You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Loic Hilliou and Version 2 by Sirius Huang.
Hybrid carrageenans, also called kappa-2 (K2) or weak kappa, are a class of sulfated polysaccharides with thermo-reversible gelling properties in water and are extracted from a specific family of red seaweeds. K2 are known in the industry for their texturizing properties which are intermediate between those of kappa-carrageenans (K) and iota-carrageenans (I). As such, K2 are gaining industrial interest, as they can replace blends of K and I (K + I) in some niche applications.
- hybrid carrageenan
- kappa-2 carrageenan
- polysaccharide
- seaweed
- hydrogel
- rheology
1. Introduction
Carrageenans are a family of sulfated polysaccharides extracted from Gigartinales, a specific order of red algae also labeled carrageenophytes. These natural biopolymers are used essentially as food additives (E407) for texturizing formulations [1][2][1,2]. More recently, carrageenans entered the cosmetics and pharmaceutics markets as excipient for capsules and tablets [2] and also for cell immobilization in drug production [3]. They also have good prospects for application in the nutraceutical and pharmaceutical industries [4] in spite of the controversy over their food safety [5][6][5,6]. Carrageenans’ gelling properties in water-based formulations enable the encapsulation of drugs for better delivery, and their bioactive attributes [7], in particular as antivirals against SARS-CoV-2 [8], are very attractive.
The food ingredients market usually classifies carrageenans into three categories based on their functionality: kappa-carrageenan (K), which forms strong gels, iota-carrageenan (I), which forms weaker gels; and lambda-carrageenan (L), which produces viscous solutions. A fourth class, hybrid carrageenans, also called kappa-2 (K2) or weak kappa [9], has gained scientific and industrial attention over the last 20 years [9][10][11][12][9,10,11,12]. The ideal chemical structures of the repeating disaccharide units found in the gelling carrageenans (i.e., K, I, and K2) are provided in Figure 1, where the nomenclature introduced by Knutsen et al. [13] is used. There are two main reasons for the increasing interest in K2. First, recent issues in farms of seaweeds cultivated for K and I production [14][15][14,15] have led to a significant drop in the production of carrageenans. As K2 are isolated from alternative carrageenophytes, they critically contribute to addressing the greater demand in the food sector [2]. Second, K2 are used in niche dairy applications to substitute mixes of intermediate strength K and I gels [9][16][9,16] but also to replace non-gelling carrageenan blends. Thus, the direct use of K2 instead of carrageenan blends avoids an additional costly mixing pro cess after carrageenan extraction [17].
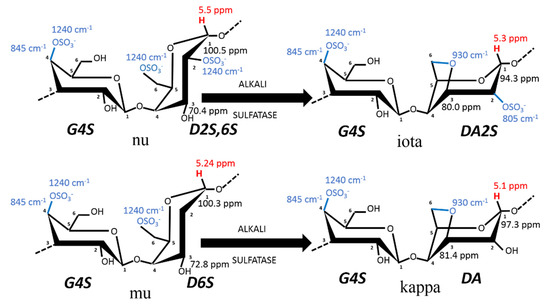
Figure 1. Chemical structure of carrageenan disaccharides comprising the sulfated polysaccharides K, I, and K2. K2 contains between 20 and 50 mol% of G4S-DA2S, as arbitrarily set by the industrial definition of kappa-2 or weak kappa [9]. The diads given for each carrageenan structure follow the nomenclature introduced by Knudsen et al. [13], where numbers close to carbons indicate their numeration in the galactopyranose. For instance, in iota-carrageenan made of alternating residues of 3-linked β-D-galactopyranose (G) sulfated at the fourth carbon (4S) and 4-linked 3,6-anhydrogalactose-D-galactopyranose (DA) sulfated at the second carbon (2S) is noted as G4S-DA2S, whereas in biological precursors, nu- and mu-carrageenan, the 4-linked α-D-galactopyranose is noted as D, with the same nomenclature for indicating the positions of the sulfates. The transformation from (D) into (DA) can be performed in the industry by alkali treatment (ALKALI) of the seaweed or occurs enzymatically (SULFATASE) in vivo, to turn the biological precursors nu-carrageenan (N) and mu-carrageenan (M) disaccharides into less sulfated I and K, respectively. Note that G4S is common to all of these disaccharides and, thus, characterizes this family of gelling carrageenans. The blue numbers refer to the FTIR absorption bands, which characterize the corresponding chemical bonds highlighted in blue, whereas the red numbers correspond to the 1H-NMR chemical shifts of the corresponding anomeric proton highlighted in red. The numbers close to C1 and C3 of the 4-linked residues correspond to the 13C-NMR chemical shifts of the corresponding carbon.
Since the last reviews on K2 [10][11][10,11], some progress has been made in the understanding of their biosynthesis and chemical structure [18] and on how to tune it through specific extraction routes or seaweeds treatments. The understanding of K2s gel’s structure–rheological properties’ relationships has also improved. Such recent progress is reported here, reviewing the literature on gelling K2 published over the last decade or so.
2. From Seaweeds to Hybrid Carrageenans
2.1. Seaweeds Used for Kappa-2 Carrageenan Production: The Carrageenophytes
Carrageenans are components of the seaweeds’ cell walls that primarily provide resistance to oceanic physical forces, osmotic stress (variation in salinity), and to desiccation during low tides, among other attributes (see, e.g., [19] and references therein). The current model for the biosynthetic pathway of carrageenan in seaweeds is described in three steps involving three different types of enzymes [18][19][18,19]: galactose residues are possibly polymerized first and then sulfated in the cells before the final cyclization of galactose 6-sulfate moieties into 3,6-anhydro derivatives occurring in the cell walls [20]. The enzymatic cyclization is now firmly established [18][19][20][18,19,20] and is thus illustrated in Figure 1. The main carrageenophytes used to produce K2 are listed in Table 1, taking into account the definition given to K2 in terms of chemical composition: carrageenans containing between 20 and 50 mol% of G4S-DA2S [9][12][16][9,12,16]. However, the list in Table 1 is extended to K2 containing between 80 and 50 mol% of G4S-DA, based on the K2 chemical structures reviewed by van de Velde [10] and documented in more recent studies, and assuming that the remaining carrageenan fraction is essentially I. Also, a 5% error in the quantification of K is considered here, since it is the uncertainty of proton NMR spectroscopy often used to characterize the extracted K2.
In a seminal paper published three decades ago, Chopin et al. [21] performed the Fourier transform infrared diffuse reflectance spectroscopy (DRIFT) of powdered algal materials to achieve a direct qualitative assessment of the main K2 contained in carrageenophytes. The DRIFT spectra of seaweeds present symptomatic absorption bands at specific wavelengths which can be assigned to specific chemical groups of the carrageenan disaccharide units (see the wavelength numbers in Figure 1). DRIFT was then used with many carrageenanophytes [22] and other infrared spectroscopic techniques (including Raman) were used over the last decade for a fast assessment of seaweeds carrageenan constituents [23][24][25][23,24,25].
Table 1. Gigartinales with the carrageenan constituents and corresponding chemical composition of K2 extracted using the listed protocol (alkali or water extraction). Data are taken from the references listed in the last column (Ref.), where blends of generic phases or reproductive plants are studied. The K content is given in mol.% computed from the NMR data reported in the corresponding reference study. L stands for lambda-carrageenan (G2S-D2S,6S) and T for theta-carrageenan (G2S-DA2S).
| Seaweed Species | Carrageenans in Seaweeds |
Extraction | K Content in K2 | Ref. |
|---|---|---|---|---|
| Ahnfeltiopsis devoniensis | K, I, M | water | 50–55 | [26] |
| alkali | 17–35 a,b | [27] | ||
| K, I, M, N | alkali | 30–50 c | [28] | |
| K, I, M, N | water | 42–50 c | [28] | |
| Chondracanthus acicularis | K, I | alkali | 76 | [10][22][10,22] |
| K, I, M, N, L, T | [23] | |||
| T | [24] | |||
| water/alkali | 60 a | [27] | ||
| Chondracanthus canaliculatus | K, I c | alkali | 78 | [10][22][10,22] |
| Chondracanthus chamissoi | K, I, T | alkali | 82 | [10][22][10,22] |
| water | 35–100 b | [29] | ||
| water | 59 | [30] | ||
| alkali | 64 | [30] | ||
| Chondracanthus corymbiferus | K, I | alkali | 74 | [10][22][10,22] |
| Chondracanthus teedei | K, I, M, N, T | alkali | 50 | [24][25][24,25] |
| water/alkali | 53–58 a | [27] | ||
| K, I, N | alkali | 50–58 c | [10][22][10,22] | |
| Chondrus canaliculata | K, L, T | alkali | 69 | [10][22][10,22] |
| Chondrus crispus | K, I, M, L, T c | alkali | 30–81 c | [10][22][10,22] |
| K, I, M, L | [24] | |||
| K, I, M | water | 60–70 | [26] | |
| alkali | 70 a | [27] | ||
| alkali | 64 a | [31] | ||
| alkali | 71–72 | [32] | ||
| K, M, N | alkali | 65–84 c | [28] | |
| K, M, N | water | 65–75 c | [28] | |
| Chondrus ocellatus | K, I, L | 85 | [10][22][10,22] | |
| Eucheuma isiforme | I | alkali | 60 | [10][22][10,22] |
| Eucheuma platycladum | K, I | alkali | 83 | [10][22][10,22] |
| Gigartina alveata | K, L | alkali | 64 | [10][22][10,22] |
| Gigartina bracteata | K, L | [10] | ||
| Gigartina chamissoi | alkali | 54 | [22] | |
| alkali | 57 | [32] | ||
| Gigartina clathrata | alkali | 80 | [22] | |
| Gigartina pistillata | K, I, M, N, L | [24] | ||
| K, I | 41–45 | [10][22][10,22] | ||
| KIM, L, T | [23] | |||
| alkali | 35–49 a | [27] | ||
| Gigartina radula | K, L | alkali | 50 | [10][22][10,22] |
| Gigartina skottsbergii d | K, I, M, N, L c | alkali | 59 | [10][22][10,22] |
| alkali | 57 | [32] | ||
| water | 62 | [33] | ||
| water | 70 | [34] | ||
| Gymnogongrus crenulatus | K, I, L | alkali | 64 c | [10][10[22],22] |
| alkali | 60–64 | [27] | ||
| Gymnogongrus humilis | K, I | alkali | 68 | [10][22][10,22] |
| Gymnogongrus tenuis | water | 14–51 b,c | [35] | |
| Gymnogongrus torulosus | alkali | 45 | [10] | |
| Gymnogongrus vermicularis | alkali | 71 | [10] | |
| Iridaea undulosa | alkali | 58–62 | [10] | |
| Iridaea cordata | water | 42 a,b,c–66 a | [36] | |
| Mastocarpus pacificus | water | >50 | [37] | |
| Mastocarpus stellatus | K, I, M, N, L c | alkali | 74 | [10][22][10,22] |
| K, I, M, N | [23][24][23,24] | |||
| K, I, M, N | water | 53–67 | [26] | |
| alkali | 62 a | [27] | ||
| water | 55–64 a | [38] | ||
| K, M, N | alkali | 62–80 a | [39] | |
| K, M, N | water | 62–65 a | [39] | |
| Mazzaella laminarioides | I, L | alkali | 55–75 c | [10][22][10,22] |
| K, I, M, N | [40] | |||
| water | 46 b | [30] | ||
| alkali | 54 | [30] | ||
| Mazzaella parksii | water | 63–79 c | [41] | |
| Mazzaella splendens | K, I, L, T | alkali | 72 | [10][22][10,22] |
| Sarcothalia crispata | K, I, M, N | [24] | ||
| K, I, M, N, L, T c | alkali | 40–63 | [10][22][10,22] | |
| water | 51 | [30] | ||
| alkali | 56 | [30] | ||
| water | 56 | [34] | ||
| Sarcothalia radula | water | 45 | [30] | |
| alkali | 53 | [30] | ||
| Turnerella mertensiana | water | 65 c | [42] | |
| alkali | 82 | [42] |
Table 1 compiles the carrageenan composition of some carrageenophytes, starting from the data reported by Chopin et al. [22] and focusing on more recent updates. Solid-state 13C NMR spectra measured on powdered algal materials has also been used for a qualitative assessment of carrageenans present in seaweeds [43][44][43,44]. However, as noted recently, such a technique is still underutilized in spite of showing good prospects for a quantitative assessment of the carrageenan composition of seaweeds [26]. The geographical variations that impact on the growing conditions of seaweeds lead to variations in the types of biosynthesized carrageenans among seaweeds populations, see for example refs. [27][31][38][27,31,38], as well as in the carrageenan content in seaweeds [45]. In addition, the reproductive and vegetative life stages of seaweeds, as listed in Table 1, are known to contain different types of carrageenans (see, e.g., [18][19][20][21][22][24][25][26][27][31][36][38][40][45][46][18,19,20,21,22,24,25,26,27,31,36,38,40,45,46] and references therein). Thus, several entries in Table 1 mirror such variation in the carrageenan composition of seaweeds, as well as in the K content of the extracted carrageenans. But this has virtually no impact on the K2 chemical structures produced in the industry from a specific carrageenophyte. This is because the large scale extraction process does not include a sorting of seaweeds, and alkali are often used to convert more sulfated disaccharide units into gelling K2 (see Figure 1).
In contrast to the direct chemical analysis of seaweeds, most of the research on the identification of the gelling K2 produced by carrageenophytes, which is reviewed elsewhere [10], relies on the extraction of polysaccharides from seaweeds. K2 extraction, thus, adds complexity to the classification of carrageenophytes for K2 production, as variability in the employed extraction protocols is intrinsically linked to the variability of the isolated K2 chemical structures. Thus, variability in the K content of K2 extracted from the seaweeds listed in Table 1 comes as no surprise to the extent that some reports suggest that certain extracted carrageenans do not qualify for the industrial definition of K2. The extraction routes for isolating K2 from carrageenophytes is detailed in the next section.
2.2. Extraction of Hybrid Carrageenans
The extraction routes used in the studies referenced in Table 1 are shown in Figure 2.
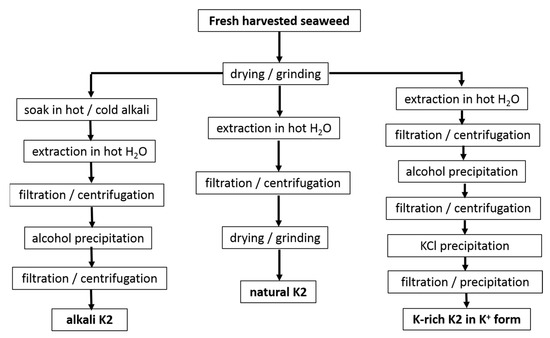
Figure 2.
Protocols used in studies reported in
Table 1
for the water extraction of K2 from seaweeds.
First, processes such as the drying and grinding of harvested seaweeds are ubiquitous in the industry before starting the extraction step. Few reports suggested that these postharvest processes affect the hybrid carrageenan characteristics. Higher temperatures used during the air drying of M. stellatus gametophytes lead to the recovery of K2 with untouched chemical structures but with smaller molecular masses [47]. The grinding of these seaweeds into smaller particles enabled the water extraction of more K2 (extraction yield increased) with larger molecular masses [48]. In contrast to this, for C. crispus gametophytes, the particle size did not impact on the extraction yield of the hybrid carrageenans [49]. Clearly, this topic deserves more research efforts. However, few studies avoid seaweeds drying and grinding to bypass any possible effect on extracted K2 characteristics, whereas larger K2 recovery yields are not needed [30][31][38][30,31,38]. Another process, the dark treatment of carrageenophytes during cultivation after harvesting, has also attracted little research, in contrast to the dark treatment of seaweeds used in the production of agar. The cultivation in the dark of C. crispus was shown to increase the amount of 3,6-anhydrogalactose in recovered K2 with correspondingly less sulfate content [50]. As such this postharvest process could be optimized for an ecofriendly in vivo enzymatic alternative to the alkali conversion of M and N into K and I [50][51][50,51]. Another ecofriendly alternative consisting in the postharvest culture of commercial seaweeds in low-nutrient condition has been suggested for the production of I [52], but this approach has not yet been tested for K2 carrageenophytes.
After seaweed postharvest treatment, K2 extraction is performed by dispersing algal material in water. Water extraction stems from the leaching of very different carrageenans (including non-gelling) from the seaweeds. Hybrid carrageenan solubility in water was recently shown to be strongly dependent on the type of cations present in the postharvested seaweeds, on the contacting duration t with water, and on the temperature, T, used during extraction [53]. Parameters t and T, as well as the type and amount of alkali, can thus be independently varied to tailor the chemical composition of the extracted K2. Lower T were consistently reported to yield more sulfated K2 for C. chamissoi [29] and C. crispus [53]. However, for G. tenui, a K2 was extracted with cold water, whereas a more sulfated hybrid carrageenan was recovered at 80 °C, which as such does not qualify as a K2 [35]. In presence of alkali, longer t was associated with the recovery of K2 containing less biological precursors (M and N) for M. stellatus [39], C. crispus, and A. devoniensis [28]. The latter two studies also showed that NaOH was more efficient than KOH to convert M and N into K and I disaccharide units, as shorter t were needed to achieve the minimum levels of more sulfated disaccharide units. Finally, the extraction parameters are also known to impact on the molecular masses of K2 [54][55][54,55], in particular with stronger and longer alkali treatments giving K2 a reduced chain size [28][39][28,39]. Overall, as both the kinetics and extent of the cyclization reactions are known to depend on K2′s chemistry [56], more studies on the optimization of the alkali extraction parameters towards the recovery of specific K2 chemical structures should be extended to other carrageenophytes.
After the extraction, solid algal residues are separated from the K2-rich solution, by filtration or centrifugation. Then, K2 is recovered from the solution usually by precipitation in alcohol, as is the case for all of the extracts listed in Table 1, except for the commercial ones [10][32][10,32]. Precipitation in KCl can be preferred to recover K2 with better gel characteristics (usually the precipitated carrageenan is less sulfated than the fraction remaining in the liquid phase [35][36][37][41][42][35,36,37,41,42]) or to partially get rid of agarans and other non-carrageenan products present in certain carrageenophytes. Alternatively, K2 can be simply recovered in film or powder form by water evaporation of the concentrated K2-rich solutions or by freeze drying [34][35][37][34,35,37]. The cooling of the concentrated hot K2-rich solution can also be performed to allow for gel formation. Various gel freezing/pressing/thawing cycles are then applied to expel water and other residues before drying. This method is, however, only amenable for K2 containing large K and salt contents to yield strong enough gels, and it was, indeed, not used in the studies referenced in Table 1. Depending on the employed separation and recovery protocols, different impurities are extracted together with K2. Thus, further K2 purification can be performed, for instance, by enzymatic treatment to get rid of Floridean starch [28][55][28,55]. In addition, K2′s chemical structure can be further modified (for instance, by alkali treatment to further reduce the number of sulfate groups on the polysaccharide chain [34][35][36][37][40][41][42][34,35,36,37,40,41,42]), a monocationic form of the polyelectrolyte can also be isolated (preferentially avoiding excessive dialysis to avoid a drop in the molecular mass [57]), or K2 can be further fractionated into less sulfated carrageenan by gelling into KCl.
Emerging greener extraction methods utilizing lower energy and water consumption, more ecofriendly solvents, and bio refinery concepts have also been tested with some of the seaweeds listed in Table 1. Microwave-assisted extraction [58][59][58,59] and ultrasound-assisted extraction [60] were shown to result in better K2 extraction yields, produce K2 with better gelling properties or, at least, to reduce the extraction time compared to K2 isolated with the conventional extraction routes provided in Figure 2. Extraction with subcritical water has also been tested but did not show promising results for K2 yields and gelling properties, at least for the seaweeds and the process parameters tested [61]. Biorefinery concepts were also applied to M. stellatus industrial wastes streamed from conventional extraction and showed that K2 and antioxidant compounds could be further extracted from the waste [62]. The scaling up and industrial intake of these greener processes are, however, still to be demonstrated for K2 extraction.
2.3. Statistical Block Copolymer Structure of Gelling Hybrid Carrageenans
The macromolecular structure of K2 has long been debated (see, e.g., [1]). Its labeling as hybrid carrageenan reflects the long discussion concerning whether K2 is a mixture of essentially K (homopolymer of G4S-DA) and I (homopolymer of G4S-DA2S) or a heteropolymer containing monomers such as G4S-DA and G4S-DA2S. An historical perspective of this topic is perhaps the best way to discuss the polymer structure of K2, since the debate has been settled only a decade ago or so.
In 1981, when Stancioff presented his diagram of K2 extracted from different seaweeds as a function of their sulfate content [63], he noted that K2 showed physical properties that differ from mixtures of K and I (K + I). Hybridity, in terms of the existence of heteropolymers, was recognized for K and I extracted from Kappaphycus alvazerii and Eucheuma denticulatum, respectively [64]; two seaweeds are mostly cultivated for the industrial production of K and I. Bellion et al. [64] found the biological precursors G4S-D2S and G4S-D2S,6S in carrageenans recovered after the enzymatic treatments of K and I, respectively [64]. Thus one could suspect a similar complexity in the macromolecular structures of K2 isolated from the seaweeds listed in Table 1. The nature of the hybridity was scrutinized by Rochas et al. [65] for the commercial carrageenan extracts from C. crispus and M. stellatus. Different macromolecular structures were hypothesized from mixtures of K and I homopolymers to copolymers showing a diblock structure or a statistical structure with short blocks of G4S-DA and G4S-DA2S randomly distributed along the polymer chain. The authors concluded from the 13C-NMR analysis of KCL soluble and insoluble fractions of carrageenans from C. crispus that both G4S-DA and G4S-DA2S were present in the fractionated products. However, these fractions were not submitted to the enzymatic treatment performed by Bellion et al. [64]. Thus, no conclusion regarding the copolymer nature of K2 from C. crispus and M. stellatus was delivered.
A decade later, KCL fractionations under similar conditions were repeated by van de Velde et al. [66] for two commercial K2s. In addition, their solution properties were systematically compared with those of K and I mixtures (K + I) of corresponding composition in G4S-DA and G4S-DA2S. KCl was not effective at separating fractions for both K2 in contrast to the corresponding K + I separated in a K-enriched gel phase and a I-enriched viscous phase. More important, the coil-to-helix transitions probed by intrinsic viscosity and optical rotation showed that K2 is characterized by a single broad transition in contrast to K + I, which showed the two transitions of both the I and K helices [65]. These marked differences prompted the authors to suspect that K2 was made of long blocks of G4S-DA and G4S-DA2S on the same polymer chain [66].
More recently, the statistical block copolymer nature of K2 (see Figure 3) was firmly established by W. Helbert’s group, who extended the enzymatic degradation studies of Bellion et al. [64] to a series of K2 and new ι-carrageenases and κ-carrageenases.
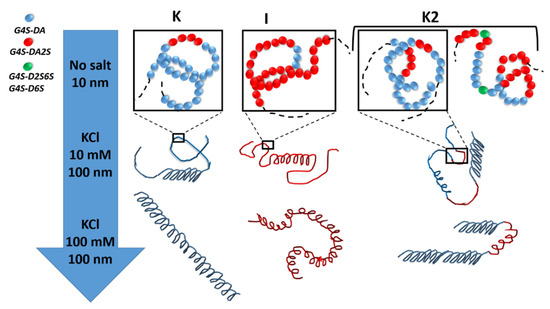
Figure 3. The statistical block copolymer structure of K, I, and K2. K and I from K. alvazerii and E. denticulatum contain 3 to 11 mol% of G4S-DA2S and G4S-DA, respectively [10][60][62][10,60,62]. The sketches in the top row of the figure illustrates the block copolymer nature of K2 in the absence of salt (coil conformer), which includes sequences of G4S-DA2S (red sphere) and G4S-DA (blue sphere) interrupted by a more sulfated G4S-D2S,6S or G4S-D6S (green sphere), respectively. With the addition of salt, each block can adopt a helical conformation [54][55][54,55]. With more salt, full helices are formed with different levels of flexibility depending on the K or I block [67][68][67,68].
In two seminal papers reporting the in-depth chemical analysis of both degraded and undigested carrageenan products, Guibet et al. [32] and Jouanneau et al. [30] showed that K2 chains were made of (1) blocks of G4S-DA, (2) blocks of G4S-DA2S, and (3) sequences where G4S-DA and G4S-DA2S are randomly distributed. Moreover, the presence of biological precursor diads G4S-D2S and G4S-D2S,6S in blocks of G4S-DA and G4S-DA2S, respectively, was demonstrated. These results, thus, confirmed earlier conclusions drawn from the modeling of coil-to-helix transitions in purified K2 (e.g., free of G4S-D2S and G4S-D2S,6S) [55]. The analysis of the experimental data with a statistical block copolymer model suggested the existence of randomly distributed blocks of at least 8 to 14 G4S-DA diads and 2 to 5 G4S-DA2S diads, depending on the experimental technique used to probe the transitions and the type of salt used to induce the helical formation and aggregation. The lengths of the blocks are overall of the same order of the lengths actually inferred from the enzymatic degradation studies [30][32][30,32]. But more importantly, the latter underlined that the distribution of block lengths along the K2 chain varies from chain to chain and that this compositional variation is specific to the seaweed producing the K2, as well as the extraction process. In other words, and using a vocabulary specific to polymer science, K2 are statistical block copolymers with polydispersity in both chain and blocks lengths.
Several of the recent studies listed in Table 1 confirm the statistical block copolymer structure of K2 extracted under various conditions from different carrageenophytes. Essentially, the chemical analysis of the products from the acidic hydrolysis of K2 [29][37][29,37] or of fractionated K2 extracts with KCl [35][37][41][35,37,41] point towards a copolymer structure, as no K and I chains can be recovered separately. Furthermore, stepping on the Stancioff’s remarks [63], two studies highlighted the differences between the phase diagrams of several K2 and of the K + I with equivalent molar compositions in G4S-DA and G4S-DA2S [69][70][69,70]. On top of confirming that K2 was not a mixture of K and I, these results pointed towards the fact that phase separation between K2 and solvent occurs at ionic strengths and carrageenan concentrations that are specific to the K2 chemical structure and do not match those found for separating K and I from mixtures. This also explains why recent KCL fractionations of seaweed extracts in increasing ionic strengths are successful in separating K2 with different chemical compositions [35][37][41][35,37,41].
