1. Small Molecule Prodrugs
Pharmacologically, prodrugs are inactive derivatives of a parent drug molecule that result from impermanent chemical modifications of bioactive compounds, which are activated at a specific target site to confer a therapeutic effect. Prodrugs should be ideally stable in the blood against enzymatic degradation, but rapidly convert into an active compound at the site of action
[1][134]. The chemical modification is usually intended to reduce drug molecule toxicity, and enhance membrane solubility or permeability
[2][135]. Typically, they consist of a small molecular inactive agent conjugated to a macromolecular carrier as a transporter or a lipophilicity enhancer
[3][136].
1.1. Folate-Modified Prodrugs
The kidneys play a crucial role in preventing folate loss. Folate is metabolized in vivo as 5-methylenetetrahydrofolate, that is filtered into the glomerulus before reabsorption at the renal vascular circulation by a folate-binding protein (FBP), localized mostly in the proximal tubular epithelium. Hence, folate can be employed to deliver drugs to proximal tubular cells as a ligand
[4][44]. Mathias et al. successfully produced [
99mTc]DTPA-folate and assessed its potential as a Folate Receptor-Targeted Radiopharmaceutical
[5][137]. The [
99mTc]DTPA-folate complex biodistribution in the kidney reached about 21% of the injected dose per gram tissue after 4 h of intravenous administration in Athymic Male Mice with KB Xenografts. The biodistribution level of a complex in the kidney was lowered to 2.3% when free folic acid was administered at 1–2 min prior to complex injection. Similarly, Trump et al. found that after 4 h of intravenous injection, the biodistribution of [
99mTc](CO)3-DTPA-folate in the kidney was nearly 47% of the injected dose per gram tissue
[6][138].
Moreover, in pioneering work by Wang et al., DTPA–folate conjugate was synthesized by attaching folic acid to a diethylenetriaminepentaacetic acid via an ethylenediamine spacer
[7][139]. The conjugate was excreted rapidly in urine following intravenous administration to a normal rat. A pharmacodynamic study on athymic tumor-bearing mice showed a good uptake of DTPA–folate in tumor tissue with a large distribution in the kidneys after 1 h of intravenous administration
[8][140]. In addition, Shan, et al., produced folic acid-conjugated paclitaxel–Evans blue prodrug for targeted cancer therapy
[9][141]. The targeting ability study was carried out using tumor-bearing mice. Their findings showed that the concentration of paclitaxel in the kidneys was increased from 5769 ng/g to 9181 ng/g after intravenous injection of free-paclitaxel and folic acid-conjugated paclitaxel, respectively. Thus, folate-conjugated small molecules may be an excellent carrier for renal drug targeting and the physicochemical properties of the complex may possess an essential role in tissue targeting.
1.2. Sugar-Modified Prodrugs
Recently, the significance of sugar-modified peptides in renal-targeted drug delivery has attracted a lot of attention in pharmacology and pathology. The structure of the sugar plays an essential role in renal selectivity and efficient uptake of the glycosylated peptides through binding to the renal microsomal fraction that enhances peptide distribution in the proximal tubules
[10][11][142,143].
Suzuki et al. synthesized glycosylated peptide (
[3H]Glc-S-C8-AVP) to determine the inhibitory glycosides effects on the kidney membrane fraction using different glycosylated derivatives. Their findings indicated that the alkylglucoside structure (Glc-S-C8-) was required for targeting of the kidney. The targeting efficiency was affected by the sugar moieties type, especially the type of linkage, structure of the peptide, and the length of the alkyl chain, as well as the charge and size of the molecule
[11][143]. On the other hand, Shirota et al. characterized the renal targeting efficiency and limitations of alkylglucoside carriers
[12][144]. The researchers combined alkylglucoside vector with acylated poly-L-lysine at different molecular weights to study the effect of the size of derivatized ligands with alkylglucoside. The study of tissue distribution found that molecular weight had a negative effect on the accumulation of alkylglucoside-acylated poly-L-lysine accumulation in the kidneys of mice. In addition, alkylglucoside was combined with anionic, neutral, or cationic tyrosine derivatives to investigate the charge effects on the specific binding to kidney membranes. According to these results, the anionic charge of the alkylglucoside-tyrosine conjugate led to reduction in the renal targeting efficiency of the conjugate.
Moreover, Lin and co-workers prepared a prednisolone succinate-glucosamine conjugate (PSG) and a 2-deoxy-2-aminodiglucose-prednisolone conjugate (DPC) as a potential targeted delivery system of prednisolone in the kidneys
[13][145]. The cytotoxicity and cellular uptake studies using HK-2 and MDCK cell lines showed that the conjugate PSG decreased the cytotoxicity, as well as improved the cellular uptake to 2.2 times higher, compared with prednisolone. DPC-enhanced kidney-specific localization in vivo, in which the drug concentration was 4.9-fold greater than that of prednisolone, was observed The researchers concluded that 2-glucosamine, as well as 2-deoxy-2-aminodiglucose, could be potential carriers for kidney-targeting drug delivery.
In addition, Liang, Zhen, et al. conjugated zidovudine with chitosan oligomers to increase the half-life and elimination time of zidovudine in human plasma and kidneys
[14][146]. The conjugate was evaluated with reference to in vitro release in mice plasma and renal homogenate after intravenous administration. The results indicated that the mean retention time of the conjugate was 1.5 h, compared with 0.59 h for zidovudine without chitosan oligomers, as well as the conjugate enhancing the accumulation of zidovudine in the kidney higher than in any other organ. Hence, in order to improve the treatment of acute kidney injury through enhanced rapid distribution in the kidney and enhanced retention time in the renal tubule, Lui et al. prepared an l-serine-modified chitosan-based carrier
[15][147]. This system showed rapid accumulation and long-term retention in renal tubules resulting from the native cationic charge of chitosan and the specific interactions between serine and kidney injury molecules.
1.3. Amino Acid-Modified Prodrugs
Some endogenous enzymes exist in a high concentration in kidneys, especially those involved in amino acids, such as γ-glutamyltranspeptidase and L-decarboxylation
[4][16][44,148]. On this basis, it was envisaged that chemical modification of drugs with substrates of these enzymes would increase the concentration of drug molecules in proximal tubular cells as a result of the effect of the relevant enzyme
[4][44]. In this regard, Wilk et al. synthesized γ-glutamyl-dopamine (GGDA) chemically and enzymatically, followed by determination of the tissue distribution of dopamine in the kidney after injection into mice
[16][148]. After equivalent dose administration, the dopamine concentration in kidneys produced by GGDA was 5-fold higher than that of L-dopa. The renal blood flow was significantly improved with an insignificant effect on the blood pressure and on the heart. In addition, by administration of GGDA orally, the results showed that the concentration of free dopamine was high in urine compared to that in the plasma
[17][18][149,150]. The researchers concluded that GGDA could be considered a potential specific strategy to deliver dopamine to the kidney.
Moreover, in order to improve the renal targeting of the parent drug prednisolone, Su et al. synthesized an N-acetyl-glutamyl prednisone prodrug for evaluating in vivo distribution and the bone mineral densities (BMD) in rats
[19][151]. The obtained findings showed that, compared with the parent prednisone, the renal concentration of prodrug was increased and its effect on bone density was reduced. Renal endothelial cells are a major part of the filtration system in the kidneys. Throughout transplant-associated ischemia, endothelial cells are the main target of complement-activated injury, resulting in delayed posttransplant function
[20][152]. Durigutto et al. generated a targeted delivery system to renal endothelium by coupling a neutralizing anti-C5 antibody with a cyclic RGD peptide
[21][153]. The novel system showed preferential localization to ischemic endothelial cells in a rat for renal ischaemia reperfusion injury (IRI). The injected anti-C5 antibody RGD reduced the renal injury level without a significant effect on circulating levels of C5. The antibody conjugate was suggested as a novel target for drugs to prevent post-transplant IRI as well as in transplant medicine.
2. Antibody Modified Carriers
Antibodies are unable to be filtered via the glomerulus because of their high molecular weight (about 150 kDa), and, thus, they cannot be applied as a drug delivery system for targeting the renal proximal tubuli. However, radiolabeled end products of antibody fragments showed prolonged renal radioactivity, as well as radiolabeled monoclonal antibody fragments, which have an accumulation in renal tubuli according to the studies of anti-cancer therapy
[22][23][24][154,155,156]. Although the prolonged and increased accumulation of fragments in the kidneys are undesirable side effects of these cancer treatment strategies, taking the use of antibody fragments as a renal carrier system into consideration could be beneficial. The advantage of incorporating antibody fragments for therapeutic administration into proximal tubular cells is the targeting of disease-related growth factor receptors, such as EGF receptors and TGF-β receptors, located in the proximal tubular cells on its basolateral and apical membranes
[25][26][157,158]. Antibody-drug conjugates may, thus, have 2-fold inhibitory effects: first, preventing natural ligands from binding to the receptor, and second, delivering the drug after internalization of the drug-antibody conjugate by target cells.
Li et al. employed an antibody fragment F(ab’)2 in order to target plasmalemma vesicle-associated protein (PV1), an endothelial cell-specific protein localized in caveolae, fenestrations, and trans-endothelial channels as a structural component
[27][159]. By finding the suitable binding affinity of an antibody toward PV1, the developed delivery system showed continual retention in mice kidneys at 24 h, whereas the isotype control F(ab′)2 was eliminated rapidly in the urine with a significant reduction in signaling in the kidney. The researchers suggested that PV1-targeted F(ab′)2 is potentially a useful system for delivering therapeutic agents to the kidneys. On the other hand, Kvirkvelia et al. attempted to treat nephritis by using a human monoclonal antibody (F1.1) as a vehicle for delivering the drug to glomeruli via directly targeting the noncollagenous-1 domain (NC1) of 3(IV) collagen
[28][160]. After coupling PGE2 and dexamethasone to F1.1, the glomerular localization, as well as the conjugation capacity to modify disease, were estimated in mice with established nephritis. The conjugates demonstrated high efficacy and reduced systemic effects and the blood urea nitrogen levels were also improved, compared to the untreated mice.
Podocytes, also known as visceral epithelial cells, are the last barrier of the kidney filtration and their function is critical in proteinuria remission. They are harmed in a variety of diseases with immune and non-immune causes. In order to target the visceral epithelial cells within the glomerulus, an anti-mouse podocyte antibody was modified by means of enzymatic cleavage and by coupling with a protamine molecule
[29][161]. Protamine has a positive surface charge, and, thus, it can bind to nucleic acids that are negatively charged, such as siRNA. Furthermore, protamine provides protection for nucleic acids against degradation. By loading siRNA specific for nephrin into this antibody, the mRNA levels of nephrin in mice were significantly decreased, pointing to the specificity of the delivery system.
3. Macromolecular Carriers
Macromolecular carriers are considered to be very useful vehicles for targeting therapeutic molecules to the kidney, in which low molecular weight glomerular protein (LMWP) can accumulate in the kidneys selectively. Generally, macromolecular carriers are small molecular weight (MW <30,000 Da), biologically active, proteins in the circulatory system, including peptide hormones (such as insulin), immune proteins (such as light chain immunoglobulin), and enzymes (such as lysozyme)
[4][44]. Ordinarily, the macromolecular carriers have a molecular weight larger than that of the encapsulated drug, and the kinetics of the protein carrier is superior to the intrinsic kinetics of the drug. In particular, the filtration of LMWP occurs through the glomerulus and the reabsorption through the renal tubules. The properties of LMWP, such as non-immunogenic properties, as well as its several functional groups, confer on LMWP the potential to be a promising delivery system for different drugs. Drugs conjugate with LMWP through different methods, such as ester, peptide, amide, and disulfide bonds
[4][30][44,162]. The macromolecular carrier–drug conjugate is removed from the circulation and then the drug is released under enzymatic or chemical hydrolysis, while the activation occurs in lysosomes. Ideally, the synthesis of the conjugate of drug–LMWP requires a high degree of skill due to the several active groups of LMWP that are extremely vulnerable to self-aggregation
[4][44].
In this regard, Kok et al. studied the impact of the chemical modification of primary amino groups on the pharmacokinetic profile of drug–LMWP conjugates. The organic anion fluorescein isothiocyanate (FITC) was used as a model drug
[31][163]. In order to study the effect of charge modifications on the FITC–LMWP lysozyme (LZM) conjugate distribution, they prepared a series of conjugates with different amounts of primary amino groups. The final prepared conjugates had several determined amounts of primary amino groups of 8.5 (FITC-cat-LZM), 6.5 (FITC-LZM), or 0.2 (FITC-Suc-LZM). The findings showed that the charge of the products had an effect on the biodistribution of FITC–LZM conjugate. The tubular reabsorption was reduced while the excretion of the conjugate was increased into the urine as a result of reducing the amount of primary amino groups. Moreover, the renal reabsorption and the extrarenal distribution of the conjugate with partial loss of renal selectivity were enhanced by increasing the amount of primary amino groups.
LMWP lysozyme is considered a suitable drug system for renal drug targeting. In another study by Kok et al., they synthesized two different FITC–LZM conjugates with and without positive charge to study urinary excretion
[32][164]. The positively charged conjugate was synthesized by reacting fluorescein isothiocyanate (FITC) with lysozyme, whereas the non-charged conjugate was synthesized by reacting succinic anhydride with the remaining free primary amino groups of the FITC–LZM. The obtained data indicated that the urinary excretion of a drug–LMWP conjugate was enhanced via decreasing the positive charge of the carrier surface. This system could be a promising candidate for drug delivery to the bladder.
4. Water Soluble Polymeric Carriers
Several research projects have shown that water soluble polymers have a beneficial effect on kidney-targeted drug delivery approaches. The accumulation in the renal tubule of any polymer is mainly related to several determinations, such as the final molecular weight, the type of the monomeric unit, the anionic group of the monomer, and the content of the anionic monomer
[33][165].
One example, polyvinylpyrrolidone (PVP), with low molecular weight, is excreted in the urine without an accumulation in the kidneys
[34][166]. However, in the study of Kodaira et al., intravenous administration of carboxylated PVP showed an improved renal accumulation compared to sulfonated PVPs, in which around 30% of the administered dose was observed in renal tubules; mainly the proximal tubular epithelial cells
[35][167].
Due to the safety property, as well as the strong kidney-targeted ability of Poly (vinylpyrrolidone-co-dimethyl maleic acid) (PVD) as a drug carrier, Yamamoto et al. studied the relationship between the molecular weight of PVD and its renal accumulation in mice
[36][168]. The findings showed that the molecular weight of 6–8 kDa led to the highest renal accumulation, in which 80% of the intravenously-administered dose accumulated in the kidneys for 3 h.
Liu et al. applied atom transfer radical polymerization to synthesize a panel of polymers in order to determine the impact of molecular weight as well as anionic charge density on kidney targeting and distribution in mice
[37][169]. The observed results showed that kidney-specific polymer accumulation improved due to the anionic monomer content, but not the molecular weight. The experimental focal segmental glomerulosclerosis enhanced kidney accumulation of anionic polymers; and anionic polymers accumulated mainly in proximal tubule cells, with little distribution in kidney glomeruli.
Kamada et al. synthesized polyvinylpyrrolidone-co-dimethyl maleic anhydride [poly(VP-co-DMMAn)] to evaluate its use as a kidney-targeted drug carrier
[34][166]. The conjugate led to an increase in accumulation and retention in the kidneys, compared to unconjugated drugs, without any toxic effect, in which the accumulation was around 80% for 24 h. with about 40% remaining in the kidneys for 96 h after intravenous administration to mice. In contrast, the distribution of polyvinylpyrrolidone with the same molecular weight showed a random distribution in vivo. In addition, modifying poly(VP-co-DMMAn) with superoxide dismutase led to a high accumulation in the kidneys of mice, conjugated with an accelerated recovery from acute renal failure. unlike the native superoxide and polyvinylpyrrolidone modified superoxide dismutase.
5. Nanoparticles
The distinctive size of nanoparticles has been widely applied to develop an effective targeted delivery system. Particles and colloids with a size of 5–7 nm can pass the glomerular filtration barrier, and, thus, they may be used in the targeting of the tubular area. Otherwise, systems with a size range of 30–150 nm do not penetrate into primary urine, unless in the case of damage in the glomerular filtration barrier by disease or the if the particles have been degraded into particles <10 nm
[38][55]. The particle size of 80 nm was attributed as being the maximum glomerular accumulation, regardless of some exceptions, such as the distinct chemical composition of each carrier system, as well as extra-small carriers (2 nm) which find it difficult to pass the glycocalyx and are usually removed by the liver
[39][170].
Gao et al. prepared low molecular weight chitosan nanoparticle-loadedsiRNA duplexes by means of ionic gelation as a therapeutic targeting strategy for various kidney diseases
[40][171]. Chitosan/siRNA nanoparticles, with a size range of 75 ± 25 nm, were administered to chimeric mice with conditional knockout of the megalin gene. The results showed a specific delivery after nanoparticles administration to proximal tubule epithelial cells in mice kidneys with a residence time of more than 48 h. The specific uptake of proximal tubule epithelial cells was mediated by megalin, and that led to a reduced expression of the water channel aquaporin 1 (AQP1) by up to 50%. This system might be a potential platform for treating many kidney diseases via targeting of siRNAs, or drugs, to proximal tubule epithelial cells.
Recently, chitosan polymer was used to synthesize nanoparticle-loaded metformin to investigate its efficiency as an oral drug delivery carrier for chronic kidney disease
[41][172]. Chitosan nanoparticles were produced by ionic gelation followed by several experiments to examine their physiochemical and muco-adhesion properties, as well as their release profile, in a low pH environment. Metformin was encapsulated into chitosan nanoparticles in order to modify the paracellular permeation and improve the oral bioavailability of metformin. Upon administration of the nanoparticles (~145 nm diameter) by oral gavage to a murine model of polycystic kidney disease, a higher metformin bioavailability, as well as a lower cyst growth, was observed compared to the free drug. The nitrogen, creatinine, and blood urea levels were found to remain similar to untreated mice, elucidating the absence of nephrotoxicity. This
res
earch tudy highlighted chitosan nanoparticles as a potential oral delivery platform for polycystic kidney disease.
PLGA nanoparticles were also applied for renal applications as a therapeutic agent delivery
[42][43][173,174]. In this approach, Tang et al. successfully employed PLGA nanoparticles in plasmid DNA (pDNA) delivery, in which pDNA was embedded in calcium phosphate (CaPi) and encapsulated into PLGA nanoparticles
[44][175]. The transfection efficiency was enhanced after optimizing pDNA loading efficiency as well as pDNA release kinetics, compared to conventional methods of plasmid delivery (e.g., lipofectamine). In addition, a significant improvement in the transfection efficiency of the applied nanoparticles on human embryonic kidney (HEK 293) cells was observed compared to pDNA-loaded PLGA nanoparticles as well as the CaPi-pDNA embedded PLGA microparticles.
In another study, Williams and his team investigated renal safety and selectivity after the administration of mesoscale nanoparticles that were prepared by conjugating poly (lactic-co-glycolic acid) with polyethylene glycol (PLGA-PEG)
[45][100]. The direct administration of these particles intravenously showed an increase of around 26-fold in renal accumulation, greater than in any other organ. The ex vivo imaging and intravital microscopy exhibited the delivery of mesoscale nanoparticles into proximal tubule cells. In addition, the mice that were treated with the nanoparticles did not show any systemic consequences, liver impairment, immune reaction, or renal impairment. This
res
earch tudy portends more developments on the targeted drug delivery of renal tubules. The recent advances in kidney-targeted drug delivery systems using nanotechnology are shown in (
Table 1).
7. Hydrogel
Hydrogels can be utilized to encapsulate various therapeutics that are useful in the treatment of kidney diseases. Existing kidney-targeted drug delivery hydrogels can be classified as follows: (1) cell-seeded to enhance paracrine activity and cell survival, (2) therapeutic-loaded for controlled release, and (3) hydrogel/NPS composites (such as micelle- and exosome-loaded hydrogels). The majority of hydrogels used for kidney therapy are administered by intrarenal/intracapsular injection. Intracapsular injection of hydrogels has gained popularity in recent developments in the utilization of hydrogels for kidney drug delivery and has proven to be effective in achieving local and controlled release of therapeutics and in protecting, as well as enhancing, the effects of concurrently transplanted pro-regenerative cells. However, the majority of the hydrogel reported on were used to deliver cell therapy and used invasive delivery through injection (Table 2).
6. Liposomes
Liposomes are used as a drug delivery platform, due to their following features: controllable release profile, stability in vitro and in vivo, targeted drug delivery, and disease site localization. Liposomes have been widely studied for drug delivery and they are used in several current marketed products. Singh et al. prepared unilamellar vesicles (SUVs) containing methotrexate [(MTX) SUVs)] linked to Dal K29
[54][182]. After 2 h of vesicle incubation with CaKi-1 cancer cells (human kidney), the binding to CaKi-1 cells increased about 8-fold more than unlinked (MTX)SUVs and around 6-fold more than nonspecific mouse myeloma IgGl-linked (MTX)SUV. In addition, the obtained results of the colony inhibition assay showed that the prepared system was, respectively, 40 and 5 times better than free MTX and Dal K29-MTX in inhibiting the growth of CaKi-1 cells.
By using prednisolone phosphate (PSLP) as a model drug, the ability of PEG-modified liposomes containing TRX-20 (TRX-liposomes) to target mesangial cells and their pharmacokinetic behavior was studied by Morimoto and his team, using a rat experimental glomerulonephritis model
[55][183]. The results showed that TRX–liposomes targeted glomeruli in the kidney cortex successfully via binding to chondroitin proteoglycans. In particular, TRX–liposomes showed their effect using a much lower dose than that required of PEG–liposomal formulations or the conventional injection of PSLP. The distinct pharmaceutical properties of TRX–liposomes support the idea that they are a candidate system for targeting inflamed glomerular mesangial cells in glomerulonephritis therapy.
In the study by Tuffin et al., mesangial cells were targeted by the prepared liposomes with Fab′ fragments of OX7 mAb (OX7-IL)
[56][184]. However, a single injection of a low dose of doxorubicin encapsulated in OX7-IL was administrated intravenously and showed extensive glomerular damage without adverse effects on other kidney tissues or other organs.
Li et al. prepared a system in order to deliver celastrol to interstitial myofibroblasts, that was related, specifically, to renal fibrogenesis
[57][185]. The loading of celastrol into linear pentapeptide Cys-Arg-Glu-Lys-Ala (CREKA)-coupled liposomes led to an alleviation in renal fibrosis induced by unilateral ureteral obstruction (UUO) in mice, with much lower toxic effect than the free drug. This
res
earch tudy suggested that loading celastrol in CREKA–liposomes could increase its therapeutic effect and decrease its systemic toxicity as a novel strategy in renal fibrosis treatment. In addition, the ability of CREKA-coupled liposomes to bind to fibronectin could permit imaging application to diagnose renal fibrosis. All the delivery system strategies are summarized in
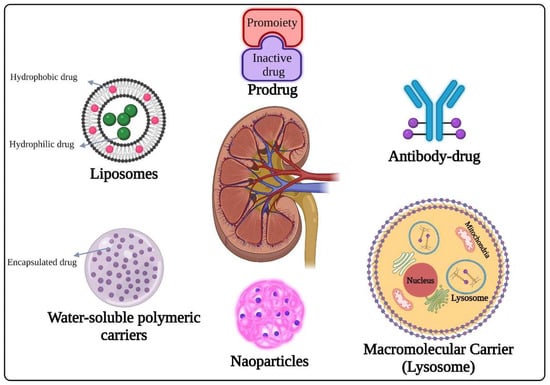
Figure 1.
Figure 1.
Different strategies of renal drug delivery system.
Stem cell therapy, including mesenchymal stem cells (MSCs), and endothelial progenitor cells (EPCs) has been demonstrated to treat chronic kidney disease. It involves the use of a paracrine activity to stimulate the repair of damaged kidney tissues
[70][71][193,194]. Nevertheless, cell therapies are often limited by the poor survival and density of transplanted cells. A number of nanomaterials have been developed to improve the efficiency of stem cell therapy for patients with kidney disease
[58][186]. Generally, these hydrogels are composed of polymeric substances with high biocompatibility and a natural origin. Some of the hydrogels used are collagen/poly γ-glutamic acid-loaded with antioxidant α-lipoic acid
[72][195]; cross-linked HA/collagen loaded with therapeutics such as stromal cell-derived factor-1
[73][74][75][196,197,198]; and decellularized bovine pericardium
[76][199]. In rodent models of adriamycin-induced nephrotoxicity
[74][197], cisplatin-induced renal dysfunction
[73][196], and lipopolysaccharide-induced endotoxemia
[73][74][75][196,197,198], hydrogels enhanced the retention of co-transplanted stem cells, as well as the paracrine effect. This helped to protect stem cells from the damaging effects of an inflammatory environment.
Another strategy using hydrogels was employed to deliver therapeutics. Using a “plum-pudding” design, Qin et al. developed an injectable micelle–hydrogel hybrid. The pudding was a hyaluronic acid (HA) hydrogel cross-linked with the plums, Pluronic F127-methacrylate self-assembled micelles. Micelles containing celastrol (anti-inflammatory medication) were introduced into HA hydrogel containing anti-transforming growth factor-1 (TGF-1) antibodies. The targeted and extended (over 21 days) release of each treatment inside the kidneys reduced inflammatory markers and slowed the formation of renal interstitial fibrosis in unilateral ureteral obstruction mice models
[68][34].
NPs are frequently being coupled with hydrogels to produce hybrid nanomaterials for multi-step controlled delivery
[77][200]. These hybrids have been utilized to treat kidney disease, but in an invasive way. Hence, delivering such hybrids in a non-invasive way may serve as a valuable reference for establishing delivery of hydrogels to the renal system. In addition, these hybrids can be used to support the infiltrating cells of patients with damaged or dysfunctional kidney tissues. The most commonly used biocompatible and biodegradable materials include natural polymers, such as gelatin, collagen, alginate, HA, and chitosan. The properties of natural and endogenous polymers should be considered when designing hydrogels. For instance, HA can be found in healthy tissues with low molecular weight form (900 kDa)
[78][201]. High molecular weight (HA) can be found in kidney tissues, which can help mediate the development of kidney diseases. On the other hand, low molecular weight HA can be detrimental to the development of kidney diseases
[79][202]. In acute kidney injury, the induction of interleukin-10 by the blood vessel stimulated high molecular weight HA. It was found that this condition could protect the kidney from damage and fibrosis. It was also observed that high molecular weight HA could serve as a biomarker for the progression of fibrosis following kidney transplantation. High molecular weight HA was shown to maintain the structure of the vascular endothelium and reduce inflammation in patients with IgA and diabetic nephropathy. The results of the study suggested that the use of high molecular weight HA hydrogels and biomimetics could be beneficial for the treatment of kidney disease.
A detailed explanation can be found in Advanced Drug Delivery Systems for Renal Disorders: 10.3390/gels9020115