Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vicenç Artigas and Version 2 by Jason Zhu.
Soft tissue sarcomas (STS) are an uncommon and biologically heterogeneous group of tumors arising from mesenchymal cells. The incidence is estimated at five cases per 100,000 people per year. Retroperitoneal sarcomas (RPS) account for 10–15% of all STS, and their management depends on their anatomical characteristics and histotype. Due to their very low incidence, it is recommended that RPS be treated in reference centers and evaluated by an experienced multidisciplinary team (MDT). In Spain, the Spanish Group for Research in Sarcomas (GEIS) brings together experts from various specialties to promote research on sarcomas and improve treatment results.
- retroperitoneal sarcoma
- soft tissue sarcoma
- retroperitoneum
1. Introduction
Soft tissue sarcomas (STS) are an uncommon and heterogeneous group of tumors of mesenchymal cell origin, with an estimated incidence of five cases per 100,000 per year in Europe [1][2][1,2]. Approximately 10–15% of all STS are retroperitoneal sarcomas (RPS). RPS usually presents at an advanced stage with nonspecific symptoms, such as increased abdominal perimeter, abdominal pain, and a change in bowel habits. Although STS comprise more than 100 histopathologic subtypes, in the retroperitoneum, the most frequent subtypes are, in order of frequency, well-differentiated liposarcoma (WDLPS)/dedifferentiated liposarcoma (DDLPS), leiomyosarcoma (LMS), solitary fibrous tumor (SFT) and malignant peripheral nerve sheath tumor (MPNST) [3]. Each of these entities has its own distinct biological behavior in terms of risks of local (LR) or distant recurrence (DR) and overall survival (OS) [4]. Recent advances in the understanding of the biological variability of RPS have led to more personalized histology-based management that includes surgical and non-surgical treatments such as radiotherapy and chemotherapy.
RPS requires a multidisciplinary and complex therapeutic approach and should preferably be treated in specialized centers with a team of radiologists, pathologists, surgeons, and medical and radiation oncologists with expertise in the treatment of this disease. For this reason, there has been increasing interest in centralizing the management of these patients in national reference centers. In addition, international cooperation has led to the creation of collaborative groups, such as the Trans-Atlantic Australasian Retroperitoneal Sarcoma Working Group (TARPSWG), to improve knowledge of this disease and advance its treatment.
2. Imaging Diagnosis
The imaging technique of choice for the diagnosis of RPS is a computed tomography (CT) scan with intravenous iodinated contrast. The administration of intravenous iodinated contrast is highly recommended because it allows the identification of areas of enhancement, not necrotic, and to obtain a biopsy with greater diagnostic accuracy [5][6][7][8][10,11,12,13]. It is important to determine whether the tumor arises from retroperitoneal soft tissues or from a retroperitoneal organ as the former are less frequent but typically malignant entities (e.g., a heterogeneous fatty mass arising from retroperitoneal tissue may be an LPS, whereas a renal fatty lesion suggests an angiomyolipoma) (Figure 1) [6][8][11,13]. Certain morphological signs allow the radiologist to deduce the origin of a retroperitoneal mass, such as the phantom organ sign, the embedded organ sign, the beak sign, and the prominent feeding artery sign [6][11].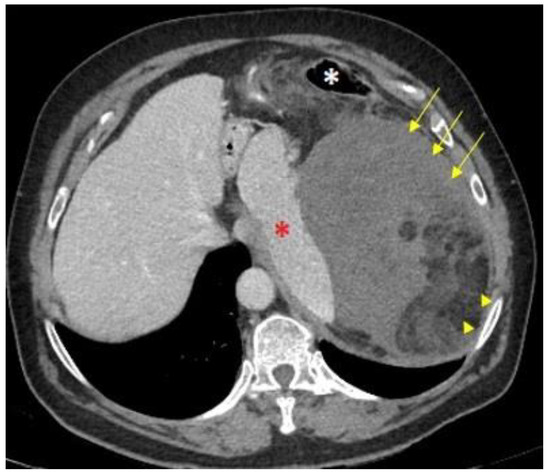
Figure 1. CT Morphological signs of DD-LPS: An axial computed tomography (CT) scan shows a giant mass in the left hypochondrium. The medial displacement of the spleen (red asterisk) and the anteromedial position of the descending colon (white asterisk) indicate it is a retroperitoneal-originated neoplasm. The mix of macroscopic fat (arrowheads) and soft-tissue components (arrows) suggests a dedifferentiated liposarcoma.
-
Recommendations
-
A CT scan is the imaging technique of choice for the diagnosis and evaluation of resection of retroperitoneal sarcomas (IV, A).
-
MRI is also recommended to evaluate pelvic tumors (IV, A).
3. Biopsy
In patients with a suspected RPS, histological sampling should be conducted before any treatment is undertaken, except in exceptional cases where biopsy is high risk and a clear radiological diagnosis is available, e.g., well-differentiated liposarcoma (WDLPS) [10][11][15,16]. A percutaneous image-guided core needle biopsy is usually preferred to a surgical biopsy. The biopsy target and the needle trajectory should be determined following a thorough review of all the patient’s radiological studies and ideally after discussion by an MDT. Most retroperitoneal tumors can be percutaneously biopsied without entering the peritoneal space. When a retroperitoneal mass is heterogeneous in the radiological examinations, the biopsy should be directed to the most “dedifferentiated” solid areas [12][17]. Multiple (3–4) biopsies using a 14–16 G trucut needle are recommended. A coaxial biopsy system may be used to obtain multiple tumor samples with a single percutaneous access. Percutaneous biopsy of deep retroperitoneal tumors is usually performed under CT image guidance, but large or superficial tumors can be safely biopsied using ultrasound guidance. If the standard safety requirements for radiological interventional procedures are fulfilled, the rate of early complications of percutaneous needle core biopsy in RPS is low, and the risk of tumor seeding also seems to be low (0.5–2%) [13][14][18,19]. In metastatic retroperitoneal sarcomas, a biopsy of the metastatic sites could be considered, as it would allow people to confirm the histological diagnosis and the advanced stage in a single procedure.-
Recommendations
-
Before any treatment for a suspected RPS, a core needle biopsy should be performed (IV, A).
-
It should be directed to the most “dedifferentiated” solid areas (IV, A).
-
In RPS with metastases, a biopsy of the metastatic sites could be considered to reach a histological diagnosis if they are more easily accessible (V, B).
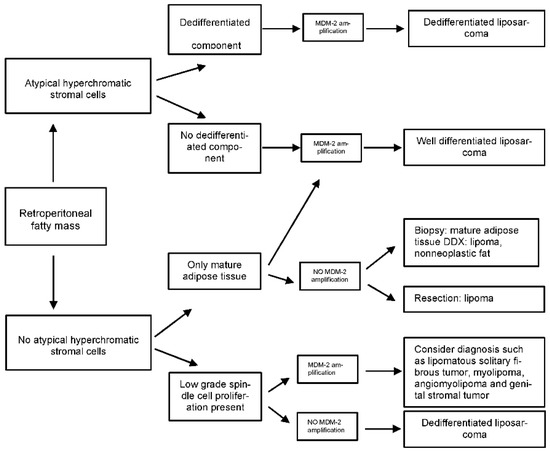
4. Pathological Diagnosis of Soft Tissue Sarcomas—Indication of Molecular Studies
The pathology report of a trucut biopsy should include the histological type, or if this is not possible, at least establish the morphologic category (spindle cell, myxoid, pleomorphic, round cell, etc.), the histologic grade and the results of the complementary studies performed (immunohistochemistry (IHC) and/or molecular biology). From the resection specimen, the pathologist must provide the following information:-
A macroscopic description: measurements of the surgical specimen, type of surgical specimen, and identification of the tissues and organs included.
-
Description of the tumor: size, appearance, location, presence of necrosis, and invasion of neighboring structures.
-
Resection margins: the distance of the tumor to the margins should be measured and those that are less than 2 cm should be specified. It should be indicated whether the margin is formed by fascia, visceral, adventitial, or periosteal tissue.
-
Presence and description of satellite nodules.
-
Lymph nodes: although lymph node involvement is rare in STS (except for rhabdomyosarcoma (RMS), angiosarcoma, or epithelioid sarcoma), the status of any lymph nodes present should be included.
-
Any additional techniques performed should be reported: IHC, reverse transcriptase-polymerase chain reaction (RT-PCR), next generation sequencing (NGS), multiplex ligation-dependent probe amplification (MLPA), fluorescence in situ hybridization (FISH), and their results.
Table 1. Recommendation of Immunohistochemistry in RPS.
| Recommendation for Immunohistochemistry in Adipocytic Tumors or Tumor with Fatty Areas in Retroperitoneum | |
|---|---|
| MDM2/CDK4 | To distinguish between benign and malignant adipocytic tumors or to subclassify LPS |
| HMB-45/STAT-6 | Angiomyolipoma or SFT |
| MYOGENIN | Allows recognition of rhabdomioblastic differentiation in DD-LPS |
| Immunohistochemistry techniques to consider in retroperitoneal fusocellular tumors | |
| MDM2/CDK4 | LPS (DD-LPS or WD-LPS), IS, MPNST |
| SMA/Desmin/H-Caldesmon | LMS or IS |
| CD34/STAT6 | SFT |
| S100/SOX10/H3K27me3 | MPNST/neural tumor |
| CKIT/DOG-1 | GIST |
| SS18-SSX/TLE-1/EMA | SS |
| HMB-45/MELAN-A | PEComa or metastatic melanoma |
| MYOGENIN | RMS or rhabdomyoblastic differentiation in other STS |
-
Recommendations
-
Detection of MDM2 amplification by FISH is currently the gold standard for the diagnosis of WD/DDLPS (I, A).
-
Molecular testing has no diagnostic role in leiomyosarcoma, SFT, or MPNST (I, A).
