water-soluble (B group and C).
The red fruit is a set of fruits of black or red color, primarily arranged in berries, like strawberry, cherry, red raspberry, black raspberry, blackberry, cranberry, blueberry, blackcurrants, and grapes. Vitamin C, or ascorbic acid, is the most quantified in red fruits and is one of the main antioxidant compounds in this type of fruit. It is a water-soluble carbohydrate-derived compound known for its high antioxidant activity due to neutralizing free radicals and other reactive oxygen species and acidic properties due to a 2,3-enediol moiety (Figure 1) [3][5].
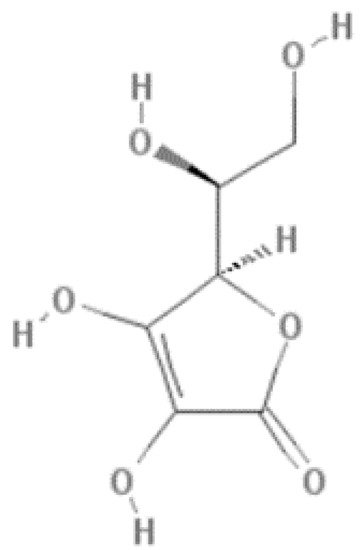
Figure 1.
Structure of ascorbic acid.
Contrary to vitamin C, vitamin A is not found in fruits, at least in large quantities, with some exceptions such as the mango, the cantaloupe, and even the watermelon. A total of 1–2 mg daily is the human requirement for vitamin B2 (riboflavin). The largest source of this vitamin is green vegetables, unlike fruits, which are relatively poor in riboflavin [3][5]. Vitamin B6 (riboflavin) is not present in large quantities in red fruits but in appreciable amounts in grapes, prunes, avocados, and bananas [3][5].
1.2. Minerals
Usually, fruits are not recognized as primary sources of mineral intake. Even so, according to the Dietary Approaches to Stop Hypertension (DASH), fruits contribute an average of 5.8%, 17.3%, 33.0%, and 6.6% to the intakes of calcium, magnesium, potassium, and zinc, respectively [29][6]. Red fruits are not only a source of vitamins [1] but are also a rich source of minerals (phosphorus, calcium, iron, potassium, magnesium, manganese, sodium, and copper) [2].
Among the fruits analyzed, blackberries and cherries have the highest concentration of minerals. There are essential differences between the different researchers regarding the concentration of minerals [2,19,23,30][2][7][8][9]. This is explained, as previously mentioned, since, in most cases, comparisons are made between fruits of different cultivars, subject to foreign soil and climatic conditions and post-harvest handling techniques [2]. The effects of cultivars and cultivation conditions on the composition of strawberries are demonstrated by Hakala et al. [31][10].
2. Sugars and Organic Acids
Generally, fructose and glucose are the main sugars in red fruits, while citric and malic acids are the primary organic acids in this type of fruit. Mikulic-Petkovsek et al. [32][11] studied the sugars and organic acids content in the fruit of 25 wild and cultivated berry species. They found that glucose and fructose were the most abundant sugars in berry fruits, and the primary organic acids were citric and malic acid. Instead, Viljakainen et al. [33][12] also showed significant variations in sugars and organic acids between different berry cultivars.
In cherries, the most abundant soluble sugars observed were glucose, fructose, and sugar alcohol sorbitol, while malic, oxalic, and shikimic acids were the most abundant organic acids [37][13].
In strawberries, Urün et al. [38][14] found high content of citric (522.4–711.5 mg/g FW) and malic acid (159.8–266.7 mg/g FW), and fructose was the sugar present in the highest concentration (2.17–4.43% of total sugars). Also, Morais et al. [39][15] showed enhancement in the total soluble solids content in strawberry plants inoculated with the PGPB strain Pedobacter sp. CC1.
Tartaric, malic, and citric acids are the most preponderant organic in white and red grapes. In a study of 24 red grape varieties [40][16], the titratable acidity, expressed as equivalent of tartaric acid, varied from 3.9 (‘Moreto Boal’) to 13.5 g/L (‘Tinta Miúda’ and ‘Jean’), these results are following the tartaric acid concentration that ranged from 2.49 to 7.70 g/L of tartaric acid. In another study with red grapes from the Douro and Dão regions, Portugal, it was found that red grapes from the Douro Region had a higher content of tartaric acid (average values 6.21 g/L) compared to red grapes from Dão (4.96 g/L) [41][17].
3. Dietary Fibers
There are several definitions of dietary fibers [46][18]; according to the Institute of Medicine [47][19], dietary fiber consists of non-digestible carbohydrates and lignin that are intrinsic and intact in plants. The term includes cellulose, hemicellulose, lignin, pectins, gums, mucilages, and a non-carbohydrate component [1]. Anita and Abraham [48][20] classified the dietary fiber into two categories: cellulose, hemicellulose, and lignin, which are water-insoluble/less fermented, being the pectins, gums, and mucilages water-soluble/well-fermented fibers.
The benefits of a high-fiber diet have long been known [49][21], and we are always incited to eat fiber-rich foods [50][22]. Thus, in addition to the direct consumption of fiber-rich products and ingredients, the food industry has been searching for new sources of dietary fiber and developing new products with fiber supplementation [51][23]. Diets rich in fruits with a high content of threads have been related to the decreased incidence of several types of diseases [52][24]. Indeed, the modulation of function of the intestinal tract [50][22], lower risk of colorectal cancer [52][24], reduction in the total and LDL cholesterol [52][24] and cardiovascular disease, reduction onset risk or symptoms of metabolic syndrome and type 2 diabetes, are benefits of a fiber-rich diet. In addition, these types of diets are usually relatively low in calories compared to meals rich in other food types [53][25]. It is recommended that adults should ingest about 20 to 35 g of dietary fiber per day [54][26].
4. Lipids and Fatty Acids
The increased consumption of red fruits is associated with their nutritional value, which offers many health benefits, particularly in the prevention of cardiovascular diseases and reduction in cancer risks. When consumed raw, red fruits present reduced fat levels, generally below 1%. Pacifico et al. [59][27] studied two sweet cherry cultivars (‘Del Monte’ and ‘Della Recca’) and found differences in their lipid composition. Similar findings were described by Kafkas et al. [60][28] in nine strawberry cultivars (‘Call-Giant4′, ‘Camarosa,’ ‘Fern,’ ‘Festival,’ ‘Kabarla,’ ‘Redlands Hope,’ ‘Sweet Charlie,’ ‘Whitney,’ and ‘Gianna’) of Turkey. Kafkas et al. [61][29] in seven raspberry cultivars (‘Heritage,’ ‘Canby,’ ‘Willamette,’ ‘Hollanda Boduru,’ ‘Newburgh,’ ‘Tulameen,’ and ‘Meeker’) and Fadavi et al. [62][30] in 25 pomegranates varieties grown in Iran also found differences in the lipid percentage and fatty acid composition. According to Al Juhaimi et al. [63][31], the rich design of grapes depends on the local origin. The same conclusion was achieved by Melgarejo and Artés [64][32] when comparing the oil content and fatty acid composition of the oilseed of seven sweet Spanish varieties with some Oriental pomegranate varieties. Al-Maiman and Ahmad [65][33] also observed a slight modification in lipid and fatty acid content during different stages of pomegranate maturation (unripe, half-ripe, and full-ripe fruits). Wang and Wang [66][34] analyzed the effect of storage conditions on several cranberry varieties and concluded that storage temperature greatly affected their fatty acid profile. The types of product processed and its residues also affect the lipid content and the fatty acid composition. Recently, Zafra-Rojas et al. [67][35] found that blackberry residues comprised of peel, seeds, and pulp exhibited lower content of fatty acids when compared with the commercial product.
Lipids in fruits are crucial in the metabolism since they are included in cell membranes, increasing resistance to viral infections and catarrhal diseases [78][36]. In red fruits, the low lipid content associated with low cholesterol levels makes these fruits highly appreciated by consumers. Consumers have increasingly become more concerned with their food's nutritional and caloric value, looking for healthier, innovative, safe, and easy-to-use products. Therefore, the consumption of red fruits has increased, in part due to an array of health benefits. Additionally, they present some fatty acids with numerous positive effects on human health, as an anticancer and neuroprotective agent, is also associated with cardiovascular disease protection [79][37]. Fatty acids in red fruits are mainly polyunsaturated acids necessary to build cell membranes and cover nerves for proper blood clotting, muscle movement, and protection against inflammation [80][38]. Polyunsaturated fatty acids are essential for normal body functions and are associated with significant beneficial cardiovascular effects [81][39].
According to the Food and Agriculture Organization/World Health Organization, about 2–4% of daily energy should come from essential fatty acids, with an additional 3% energy for pregnant or breastfeeding mothers [82][40]. The dominant fatty acids in red fruits are omega-6 linoleic acid, omega-9 oleic acid, and omega-3 linolenic acid, which are linked to various health benefits. Omega-3 and omega-6 are crucial for preventing and treating cardiovascular disease [81][39]. Their incorporation into a diet can decrease mortality from coronary artery and cardiovascular diseases [81][39]. Still linked to the reduction in cardiovascular disorders is omega-9, the most common monounsaturated fatty acid, which has also been linked to beneficial effects for diabetes [83][41].
5. Polyphenols
5.1. Phenolic Acids and Flavonoids
Phenolic compounds are ubiquitous in plants, and as in other fruits, they are in high amounts in red fruits. They are among the most studied secondary metabolites due to their bioactive functions, such as anti-proliferative, anti-diabetic, anticancer, anti-microbial, anti-inflammatory, and antiviral, along with their high antioxidant capacity [84][42]. Phenolics are a group of hydroxylated molecules gathered in different types of structures with a typical aromatic ring (Figure 2), and currently, about 8,000 other forms of plant phenolics are known [85][43].
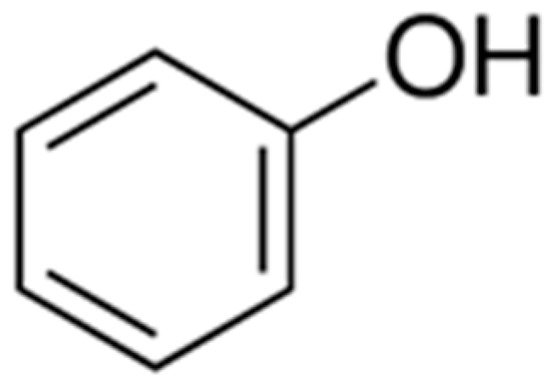
Figure 2.
The bare carbon skeleton of phenolics.
There are many criteria to classify or distinguish phenolics. However, the most commonly used is the division of phenolics into flavonoids and non-flavonoids. According to Działo et al. [85][43], flavonoids are based on two aromatic rings connected by a bridge consisting of three carbons (C6-C3-C6), which are divided into six main sub-classes: flavonols, flavones, flavanones, flavan-3-ols, isoflavones, and anthocyanidins. In nature, flavonoids occur usually in association with sugar as glycosides. The second class of phenolics is the non-flavonoid molecules [85][43], which can be divided into three subgroups: phenolic acids, lignans (C6-C3)2, and stilbenes C6-C2-C6 [86][44]. Phenolic acids can be divided into hydroxybenzoic (C6-C1) acid derivatives and hydroxycinnamic (C6-C3) acid derivatives. The first group includes molecules such as hydroxybenzoic, gallic, vanillic, and ellagic acid, and in the second group, p-coumaric, caffeic, ferulic, and chlorogenic acids are the most representative [87][45]. Flavonoids, the most relevant compounds, include catechin, quercetin, kaempferol, luteolin, and myricetin [85,87,88][43][45][46]. Anthocyanidins have cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin as the most representative molecules [87,89][45][47]. Figure 3 illustrates the chemical structures of the most common phenolics found in fruits.
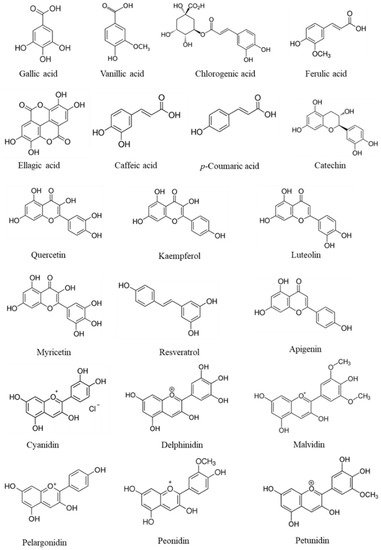
Figure 3.
Chemical structures of phenolics that are commonly found in fruits.
According to the literature, in red fruits, diverse and high amounts of phenolics can be found; however, each type of fruit contains a typical phenolic profile.
Certain similarities in phenolic content can be observed within the same plant family and genus. For example, flavonols and hydroxycinnamic acids are typical of the Ericaceae family, genus Vaccinium (bilberry, blueberry, cranberry, and lingonberry) [90,92,94,95,96][48][49][50][51][52]. In contrast, flavonols dominate in gooseberry, black, and red currant (Grossulariaceae family, genus Ribes) [98,101][53][54]. Instead, ellagic acid is the main phenolic compound in fruits from the Rosaceae family, genus Fragaria, and Rubus (strawberry and red raspberry) [93,101,102][54][55][56].
Despite the similarity between red fruits, each species has its typical profile. For example, Häkkinen et al. [111][57] reported that blueberry is rich in quercetin and caffeic acid, while bilberry and lingonberry have residual concentrations of quercetin. Hydroxycinnamates dominated in all cherry samples and represented 60–74% by weight of the phenols in the fresh and stored models of the varieties ‘Saco,’ ‘Summit,’ and ‘Van,’ and 45% by weight of the phenols in the cv—bullet samples, which were richer in anthocyanins. The relative and total levels of hydroxycinnamates, anthocyanins, flavonols, and flavan-3-ols varied among sweet cherry cultivars and during storage. Moreover, cold storage induced decreased total phenol levels in the varieties ‘Summit’ and ‘Van´; however, they increased total phenol levels in the ‘Burlat’ and ‘Saco’ [112,113][58][59].
5.2. Anthocyanins
Anthocyanins are generally accepted as red fruits' most significant group of phenolics. Anthocyanins are water-soluble compounds responsible for the blue, purple, red, or black color of many fruits, including red fruits. Until now, there are about 17 anthocyanidins identified adequately in nature. However, only six of them, cyanidin, delphinidin, petunidin, peonidin, pelargonidin, and malvidin, are found in most foods and plants [89][47]. When anthocyanidins are coupled with sugars, anthocyanins are formed [87][45].
Cyanidin-3-O-rutinoside and cyanidin-3-O-xylosylrutinoside were the main anthocyanins found in black raspberries [117][60], cyanidin-3-O-rutinoside and cyanidin-3-O-glucoside were the main anthocyanins found in sweet cherry [112,118][58][61], and delphinidin-3-O-galactoside was the main anthocyanin in blueberries [119][62]. Applying gibberellic acid, abscisic acid, and glycine-betaine at pre-harvest increased anthocyanin content in cherries [120][63]. Red raspberry cyanidin-3-O-sophoroside and cyanidin-3-O-glucoside were the main phenolics found [16,110,121][64][65][66]. Guiné et al. [93][55] found that red raspberry was also rich in ferulic acid, vanillic acid, and delphinidin-3-O-glucoside, while gooseberry was rich in chlorogenic acid and cyanidin-3-O-glucoside. Strawberry was shown to be rich in pelargonidin and ellagic acid [110,122][65][67].
Grapes are the primary dietary source of anthocyanins. Therefore, they have diverse biological properties and are regarded as secondary metabolites with potential nutritional value. Anthocyanins are mainly localized in berry skin. Grape anthocyanins are the 3-O-monoglucosides of delphinidin, cyanidin, petunidin, peonidin, and malvidin. Glucosylated derivatives of these anthocyanins, esterified at the C6 position of glucose with acetyl or coumaroyl groups, have also been found, albeit at low concentrations. The monomeric anthocyanins in grape skin extracts were mainly malvidin (1.40–7.09 mg/g of skin), in particular, malvidin-3-glucoside (0.62–6.09 mg/g of skin) [40][16].
In cherry, extracts from stems of cv. Lapins and kernels of cv. Early Bigi presented high levels of total phenolics, flavonoids, and ortho-diphenols. The major phenolic compounds identified in stems and seeds were sakuranetin and catechin. Moreover, antioxidant activities positively correlated with the increments in phenolic compounds [130][68].
During production, the correct combination of scion × rootstock can produce fruits with higher firmness, weight, sugars, vitamins, and phenolic compounds that boost the fruit’s antioxidant activity. Orchard management, such as drip irrigation and summer pruning, can also increase the total phenolic content. At the same time, applying growth regulators can improve storability, increase red coloring, increase fruit size, and reduce cracking. Salicylic acid, oxalic acid, acetylsalicylic acid, and methyl salicylate are promising growth regulators as they also grow total phenolics anthocyanins and induce the higher activity of antioxidant enzymes [131][69]. Biostimulants, such as glycine-betaine and Ascophyllum nodosum extracts, increased cherries' polyphenols content and antioxidant capacity [132,133][70][71]. In grapes, it was also observed that the application of chitosan on the whole vine before and after veraison led to the increased concentration of total phenolic compounds, anthocyanins, and tannins [134][72]. In this study, it was also observed that chitosan application not only induced the synthesis of phenolic compounds but also acted as a facilitator of phenolic transfer from leaves towards grape berries. Furthermore, the application of other foliar mitigation treatments (kaolin (5%) and potassium silicates (0.1 and 0.05%)) influenced the grape berry quality, namely the concentration of total anthocyanins and monomeric anthocyanins [135][73].
The tannin profiles of five grapes (Vitis vinifera L.) varieties were studied by Cosme et al. [136][74]. Depending on the grape variety, the polymeric fractions in skins represented 91–99%, and the distribution of the mean degree of polymerization (mDP) of the skin proanthocyanidins ranged from 3.8 to 81.0.
Although it is common to find this profile in red fruits, the differences in their levels often found within the same genus and species are due to genotype, cultural practices, environmental conditions, fruit ripeness, and postharvest and storage conditions. Howard et al. [137][75] found that total phenolics, hydroxycinnamic acids, and anthocyanidins in blueberries can vary significantly between genotypes and between growing seasons, and from their point of view, different genotypes should constantly be screened over multiple seasons to identify phenolic-rich germplasm. Similar results were achieved by Nour et al. [99][76] when they studied the variation in anthocyanins profile and content along with different genotypes of blackcurrant, and they reported that genotype is one factor that significantly influences the content of phenolics. In addition, Vagiri et al. [98][53] found a significant variation in phenolics in blackcurrants due to genotypes, ontogenic stage, and location. In 2012, Aaby et al. [102][56] found a considerable variation in phenolic profile and content in 27 strawberry cultivars during ripening, a crucial stage of fruit development and vital in phenolic accumulation. Zhang et al. [138][77], studying the effect of maturation in the collection of anthocyanins in strawberry (Fragaria × ananassa Duch.), found that the major groups of compounds such as anthocyanins, amino acids, and sugars alter during growth and maturation. The same authors concluded that each stage of strawberry development has its unique metabolic profile, with the most drastic changes occurring at the transition toward the red-ripened stage. Beekwilder et al. [139][78] reported an increment in the level of anthocyanins during raspberry fruit ripening. In contrast, the levels of ellagitannins and proanthocyanidins decreased in the same period, probably due to differences in enzymatic activity of glycosyltransferases, which interfere with the biosynthetic pathways of anthocyanins. Atkinson et al. [140][79] also found that cultural practices could interfere significantly with the content of phenolics. They found that using mulches in strawberry cultivation can increase the concentration of ellagic acid and ascorbic acid due to significant increments in light reflection. Vyas et al. [141][80] showed that environmental conditions had substantial effects on the concentration of anthocyanins and proanthocyanidins in lingonberry, and the levels of these two types of antioxidant compounds were positively correlated with latitude, altitude, reduced temperature, and increased precipitation of the collection sites.
Another essential set of factors that affects the content and availability of phenolics in red fruits are the post-harvest factors, including storage conditions and processing. Kozos et al. [142][81] found that storing blueberries in a controlled atmosphere for six weeks decreases the loss of anthocyanins compared to a standard atmosphere. The same authors found that to preserve the high quality of blueberries; the fruits must be cooled quickly after harvest and stored in a cold room with a controlled atmosphere. Also, Srivastava et al. [143][82] observed retention higher than 40% in ellagic acid and quercetin, two essential antioxidant phenolics, when blueberries were stored at one °C for 35 days. Mullen et al. [144][83] observed an increment in ellagitannins of red raspberries when they were held at four °C for three days. In addition, Ayala-Zavala [145][84] found that temperatures of 0 °C retained higher contents of antioxidant compounds in strawberries for extended periods than those stored at five °C or ten °C.
The loss of phenolic content in fruits is expected when converted into juices or jams due to the complexity of the processing steps involved. When fruits are converted into liquids, a frozen or refrigeration step is followed by blanching, milling, depectinization, pressing, pasteurization, and, if necessary, clarification and concentration. All these steps can play a significant role in phenolic degradation. For example, anthocyanin retention in cranberry juice is generally lower than 50% due to losses during various processing stages [146][85]. Buchert et al. [147][86], comparing the effects of the usage of different enzyme preparations on the anthocyanin composition of bilberry and blackcurrant juices during the juice preparation, observed that the enzyme activities of β-galactosidase, α-arabinoside, and β-glucosidase varied significantly. The presence of β-galactosidase resulted in the juice's complete loss of delphinidin, cyanidin, petunidin, and malvidin galactosides. They also observed that the pressing step resulted in marked losses of anthocyanins due to the physical removal of the anthocyanin-rich skins and the anthocyanins binding to cell wall polysaccharides. Furthermore, another study reported that frozen highbush blueberries (Vaccinium corymbosum) [148][87] showed a pronounced deterioration of phenolic compounds when frozen blueberries were processed into juice and concentrate. Nonetheless, Lee et al. [149][88] observed an increment of malvidin-glycosides and total anthocyanins increased by 51% and 60%, respectively, in pasteurized juices and concentrates compared to the fresh fruit, probably caused by the step of concentration. However, they noted that delphinidin-glycosides with pasteurization decreased by 26%. Pasteurization was found to exert a significantly destructive effect on the anthocyanin content of strawberry juice in 60–70% [150][89] due to the high temperatures used in this processing step. In jams, another way to process fruits, the tendencies are similar to those observed in juices. Kim and Padilla-Zakour [151][90], studying the processing effect on phenolics and antioxidant capacity in anthocyanin-rich cherry, plum, and raspberry, found that the processing steps and heating during jam-making decreased the contents of total phenolics by 48% for cherry, 57% for plum and 36% for raspberry. The content of total anthocyanins decreased by 86% in cherry, 84% in plum, and 45% in raspberry [151][90]. Jam making generally involves the disruption of fruit tissues followed by heating under a highly acidic environment to avoid bacterial contamination, resulting in considerable losses of anthocyanins [152][91]. However, some phenolics, such as ellagic acid, can increase between 4.6 fold in black raspberry [152][91] and 1.5 to 2.5 fold during the processing of raspberry [153][92] and strawberry [154][93] jams. This increase may be correlated to an easier extractability of ellagic acid in this heat and high-acid environment [151][90].
Despite these variations, numerous studies demonstrated that various phytochemical constituents of red fruits exhibit a wide range of biological effects [84[42][94],155], including antioxidant activities, which will be addressed in point three of this work.
2.6. Aroma and Flavor Compounds
6. Aroma and Flavor Compounds
Due to the increased interest in the health benefits of small-berry fruits, research on berries’ flavor quality has also increased. Volatiles found in small berries are diverse and give unique flavors to different fruits. Fruit flavor also depends upon taste (sweetness, sourness, and low or no astringency) and aroma (concentration of volatile organic compounds). Nevertheless, while the environment may alter the flavor quality of small fruits, genetic factors seem to determine the flavor profile quantitatively and qualitatively [156][95].
Strawberries (Fragaria × ananassa) are among the most appreciated fruits worldwide. The modern garden strawberry varieties present largeness, beautiful red color, and extended shelf life. However, consumers often censure the sensory quality, as they seem deficient in flavor and fragrance [157][96].
Numerous volatiles of several chemical natures have been identified in ripe strawberries, including alcohols, aldehydes, esters, furanones, ketones, and terpenes [158][97]. Wild strawberries, opposite to garden varieties, present intense flavor and fragrance [159][98]. These varieties provide an appreciated source of volatile compounds for breeding new commercial strawberries with improved aroma [160][99]. One example is Fragaria moschata or musk strawberries, recognized for their well-known smell.
At present, few musk strawberries survive in farm plantings. One example is the Italian clone ‘Profumata di Tortona,’ considered one of the most fragrant strawberries [161][100]. The berries of this variety are characterized by an intense red color, pale flesh, a delightful sour, sweet taste, and an astringent flavor, presenting aromas of caramel, mango, and tropical scent [158,162][97][101].
Ulrich and Olbricht [163,164][102][103] identified significant differences in the presence of individual esters, ketones, and terpenes between Fragaria × vesca samples and Fragaria × ananassa cultivars. Mainly in esters (ethyl hexanoate, methyl butanoate, and methyl hexanoate) found in higher concentrations in Fragaria × ananassa compared with wild samples. Contrariwise, the ester methyl anthranilate (2-aminobenzoic acid methyl ester), the aroma compound associated with strawberry aroma/flavor [165][104], presents the characteristic fragrance of wild strawberries with a fruity, concord grape, musty with a floral powdery nuance, was more abundant in Fragaria × vesca. Likewise, ketones (2-pentanone, 2-heptanone, and 2-nonanone) and terpenes (myrtenal, myrtenil acetate, α-terpineol) are present in higher levels in F. vesca, excepting the monoterpene linalool, more abundant in cultivated strawberries.
As mentioned before, esters are important volatile compounds in fruit flavor, and in strawberries, several esters have been identified [166][105]. The reaction of transacylation from acyl-CoA into alcohol is called esterification (Figure 4). The enzyme that catalyzes the reaction is alcohol acyltransferase (AAT). Ueda et al. [167][106] concluded that, in strawberries, the alcohol moieties of the esters produced revealed the alcohols mainly synthesized in the fruit, and the acid moieties reflected the acyl-CoA specificity of the AAT enzyme. The strawberry AAT enzyme had a high activity with hexanol and acetyl- or butyl-CoAs [168,169][107][108].
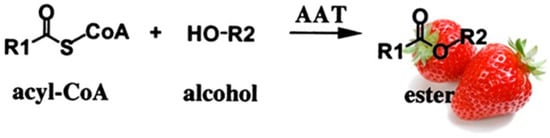
Figure 4.
Schematic representation of the esterification reaction catalyzed by the alcohol acyltransferase (ATT).
Recently, Negri et al. [159][98] identified 131 volatile compounds in ripe ‘Profumata di Tortona’ and F. vesca cv berries. ‘Regina delle Valli,’ a number exceeding the aroma compounds usually found in commercial strawberries. Moreover, 80 volatile compounds have been already identified by Schwieterman et al. [170][109] in 35 strawberry-garden varieties. In total, and according to Ulrich et al. [171][110], 979 volatile compounds were identified, 590 of which were found since 1997.
Raspberry volatiles are essential for perceiving their sensory quality and mold resistance [172][111], Figure 5. As to other fruits, volatile compounds are influenced by numerous factors, including ripeness, climate, soil, and cultivar variation [173][112].
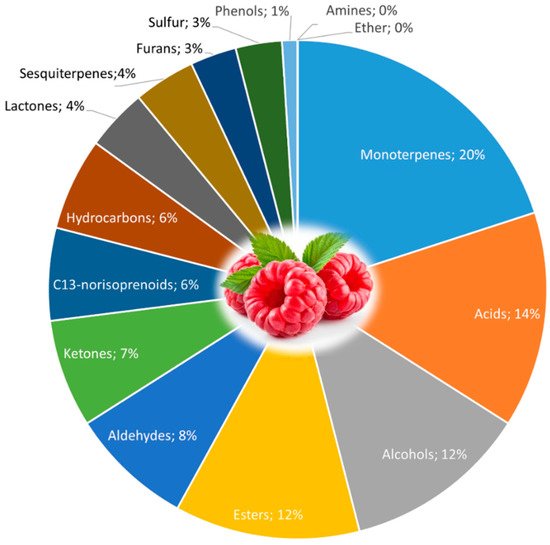
Figure 5.
Volatile compounds in raspberry fruit (
The volatile compounds in raspberry are mostly free forms of different metabolites, most of them present in the form of glycoside bound to sugars, able to release free volatile compounds by enzymatic or chemical cleavage. This process can occur during plant maturation, industrial treatments, and fruit processing [174][113].
References
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706.
- De Souza, V.R.; Pereira, P.A.; Da Silva, T.L.; Lima, L.C.O.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368.
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144.
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 1-57881-072-8.
- Gomes-Rochette, N.F.; Da Silveira Vasconcelos, M.; Nabavi, S.M.; Mota, E.F.; Nunes-Pinheiro, D.C.; Daglia, M.; De Melo, D.F. Fruit as potent natural antioxidants and their biological effects. Curr. Pharm. Biotechnol. 2016, 17, 986–993.
- Lin, P.-H.; Aickin, M.; Champagne, C.; Craddick, S.; Sacks, F.M.; McCarron, P.; Most-Windhauser, M.M.; Rukenbrod, F.; Haworth, L. Food group sources of nutrients in the dietary patterns of the DASH-Sodium trial. J. Am. Diet. Assoc. 2003, 103, 488–496.
- Jabłońska-Ryś, E.; Zalewska-Korona, M.; Kalbarczyk, J. Antioxidant Capacity, Ascorbic Acid and Phenolics Content in Wild Edible Fruits. J. Fruit Ornam. Plant Res. 2009, 17, 115–120.
- Dorofejeva, K.; Rakcejeva, T.; Galoburda, R.; Dukalska, L.; Kviesis, J. Vitamin C content in Latvian cranberries dried in convective and microwave vacuum driers. Procedia Food Sci. 2011, 1, 433–440.
- USDA-ARS (US Department of Agriculture, Agricultural Research Service). USDA Nutrient Database for Standard Reference, Release 25, Software 1.2.2, from the Nutrient Data Laboratory. Available online: http://www.nal.usda.gov/fnic/foodcomp (accessed on 28 December 2021).
- Hakala, M.; Lapvetelainen, A.; Houpalahti Kallio, H.; Tahvonen, R. Effects of varieties and cultivation conditions on the composition of strawberries. J. Food Compost. Anal. 2003, 16, 67–80.
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, 10.
- Viljakainen, S.; Visti, A.; Laakso, S. Concentrations of organic acids and soluble sugars in juices from Nordic berries. Acta Agric. Scand. B Soil Plant Sci. 2010, 52, 101–109.
- Correia, S.; Queirós, F.; Ribeiro, C.; Vilela, A.; Aires, A.; Barros, A.I.; Schouten, R.; Silva, A.P.; Gonçalves, B. Effects of calcium and growth regulators on sweet cherry (Prunus avium L.) quality and sensory attributes at harvest. Sci. Horti. 2019, 248, 231–240.
- Urün, I.; Attar, S.H.; Sönmez, D.A.; Gündesli, M.A.; Ercişli, S.; Kafkas, N.E.; Bandić, L.M.; Duralija, B. Comparison of polyphenol, sugar, organic acid, volatile vompounds, and antioxidant capacity of commercially grown strawberry cultivars in Turkey. Plants 2021, 10, 1654.
- Morais, M.C.; Mucha, Â.; Ferreira, H.; Gonçalves, B.; Bacelar, E.; Marques, G. Comparative study of plant growth-promoting bacteria on the physiology, growth and fruit quality of strawberry. J. Sci. Food Agric. 2019, 99, 5341–5349.
- Costa, E.; Cosme, F.; Jordão, A.M.; Mendes-Faia, A. Anthocyanin profile and antioxidant activity from 24 grape varieties cultivated in two Portuguese wine regions. OENO One 2014, 48, 51–62.
- Costa, E.; Cosme, F.; Rivero-Pérez, M.D.; Jordão, A.M.; González-SanJosé, M.L. Influence of wine region provenance on phenolic composition, antioxidant capacity and radical scavenger activity of traditional Portuguese red grape varieties. Eur. Food Res. Technol. 2015, 241, 61–73.
- Ha, M.A.; Jarvis, M.; Mann, J. A definition for dietary fibre. Eur. J. Clin. Nutr. 2000, 54, 861–864.
- Institute of Medicine. Dietary Reference Intakes Proposed Definition of Dietary Fiber. A Report of the Panel on the Definition of Dietary Fiber and The Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Food and Nutrition Board, National Academy Press: Washington, DC, USA, 2001.
- Anita, F.P.; Abraham, P. Clinical Dietetics and Nutrition; Delhi Oxford University Press: Calcutta, India, 1997; pp. 73–77.
- Fayet-Moore, F.; Cassettari, T.; Tuck, K.; McConnell, A.; Petocz, P. Dietary fibre intake in Australia. Paper II: Comparative examination of food sources of fibre among high and low fibre consumers. Nutrients 2018, 10, 1223.
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fibre. Nutr. Rev. 2009, 67, 188–205.
- Chau, C.F.; Huang, Y.L. Comparison of the chemical composition and physicochemical properties of different fibres prepared from peel of the Citrus sinensis L. Cv. Liucheng. J. Agric. Food Chem. 2003, 51, 2615–2618.
- Terry, P.; Giovannucci, E.; Michels, K.B.; Bergkvist, L.; Hansen, H.; Holmberg, L.; Wolk, A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J. Nat. Canc. Instit. 2001, 93, 525–533.
- Marlett, J.A.; McBurney, M.I.; Slavin, J.L. Position of the American Dietetic Association health implications of dietary fiber. J. Am. Diet. Assoc. 2002, 102, 993–1000.
- Tutelyan, V.A. Norms of physiological needs in energy and nutrients for different groups of the population of the Russian Federation. Vopr. Pitan. 2009, 78, 4–15.
- Pacifico, S.; Di Maro, A.; Petriccione, M.; Galasso, S.; Piccolella, S.; Di Giuseppe, A.M.A.; Scortichini, M.; Monaco, P. Chemical composition, nutritional value and antioxidant properties of autochthonous Prunus avium cultivars from Campania Region. Food Res. Int. 2014, 64, 188–199.
- Kafkas, E.; Gunaydin, S.; Ercisli, S.; Ozogu, Y.; Unlu, M.A. Fat and fatty acid composition of strawberry cultivars. Chem. Nat. Compd. 2009, 45, 861–863.
- Kafkas, E.; Ozgen, M.; Ozogul, Y.; Turemis, N. Phytochemical and fatty acid profile of selected red raspberry cultivars: A comparative study. J. Food Qual. 2008, 31, 67–78.
- Fadavi, A.; Barzegar, M.; Azizi, M.H. Determination of fatty acids and total lipid content in oilseed of 25 pomegranates varieties grown in Iran. J. Food Compos. Anal. 2006, 19, 676–680.
- Al Juhaimi, F.; Geçgel, Ü.; Gülcü, M.; Hamurcu, M.; Özcan, M.M. Bioactive properties, fatty acid composition and mineral contents of grape seed and oils. S. Afr. J. Enol. Vitic. 2017, 38, 103–108.
- Melgarejo, P.; Artés, F. Total lipid content and fatty acid composition of oilseed from lesser known sweet pomegranate clones. J. Sci. Food Agric. 2000, 80, 1452–1454.
- Al-Maiman, S.A.; Ahmad, D. Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chem. 2002, 76, 437–441.
- Wang, C.Y.; Wang, S.Y. Effect of storage temperatures on fruit quality of various cranberry cultivars. Acta Hortic. 2009, 810, 853–862.
- Zafra-Rojas, Q.; Cruz-Cansino, N.; Delgadillo-Ramírez, A.; Alanis, E.; Añorve-Morga, J.; Quintero-Lira, A.; Castañeda, A.; Ramírez-Moreno, E. Organic acids, antioxidants, and dietary fiber of Mexican blackberry (Rubus fruticosus) residues cv. Tupy. J. Food Qual. 2018, 1, 5950761.
- Luginina, E.A.; Egoshina, T.L. Biochemical composition of fruits of wild growing berry plants. In Temperate Horticulture for Sustainable Development and Environment: Ecological Aspects; Weisfeld, L., Opalko, A.I., Bekuzarova, S.A., Eds.; Apple Academic Press Inc.: New York, NY, USA, 2018; 20p.
- Nagy, K.; Tiuca, I.-D. Importance of fatty acids in physiopathology of human body. IntechOpen 2017. Available online: https://www.intechopen.com/books/fatty-acids/importance-of-fatty-acids-in-physiopathology-of-human-body (accessed on 28 August 2018).
- Dominguez, L.J.; Barbagallo, M. Not All Fats Are Unhealthy. In The Prevention of Cardiovascular Disease through the Mediterranean Diet; Sánchez-Villegas, A., Sánchez-Tainta, A., Eds.; Academic Press: London, UK, 2018; pp. 35–58.
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172.
- Sanjeev, P.; Chaudhary, D.P.; Sreevastava, P.; Saha, S.; Rajenderan, A.; Sekhar, J.C.; Chikkappa, G.K. Comparison of fatty acid profile of specialty maize to normal maize. J. Am. Oil Chem. Soc. 2014, 91, 1001–1005.
- Johnson, M.; Bradford, C. Omega-3, Omega-6 and Omega-9 fatty acids: Implications for cardiovascular and other diseases. J. Glycom. Lipidom. 2014, 4, 123.
- Johansson, E.; Hussain, A.; Kuktaite, R.; Andersson, S.C.; Olsson, M.E. Contribution of organically grown crops to human health. Int. J. Environ. Res. Public Health 2014, 11, 3870–3893.
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160.
- Balasundrama, N.; Sundramb, K.; Sammana, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203.
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- Colak, N.; Primetta, A.K.; Riihinen, K.R.; Jaakola, L.; Grúz, J.; Strnad, M.; Torun, H.; Ayaz, F.A. Phenolic compounds and antioxidant capacity in different-colored and non-pigmented berries of bilberry (Vaccinium myrtillus L.). Food Biosci. 2017, 20, 67–78.
- Wang, L.J.; Wu, J.; Wang, H.-X.; Li, S.-S.; Zheng, X.-C.; Du, H.; Xu, Y.-J.; Wang, L.-S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods 2015, 16, 295–304.
- Brown, P.N.; Shipley, P.R. Determination of anthocyanins in cranberry fruit and cranberry fruit products by high-performance liquid chromatography with ultraviolet detection: Single-laboratory validation. J. AOAC Int. 2011, 94, 459–466.
- Biswas, N.; Balac, P.; Narlakanti, S.K.; Enamul Haque, M.D.; Mehedi Hassan, M.D. Identification of phenolic compounds in processed cranberries by HPLC method. J. Nutr. Food Sci. 2013, 3, 181.
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842.
- Vagiri, M.; Ekholm, A.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Major phenolic compounds in black currant (Ribes nigrum L.) buds: Variation due to genotype, ontogenetic stage and location. Food Chem. 2015, 63, 1274–1280.
- Adina, F.; Cecilia, G.; Felicia, G.; Carmen, D.; Ovidiu, T. Identification and quantification of phenolic compounds from red currant (Ribes rubrum L.) and raspberries (Rubus idaeus L.). Int. J. Pharm. Phytoch. Ethnomed. 2017, 6, 30–37.
- Guiné, R.P.F.; Soutinho, S.M.A.; Gonçalves, F.J. Phenolic compounds and antioxidant activity in red fruits produced in organic farming. Croat. J. Food Sci. Technol. 2014, 6, 15–26. Available online: http://hdl.handle.net/10400.19/2233 (accessed on 20 August 2020).
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97.
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353.
- Gonçalves, B.; Landbo, A.-K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530.
- Gonçalves, B.; Landbo, A.-K.; Let, M.; Silva, A.P.; Rosa, E.; Meyer, A.S. Storage affects the phenolic profiles and antioxidant activities of cherries (Prunus avium L.) on human low-density lipoproteins. J. Sci. Food Agric. 2004, 84, 1013–1020.
- Tulio, A.Z., Jr.; Reese, R.N.; Wyzgoski, F.J.; Rinaldi, P.L.; Fu, R.; Scheerens, J.C.; Miller, A.R. Cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside as primary phenolic antioxidants in black raspberry. J. Agric. Food Chem. 2008, 56, 1880–1888.
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984.
- Aires, A.; Carvalho, R.; Matos, M.; Carnide, V.; Silva, A.P.; Gonçalves, B. Variation of chemical constituents, antioxidant activity, and endogenous plant hormones throughout different ripening stages of highbush blueberry (Vaccinium corymbosum L.) cultivars produced in centre of Portugal. J. Food Biochem. 2017, 41, e12414.
- Correia, S.; Aires, A.; Queirós, F.; Carvalho, R.; Schouten, R.; Silva, A.P.; Gonçalves, B. Climate conditions and spray treatments induce shifts in health promoting compounds in cherry (Prunus avium L.) fruits. Sci. Hortic. 2020, 263, 109147.
- Anjos, R.; Cosme, F.; Gonçalves, A.; Nunes, F.M.; Vilela, A.; Pinto, T. Effect of agricultural practices, conventional vs organic, on the phytochemical composition of ‘Kweli’ and ‘Tulameen’ raspberries (Rubus idaeus L.). Food Chem. 2020, 2020, 126833.
- Pinto, M.S.; Lajolo, F.M.; Genovese, M.I. Bioactive compounds and quantification of total ellagic acid in strawberries (Fragaria x ananassa Duch.). Food Chem. 2008, 107, 1629–1635.
- Kassim, A.; Poette, J.; Paterson, A.; Zait, D.; McCallum, S.; Woodhead, M.; Smith, K.; Hackett, C.; Graham, J. Environmental and seasonal influences on red raspberry anthocyanin antioxidant contents and identification of quantitative traitsloci (QTL). Mol. Nutr. Food Res. 2009, 53, 625–634.
- Corona, G.; Tang, F.; Vauzour, D.; Rodriguez-Mateos, A.; Spencer, J.P.E. Assessment of the anthocyanidin content of common fruits and development of a test diet rich in a range of anthocyanins. J. Berry Res. 2011, 1, 209–216.
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic profile and bioactive potential of stems and seed kernels of sweet cherry fruit. Antioxidants 2020, 9, 1295.
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Factors affecting quality and health promoting compounds during growth and postharvest life of sweet cherry (Prunus avium L.). Front. Plant Sci. 2017, 8, 2166.
- Gonçalves, B.; Moutinho-Pereira, J.; Santos, A.; Silva, A.P.; Bacelar, E.; Correia, C.; Rosa, E. Scion-rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol. 2006, 26, 93–104.
- Gonçalves, B.; Morais, M.C.; Sequeira, A.; Ribeiro, C.; Guedes, F.; Silva, A.P.; Aires, A. Quality preservation of sweet cherry cv. ‘Staccato’ by using glycine-betaine or Ascophyllum nodosum. Food Chem. 2020, 322, 126713.
- Singh, R.K.; Soares, B.; Goufo, P.; Castro, I.; Cosme, F.; Pinto-Sintra, A.L.; Inês, A.; Oliveira, A.A.; Falco, V. Chitosan upregulates the genes of the ROS pathway and enhances the antioxidant potential of Grape (Vitis vinifera L. ‘Touriga Franca’ and ’Tinto Cão’) tissues. Antioxidants 2019, 8, 525.
- Singh, R.K.; Afonso, J.; Nogueira, M.; Oliveira, A.A.; Cosme, F.; Falco, V. Silicates of potassium and aluminium (kaolin); comparative foliar mitigation treatments and biochemical insight on grape berry quality in Vitis vinifera L. (cv. Touriga National and Touriga Franca). Biology 2020, 9, 58.
- Cosme, F.; Ricardo-da-Silva, J.M.; Laureano, O. Tannin profiles of Vitis vinifera L. cv. red grapes growing in Lisbon and from their monovarietal wines. Food Chem. 2009, 112, 197–204.
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247.
- Nour, V.; Stampar, F.; Veberic, R.; Jakopic, J. Anthocyanins profile, total phenolics and antioxidant activity of black currant ethanolic extracts as influenced by genotype and ethanol concentration. Food Chem. 2013, 141, 961–966.
- Zhang, J.; Wang, X.; Yu, O.; Tang, J.; Gu, X.; Wan, X.; Fang, C. Metabolic profiling of strawberry (Fragaria × ananassa Duch.) during fruit development and maturation. J. Exp. Bot. 2011, 62, 1103–1118.
- Beekwilder, J.; Jonker, H.; Meesters, P.; Hall, R.D.; van der Meer, I.M.; Ric de Vos, C.H. Antioxidants in raspberry: on-line analysis links antioxidant activity to a diversity of individual metabolites. J. Agric. Food Chem. 2005, 53, 3313–3320.
- Atkinson, C.J.; Dodds, P.A.A.; Ford, Y.Y.; Le Mière, J.; Taylor, J.M.; Blake, P.S.; Paul, N. Effects of cultivar, fruit number and reflected photosynthetically active radiation on Fragaria x ananassa productivity and fruit ellagic acid and ascorbic acid concentrations. Ann. Bot. 2006, 97, 429–441.
- Vyas, P.; Curran, N.H.; Igamberdiev, A.U.; Debnath, S.C. Antioxidant properties of lingonberry (Vaccinium vitis-idaea L.) leaves within a set of wild clones and cultivars. Can. J. Plant Sci. 2015, 95, 663–669.
- Kozos, K.; Ochmian, I.; Chełpiński, P. The effects of rapid chilling and storage conditions on the quality of Brigitta Blue cultivar highbush blueberries. Folia Hortic. 2014, 26, 147–153.
- Srivastava, A.; Akoh, C.C.; Yi, W.; Fischer, J.; Krewer, G. Effect of storage conditions on the biological activity of phenolic compounds of blueberry extract packed in glass bottles. J. Agric. Food Chem. 2007, 55, 2705–2713.
- Mullen, W.; Stewart, A.J.; Lean, M.E.J.; Gardner, P.; Duthie, G.G.; Crozier, A. Effect of freezing and storage on the phenolics, ellagitannins, flavonoids, and antioxidant capacity of red raspberries. J. Agric. Food Chem. 2002, 50, 5197–5201.
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT Food Sci. Technol. 2004, 37, 687–695.
- Pappas, E.; Schaich, K. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009, 49, 741–781.
- Buchert, J.; Koponen, J.M.; Suutarinen, M.; Mustranta, A.; Lille, M.; Törrönen, R.; Poutanen, K. Effect of enzyme-aided pressing on anthocyanin yield and profiles in bilberry and blackcurrant juices. J. Sci. Food Agric. 2005, 85, 2548–2556.
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–364.
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Impact of juice processing on blueberry anthocyanins and polyphenolics: Comparison of two pretreatments. J. Food Sci. 2002, 67, 1660–1667.
- Narwojsz, A.; Borowska, E.J. Cranberry and strawberry juices-influence of method production on antioxidants content and antioxidative capacity. Pol. J. Nat. Sci. 2010, 25, 209–214.
- Kim, D.-O.; Padilla-Zakour, O.I. Jam processing effect on phenolics and antioxidant capacity in anthocyanin-rich fruits: Cherry, plum, and raspberry. J. Food Sci. 2004, 69, S395–S400.
- Savikin, K.; Zdunić, G.; Janković, T.; Tasić, S.; Menković, N.; Stević, T.; Dordević, B. Phenolic content and radical scavenging capacity of berries and related jams from certificated area in Serbia. Plant Foods Hum. Nutr. 2009, 64, 212–217.
- Amakura, Y.; Umino, Y.; Tsuji, S.; Tonogai, Y. Influence of jam processing on the radical scavenging activity and phenolic content in berries. J. Agric. Food Chem. 2000, 48, 6292–6297.
- Pinto, M.S.; Lajolo, F.M.; Genovese, M.I. Bioactive compounds and antioxidant capacity of strawberry jams. Plant Foods Hum. Nutr. 2007, 62, 127–131.
- Paredes-López, O.; Cervantes-Ceja, M.L.; Vigna-Pérez, M.; Hernández-Pérez, T. Berries: Improving human health and healthy aging, and promoting quality life-A Review. Plant Foods Hum. Nutr. 2010, 65, 299–308.
- Du, X.; Qian, M. Flavor Chemistry of Small Fruits: Blackberry, Raspberry, and Blueberry. Flavor and Health Benefits of Small Fruits, In. Flavor and Health Benefits of Small Fruits; Du, X., Qian, M., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; pp. 27–43.
- Colquhon, T.; Levin, L.; Mosckowitz, H.; Whiteker, V.; Clark, D.; Folta, K. Framing the perfect strawberry: An exercise in a consumer-assisted selection of fruit crops. J. Berry Res. 2012, 2, 45–61.
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, S487–S496.
- Negri, A.S.; Allegra, D.; Simoni, L.; Rusconi, F.; Tonelli, C.; Espen, L.; Galbiati, M. Comparative analysis of fruit aroma patterns in the domesticated wild strawberries “Profumata di Tortona” (F. moschata) and “Regina delle Valli” (F. vesca). Front. Plant Sci. 2015, 6, 56.
- Ulrich, D.; Hoberg, E. Flavour analysis in plant breeding research on strawberries. In Frontiers of Flavour Sciences; Schieberle, P., Engel, K.-H., Eds.; Deutsche Forschungsanstalt Lebensmittelchemie: Garching, Germany, 2000; pp. 161–163.
- Urruty, L.; Giraudel, J.L.; Lek, S.; Roudeillac, P.; Montury, M. Assessment of strawberry aroma through SPME/GC and ANN methods. Classification and discrimination of varieties. J. Agric. Food Chem. 2002, 50, 3129–3136.
- Pet’ka, J.; Leitner, E.; Parameswaran, B. Musk strawberries: The flavor of a formerly famous fruit reassessed. Flavour Fragr. J. 2002, 27, 273–279.
- Ulrich, D.; Olbricht, K. Diversity of volatile patterns in sixteen Fragaria vesca L. accessions in comparison to cultivars of Fragaria × ananassa. J. App. Bot. Food Qual. 2013, 86, 37–46.
- Ulrich, D.; Olbricht, K. Diversity of metabolite patterns and sensory characters in wild and cultivated strawberries. J. Berry Res. 2014, 4, 11–17.
- Barbey, C.R.; Hogshead, M.H.; Harrison, B.; Schwartz, A.E.; Verma, S.; Oh, Y.; Lee, S.; Folta, K.M.; Whitaker, V.M. Genetic Analysis of Methyl Anthranilate, Mesifurane, Linalool, and Other Flavor Compounds in Cultivated Strawberry (Fragaria × ananassa). Front Plant Sci. 2021, 12, 615749.
- Fan, Z.; Hasing, T.; Johnson, T.; Garner, D.M.; Schwieterman, M.L.; Barbey, C.R.; Colquhoun, T.A.; Sims, C.A.; Resende, M.F.R.; Whitaker, V.M. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 2021, 8, 66.
- Ueda, Y.; Tsuda, A.; Bai, J.H.; Fujishita, N.; Chachin, K. Characteristic pattern of aroma ester formation from banana, melon, and strawberry with reference to the substrate specificity of ester synthetase and alcohol contents in pulp. J. Jpn. Soc. Food Sci. Technol. 1992, 39, 183–187.
- Olias, J.M.; Sanz, C.; Rios, J.J.; Perez, A.G. Substrate specificity of alcohol acyltransferase from strawberry and banana fruits. In Fruit Flavors: Biogenesis, Characterization and Authentication; Rouseff, R.L., Leahy, M.M., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 134–141.
- Aharoni, A.; Keizer, L.C.; Bouwmeester, H.J.; Sun, Z.; Alvarez-Huerta, M.; Verhoeven, H.A.; Blaas, J.; van Houwelingen, A.M.; De Vos, R.C.; van der Voet, H.; et al. Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 2000, 12, 647–662.
- Schwieterman, M.L.; Colquhoun, T.A.; Jaworski, E.A.; Bartoshuk, L.M.; Gilbert, J.L.; Tieman, D.M.; Odabasi, A.Z.; Moskowitz, H.R.; Folta, K.M.; Klee, H.J.; et al. Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS ONE 2014, 9, e88446.
- Ulrich, D.; Kecke, S.; Olbricht, K. What do we know about the chemistry of strawberry aroma? J. Agric. Food Chem. 2018, 66, 3291–3301.
- Aprea, E.; Carlin, S.; Giongo, L.; Grisenti, M.; Gasperi, F. Characterization of 14 raspberry cultivars by solid-phase microextraction and relationship with Gray Mold susceptibility. J. Agric. Food Chem. 2010, 58, 1100–1105.
- Forney, C.F.; Kalt, W.; Jordan, M.A. The composition of strawberry aroma is influenced by cultivar, maturity, and storage. HortScience 2000, 35, 1022–1026.
- Ikan, R. Naturally Occurring Glycosides; John Wiley: Chichester, UK; New York, NY, USA, 1999.
More
