Ovarian cancer is considered one of the most aggressive and deadliest gynecological malignancies worldwide. Unfortunately, the therapeutic methods that are considered the gold standard at this moment are associated with frequent recurrences. Survival in ovarian cancer is associated with the presence of a high number of intra tumor infiltrating lymphocytes (TILs). Therefore, immunomodulation is considered to have an important role in cancer treatment, and immune checkpoint inhibitors may be useful for restoring T cell-mediated antitumor immunity. PD-1 is a 50–55 kDa, 288 aa transmembrane glycoprotein that lacks the membrane-proximal cysteine residue required for homodimerization that is present in the structure of the other members of the CD28 family, thus rendering PD-1 monomeric, both in solution and on cell surface, as structural and biochemical analyses show [26]. The cytoplasmic domain presents two tyrosine residues, with the membrane-proximal one constituting an immunoreceptor tyrosine-based inhibitory motif (ITIM) and the second one constituting an immunoreceptor tyrosine-based switch motif (ITSM).
- PD-1/PD-L1
- ovarian cancer
- gynecological malignancy
- miRNAs
- lncRNAs
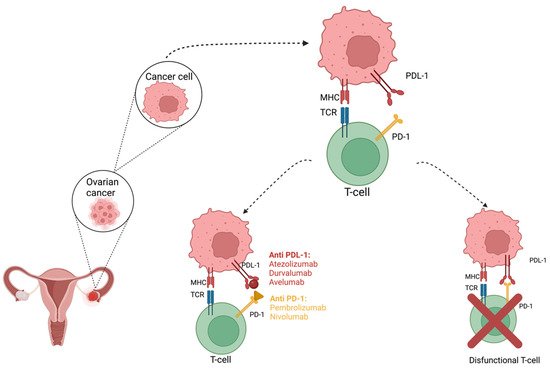
2. Efficacy of PD-1/PD-L1 Inhibitors in Ovarian Cancer
References
- Breast Cancer—Statistics. Available online: https://www.cancer.net/cancer-types/breast-cancer/statistics (accessed on 14 May 2021).
- Lim, D.; Oliva, E. Precursors and pathogenesis of ovarian carcinoma. Pathology 2013, 45, 229–242.
- Kurman, R.J.; Shih Ie, M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am. J. Pathol. 2016, 186, 733–747.
- Kurman, R.J.; Shih Ie, M. Pathogenesis of ovarian cancer: Lessons from morphology and molecular biology and their clinical implications. Int. J. Gynecol. Pathol. 2008, 27, 151–160.
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61.
- Okazaki, T.; Honjo, T. PD-1 and PD-1 ligands: From discovery to clinical application. Int. Immunol. 2007, 19, 813–824.
- Nhokaew, W.; Kleebkaow, P.; Chaisuriya, N.; Kietpeerakool, C. Programmed Death Ligand 1 (PD-L1) Expression in Epithelial Ovarian Cancer: A Comparison of Type I and Type II Tumors. Asian Pac. J. Cancer Prev. 2019, 20, 1161–1169.
- Wilczynski, N.L.; Morgan, D.; Haynes, R.B.; Hedges, T. An overview of the design and methods for retrieving high-quality studies for clinical care. BMC Med. Inform. Decis. Mak. 2005, 5, 20.
- Pedoeem, A.; Azoulay-Alfaguter, I.; Strazza, M.; Silverman, G.J.; Mor, A. Programmed death-1 pathway in cancer and autoimmunity. Clin. Immunol. 2014, 153, 145–152.
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561.
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148.
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723.
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264.
- Lee, H.T.; Lee, S.H.; Heo, Y.S. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules 2019, 24, 1190.
- Chin, C.D.; Fares, C.M.; Campos, M.; Chen, H.W.; Shintaku, I.P.; Konecny, G.E.; Rao, J. Association of PD-L1 expression by immunohistochemistry and gene microarray with molecular subtypes of ovarian tumors. Mod. Pathol. 2020, 33, 2001–2010.
- Cancer Statistics Review, 1975-2015—Previous Version—SEER Cancer Statistics Review. Available online: https://seer.cancer.gov/archive/csr/1975_2015/index.html (accessed on 16 May 2021).
- Chen, S.; Zhang, Z.; Zheng, X.; Tao, H.; Zhang, S.; Ma, J.; Liu, Z.; Wang, J.; Qian, Y.; Cui, P.; et al. Response Efficacy of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 562315.
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019, 30, 1080–1087.
- Doo, D.W.; Norian, L.A.; Arend, R.C. Checkpoint inhibitors in ovarian cancer: A review of preclinical data. Gynecol. Oncol. Rep. 2019, 29, 48–54.
- Hinchcliff, E.; Hong, D.; Le, H.; Chisholm, G.; Iyer, R.; Naing, A.; Hwu, P.; Jazaeri, A. Characteristics and outcomes of patients with recurrent ovarian cancer undergoing early phase immune checkpoint inhibitor clinical trials. Gynecol. Oncol. 2018, 151, 407–413.
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413.
- Leskela, S.; Romero, I.; Cristobal, E.; Perez-Mies, B.; Rosa-Rosa, J.M.; Gutierrez-Pecharroman, A.; Caniego-Casas, T.; Santon, A.; Ojeda, B.; Lopez-Reig, R.; et al. Mismatch Repair Deficiency in Ovarian Carcinoma: Frequency, Causes, and Consequences. Am. J. Surg. Pathol. 2020, 44, 649–656.
- Gonzalez-Martin, A.; Sanchez-Lorenzo, L. Immunotherapy with checkpoint inhibitors in patients with ovarian cancer: Still promising? Cancer 2019, 125 (Suppl. 24), 4616–4622.
- Borella, F.; Ghisoni, E.; Giannone, G.; Cosma, S.; Benedetto, C.; Valabrega, G.; Katsaros, D. Immune Checkpoint Inhibitors in Epithelial Ovarian Cancer: An Overview on Efficacy and Future Perspectives. Diagnostics 2020, 10, 146.
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543.
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213.
- Hwang, W.T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198.
- Ghisoni, E.; Imbimbo, M.; Zimmermann, S.; Valabrega, G. Ovarian Cancer Immunotherapy: Turning up the Heat. Int. J. Mol. Sci. 2019, 20, 2927.
- A Study Of Avelumab Alone Or In Combination With Pegylated Liposomal Doxorubicin Versus Pegylated Liposomal Doxorubicin Alone In Patients with Platinum Resistant/Refractory Ovarian Cancer (JAVELIN Ovarian 200). Available online: https://clinicaltrials.gov/ct2/show/NCT02580058 (accessed on 26 September 2021).
- De Felice, F.; Marchetti, C.; Palaia, I.; Musio, D.; Muzii, L.; Tombolini, V.; Panici, P.B. Immunotherapy of Ovarian Cancer: The Role of Checkpoint Inhibitors. J. Immunol. Res. 2015, 2015, 191832.
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015, 33, 4015–4022.
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454.
- Crotzer, D.R.; Sun, C.C.; Coleman, R.L.; Wolf, J.K.; Levenback, C.F.; Gershenson, D.M. Lack of effective systemic therapy for recurrent clear cell carcinoma of the ovary. Gynecol. Oncol. 2007, 105, 404–408.
- Pawlowska, A.; Suszczyk, D.; Okla, K.; Barczynski, B.; Kotarski, J.; Wertel, I. Immunotherapies based on PD-1/PD-L1 pathway inhibitors in ovarian cancer treatment. Clin. Exp. Immunol. 2019, 195, 334–344.
- Webb, J.R.; Milne, K.; Kroeger, D.R.; Nelson, B.H. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol. Oncol. 2016, 141, 293–302.
- Grel, H.; Ratajczak, K.; Jakiela, S.; Stobiecka, M. Gated Resonance Energy Transfer (gRET) Controlled by Programmed Death Protein Ligand 1. Nanomaterials 2020, 10, 1592.
- Santoiemma, P.P.; Powell, D.J., Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol. Ther. 2015, 16, 807–820.
- Abiko, K.; Mandai, M.; Hamanishi, J.; Yoshioka, Y.; Matsumura, N.; Baba, T.; Yamaguchi, K.; Murakami, R.; Yamamoto, A.; Kharma, B.; et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin. Cancer Res. 2013, 19, 1363–1374.
- Varga, A.P.-P.; Piha-Paul, S.A.; Ott, P.A.; Mehnert, J.M.; Berton-Rigaud, D.; Johnson, E.A.; Cheng, J.D.; Yuan, S.; Rubin, E.H.; Matei, D.E. Antitumor Activity and Safety of Pembrolizumab in Patients (Pts) with PD-L1 Positive Advanced Ovarian Cancer: Interim Results from a Phase Ib Study. J. Clin. Oncol. 2015, 33, 5510.
- Gadducci, A.; Guerrieri, M.E. Immune Checkpoint Inhibitors in Gynecological Cancers: Update of Literature and Perspectives of Clinical Research. Anticancer Res. 2017, 37, 5955–5965.
- Bose, C.K. Immune Checkpoint Blockers and Ovarian Cancer. Indian J. Med. Paediatr. Oncol. 2017, 38, 182–189.
- Disis, M.L.P.; Patel, M.R.; Pant, S.; Infante, J.R.; Lockhart, A.C.; Kelly, K.; Beck, J.T.; Gordon, M.S.; Weiss, G.J.; Ejadi, S.; et al. Avelumab (MSB0010718C), an Anti-PD-L1 Antibody, in Patients with Previously Treated, Recurrent or Refractory Ovarian Cancer: A Phase Ib, Open-Label Expansion Trial. J. Clin. Oncol. 2015, 33, 5509.
- Monk, B.J.; Colombo, N.; Oza, A.M.; Fujiwara, K.; Birrer, M.J.; Randall, L.; Poddubskaya, E.V.; Scambia, G.; Shparyk, Y.V.; Lim, M.C.; et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1275–1289.
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580.
