The importance of adhesion protein sialylation was recognized by studying the changes of adhesion behavior of human tissue cells exposed in vitro to microgravity. Proteins involved in cell-cell or cell-extracellular matrix adhesion were investigated by retrieving and evaluation of information about sialylation of cell adhesion molecules detected by omics studies on cells, which change their adhesion behavior when exposed to microgravity. Using a knowledge graph created from experimental omics data and semantic searches across several reference databases, sialylation of adhesion proteins glycosylated at their extracellular domains and their impact in cellular processes were studied. This way, experimental omics data networked with the current knowledge about binding of sialic acids to cell adhesion proteins, its regulation and interactions in-between those proteins provided insights in the mechanisms behind experimental findings suggesting that balancing sialylation against de-sialylation of the terminal ends of the adhesion proteins’ glycans influences the binding activity of adhesion proteins, the interaction of cells and their aggregation. This shed light on the transition from the cells’ growth in a monolayer to spheroid formation observed in microgravity mirroring cell migration and cancer metastasis in vivo.
- semantic knowledgebase
- SPARQL
- proteome
- spheroid formation
- cell adhesion
1. Abstract
"Adhesion Protein Sialylation"
The importance of adhesion protein sialylation was recognized by studying the changes of adhesion behavior of human tissue cells exposed in vitro to microgravity. Proteins involved in cell-cell or cell-extracellular matrix adhesion were investigated by retrieving and evaluation of information about sialylation of cell adhesion molecules detected by omics studies on cells, which change their adhesion behavior when exposed to microgravity. Using a knowledge graph created from experimental omics data and semantic searches across several reference databases, sialylation of adhesion proteins glycosylated at their extracellular domains and their impact in cellular processes were studied. This way, experimental omics data networked with the current knowledge about binding of sialic acids to cell adhesion proteins, its regulation and interactions in-between those proteins provided insights in the mechanisms behind experimental findings suggesting that balancing sialylation against de-sialylation of the terminal ends of the adhesion proteins’ glycans influences the binding activity of adhesion proteins, the interaction of cells and their aggregation. This shed light on the transition from the cells’ growth in a monolayer to spheroid formation observed in microgravity mirroring cell migration and cancer metastasis in vivo.
Johann Bauer and Erich Gombocz
2. Introduction
Posttranslational modifications (PTM) of various proteins influence the adhesion behavior of tissue cells exposed to microgravity [1]. In some types of PTM, carbohydrates are bound to reactive oxygens (O-glycan) or nitrogens (N-glycan) of a protein’s amino acid side chains [2]. In many cases a monosaccharide coupled to an amino acid is extended towards the environment of a cell by a number of additional sugar monomers forming a chain, which may be branched or linear [3]. Very often the biological effect of a protein-bound carbohydrate system is exerted by carbohydrate monomers located at the terminal end of a glycan system consisting of tens or even hundreds of carbohydrate monomers. This is true regarding antigenicity of soluble or cell bound proteins as well as in regard to negative surface charges located at a cell surface and influencing the environment [3][4]. Both traits are often formed by sialic acids (SAs) that are located at the most distant end of a carbohydrate system bound to a protein [5].
(This entry is a review on the publication of Bauer, T.J.; Gombocz, E.; Wehland, M.; Infanger, M.; Grimm, D. Insights in adhesion protein sialylation and microgravity dependent cell adhesion – an omics network approach. Int J Mol Sci 2020, 21, art. no. 1749,. https://doi.org/10.3390/ijms21051749)
SA molecules bound to proteins generate negative charges [6], affect the half-life of many circulating glycoproteins and influence also the cell-cell communication, cell matrix interaction and cell adhesion [7]. As microgravity has effects on cell adhesion [8][9], it was of interest to see whether microgravity influences sialylation of human cells affecting their adhesion behavior.
Abstract
Therefore, human adhesion proteins were selected, which attracted attention, because they seemed to play a role when cancer or endothelial cells change their behavior during exposure to microgravity [8][9][10]. Using the Knowledge Explorer (KE) [11][12] the experimental data were enriched with knowledge from several fact and reference databases. A knowledge base created this way was assessed in detail to evaluate the importance of the experimental findings in context. This revealed that sialylation of adhesion proteins influences the adhesion cells’ behavior.
The importance of adhesion protein sialylation was recognized by studying the changes of adhesion behavior of human tissue cells exposed in vitro to microgravity. Proteins involved in cell-cell or cell-extracellular matrix adhesion were investigated by retrieving and evaluation of information about sialylation of cell adhesion molecules detected by omics studies on cells, which change their adhesion behavior when exposed to microgravity. Using a knowledge graph created from experimental omics data and semantic searches across several reference databases, sialylation of adhesion proteins glycosylated at their extracellular domains and their impact in cellular processes were studied. This way, experimental omics data networked with the current knowledge about binding of sialic acids to cell adhesion proteins, its regulation and interactions in-between those proteins provided insights in the mechanisms behind experimental findings suggesting that balancing sialylation against de-sialylation of the terminal ends of the adhesion proteins’ glycans influences the binding activity of adhesion proteins, the interaction of cells and their aggregation. This shed light on the transition from the cells’ growth in a monolayer to spheroid formation observed in microgravity mirroring cell migration and cancer metastasis in vivo.
3. Results
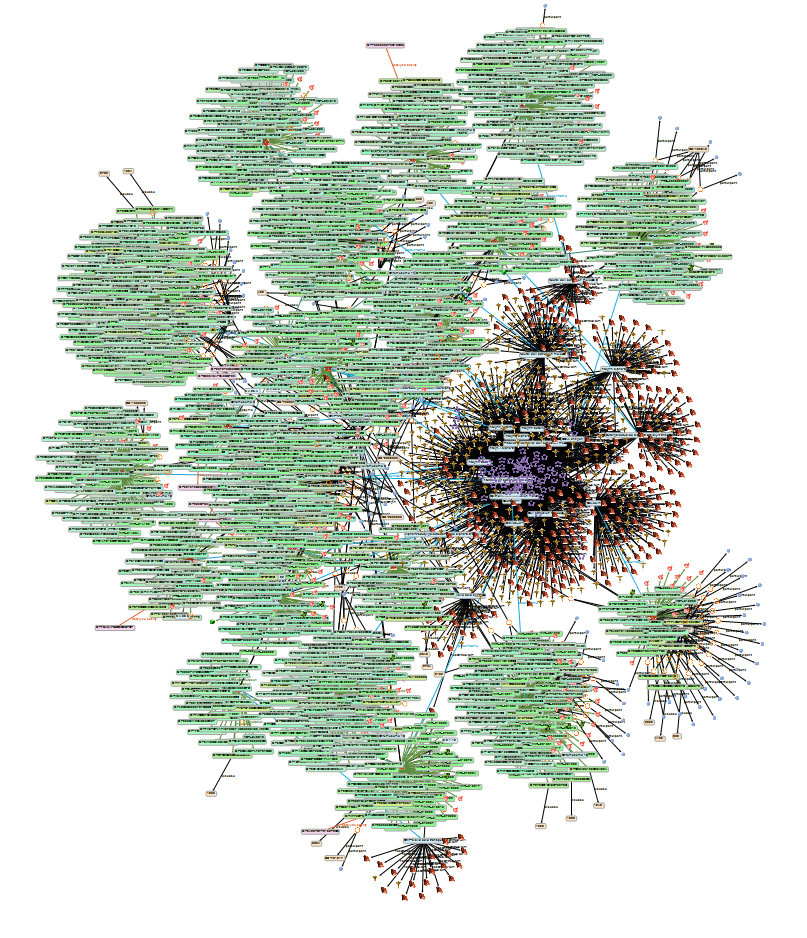
Of thousands of proteins or genes [8][9][10][13] found by mass spectrometry or microarray gene analyses of cancer cells and endothelial cells, which had been cultured for a few days on ground, in Space or on devices simulating microgravity, 17 representatives of integrins, cadherins, and other cell adhesion molecules were selected and analyzed with the aim to get information about the function of SAs bound to the tips of the glycans they bear (for details, see [14]). In a first step, names and related analysis results of the selected proteins were imported in KE via their UniProt accession numbers into an initial resource description framework (RDF) to create a knowledge base (KB) through a combination of semantic protocol and RDF query language (SPARQL) searches across several databases (Figure 1). Using this KB for selectively retrieving references revealed more than 200 publications about sialylation of these proteins.
Keywords: semantic knowledgebase; SPARQL; proteome; spheroid formation; cell adhesion
FIntroductigureon 1: Annotated experimental OMICs network in KE, enriched with linked-open data resources: The Knowledge graph shows adhesion proteins together with their genes, sequences, post-translational modifications, interactions, functions and sialylation-related annotations. (The text within the picture becomes legible after magnification.
Posttranslational modifications (PTM) of various proteins influence the adhesion behavior of tissue cells exposed to microgravity [1]. In some types of PTM, carbohydrates are bound to reactive oxygens (O-glycan) or nitrogens (N-glycan) of a protein’s amino acid side chains [2]. In many cases a monosaccharide coupled to an amino acid is extended towards the environment of a cell by a number of additional sugar monomers forming a chain, which may be branched or linear [3]. Very often the biological effect of a protein-bound carbohydrate system is exerted by carbohydrate monomers located at the terminal end of a glycan system consisting of tens or even hundreds of carbohydrate monomers. This is true regarding antigenicity of soluble or cell bound proteins as well as in regard to negative surface charges located at a cell surface and influencing the environment [3,4]. Both traits are often formed by sialic acids (SAs) that are located at the most distant end of a carbohydrate system bound to a protein [5].
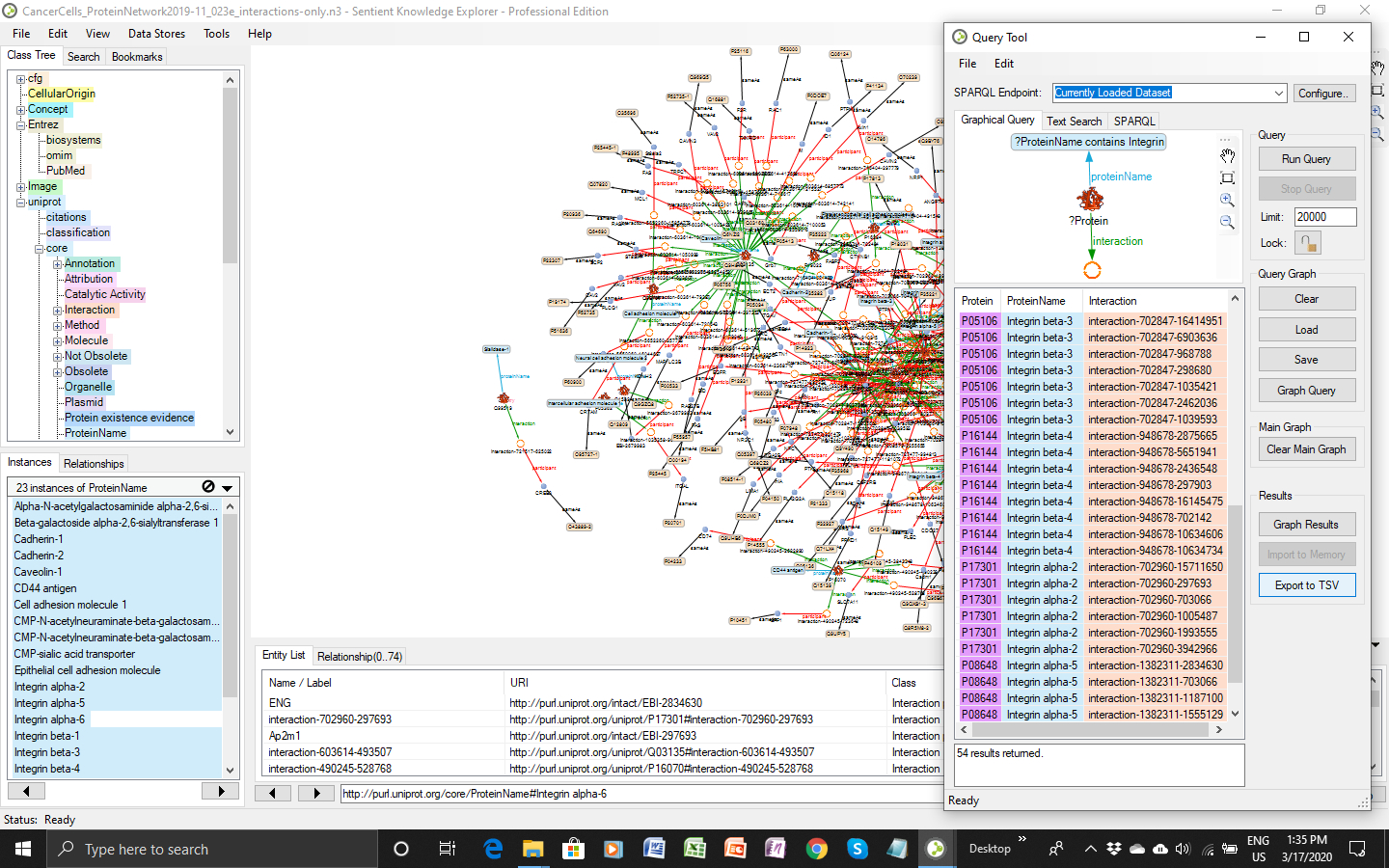
The information included in the 200 publications found indicated that the extracellular domains of various integrin dimers, of cadherins, of cell adhesion molecules like ICAM, NCAM, PeCAM and of CD44 occur on cells in a sialylated and a de-sialylated form. The different forms have influence on the cells’ adhesion and migration behavior in vivo and in vitro. They are determined by the activity of sialyltransferases, which catalyze binding of a SA moiety to the tip of an existing glycans and by the activity of neuraminidases, which cut SA moieties off the tip of sialylated glycans. Hence, the status of sialylation of adhesion proteins depends on the interplay of active sialyltransferases and neuraminidases. Iterative network queries on interaction (Figure 2) attributed to confirm that this interplay can be affected by transient quantities of sialyltransferases and neuraminidaseases, respectively as well as by the presence of scaffold proteins like caveoline 1. Under microgravity the expression of the sialyltransferase ST6GAL1 and the accumulation of caveoline 1 are changed [8][9][13]. In addition, sialyltransferases and neuraminidases not only sialylate or desialylate target glycans, they have also influence on the expression of the proteins bearing the SAs [14].
SA molecules bound to proteins generate negative charges [6], affect the half-life of many circulating glycoproteins and influence also the cell-cell communication, cell matrix interaction and cell adhesion [7]. As microgravity has effects on cell adhesion [8,9], it was of interest to see whether microgravity influences sialylation of human cells affecting their adhesion behavior.
Therefore, human adhesion proteins were selected, which attracted attention, because they seemed to play a role when cancer or endothelial cells change their behavior during exposure to microgravity [8-10]. Using the Knowledge Explorer (KE) [11,12] the experimental data were enriched with knowledge from several fact and reference databases. A knowledge base created this way was assessed in detail to evaluate the importance of the experimental findings in context. This revealed that sialylation of adhesion proteins influences the adhesion cells’ behavior.
FigResurelts 2: SPARQL query in KE across the knowledge graph for interactions on sialylated adhesion proteins.
Of thousands of proteins or genes [8,9,10,13] found by mass spectrometry or microarray gene analyses of cancer cells and endothelial cells, which had been cultured for a few days on ground, in Space or on devices simulating microgravity, 17 representatives of integrins, cadherins, and other cell adhesion molecules were selected and analyzed with the aim to get information about the function of SAs bound to the tips of the glycans they bear (for details, see [14]). In a first step, names and related analysis results of the selected proteins were imported in KE via their UniProt accession numbers into an initial resource description framework (RDF) to create a knowledge base (KB) through a combination of semantic protocol and RDF query language (SPARQL) searches across several databases (Fig. 1). Using this KB for selectively retrieving references revealed more than 200 publications about sialylation of these proteins.
Fig. 1: Annotated experimental OMICs network in KE, enriched with linked-open data resources: The Knowledge graph shows adhesion proteins together with their genes, sequences, post-translational modifications, interactions, functions and sialylation-related annotations. (The text within the picture becomes legible after magnification.)
The information included in the 200 publications found indicated that the extracellular domains of various integrin dimers, of cadherins, of cell adhesion molecules like ICAM, NCAM, PeCAM and of CD44 occur on cells in a sialylated and a de-sialylated form. The different forms have influence on the cells’ adhesion and migration behavior in vivo and in vitro. They are determined by the activity of sialyltransferases, which catalyze binding of a SA moiety to the tip of an existing glycans and by the activity of neuraminidases, which cut SA moieties off the tip of sialylated glycans. Hence, the status of sialylation of adhesion proteins depends on the interplay of active sialyltransferases and neuraminidases. Iterative network queries on interaction (Fig. 2) attributed to confirm that this interplay can be affected by transient quantities of sialyltransferases and neuraminidaseases, respectively as well as by the presence of scaffold proteins like caveoline 1. Under microgravity the expression of the sialyltransferase ST6GAL1 and the accumulation of caveoline 1 are changed [8,9,13]. In addition, sialyltransferases and neuraminidases not only sialylate or desialylate target glycans, they have also influence on the expression of the proteins bearing the SAs [14].
Fig.2: SPARQL query in KE across the knowledge graph for interactions on sialylated adhesion proteins.
Conclusion
Taking all the information provided in [14] together it was concluded that microgravity influences sialylation dependent activities of adhesion proteins on three levels: i) expression and accumulation of adhesion proteins, ii) expression and accumulation of enzymes sialylating or de-sialylation glycan systems bound to adhesion proteins and iii) expression, accumulation and localization of scaffold proteins supporting the activity of the enzymes. The gained knowledge suggests that the process of linking cells to each other or to the ECM under microgravity includes sialylation of extracellular domains of adhesion proteins.
The conclusion was achieved using an omics network approach to gain advantage from combining the results of different experiments with each other and with the knowledge described in literature while focusing on those parts of data, which are related to the topic of cell adhesion. Data experimentally obtained about the accumulation of adhesion proteins in three different types of cells by mass spectrometry [8-10] and by microarray studies on mRNA expression [13] were incorporated in a common network with current knowledge about sialylation [11,12]. This semantic network generation was made possible with the help of KE, an advanced information retrieval tool to generate and interrogate complex knowledge graphs.
References:
- Bauer, J.; Wehland, M.; Infanger, M.; Grimm, D.; Gombocz, E. Semantic analysis of posttranslational modification of proteins accumulated in thyroid cancer cells exposed to simulated microgravity. Int J Mol Sci 2018, 19, art. no. 2257, .
- Ohtsubo, K.; Marth, J.D. Glycosylation in Cellular Mechanisms of Health and Disease. Cell 2006, 126, 855-867.
- Lamblin, G.; Degroote, S.; Perini, J.-M.; Delmotte, P.; Scharfman, A.; Davril, M.; Lo-Guidice, J.-M.; Houdret, N.; Dumur, V.; Klein, A.; Roussel, P. Human airway mucin glycosylation: A combinatory of carbohydrate determinants which vary in cystic fibrosis. Glycoconjugate J 2001, 18, 661-684.
- Mehrishi, J.N.; Bauer, J. Electrophoresis of cells and the biological relevance of surface charge. Electrophoresis 2002, 23, 1984-1994.
- Du, J.; Hong, S.; Dong, L.; Cheng, B.; Lin, L.; Zhao, B.; Chen, Y.-G.; Chen, X. Dynamic sialylation in transforming growth factor-β (TGF-β)-induced epithelial to mesenchymal transition. J Biol Chem 2015, 290, 12000-12013.
- Wang, B.; Brand-Miller, J. The role and potential of sialic acid in human nutrition. Eur J Clin Nutrition 2013, 57, 1351-1369.
- Kelm, S.; Schauer, R. Sialic acids in molecular and cellular interactions. Int Rev Cytol 1997, 175, 137-240.
- Bauer, J.; Kopp, S.; Schlagberger, E.M.; Grosse, J.; Sahana, J.; Riwaldt, S.; Wehland, M.; Luetzenberg, R.; Infanger, M.; Grimm, D. Proteome analysis of human follicular thyroid cancer cells exposed to the random positioning machine. Int J Mol Sci 2017, 18, art. no. 546,
- Sahana, J.; Nassef, M.Z.; Wehland, M.; Kopp, S.; Krüger, M.; Corydon, T.J.; Infanger, M.; Bauer, J.; Grimm, D. Decreased E-Cadherin in MCF7 Human Breast Cancer Cells Forming Multicellular Spheroids Exposed to Simulated Microgravity. Proteomics 2018, 18, art. no. 1800015, .
- Ma, X.; Sickmann, A.; Pietsch, J.; Wildgruber, R.; Weber, G.; Infanger, M.; Bauer, J.; Grimm, D. Proteomic differences between microvascular endothelial cells and the EA.hy926 cell line forming three-dimensional structures. Proteomics 2014, 14, 689-698.
- Gombocz, E.A.; Stanley, R.A.; Rockey, C.; Nishimura, T. Data Integration Framework for discovery and validation: Smart merging of experimental and public data across ontologies and taxonomies. Bio-IT World, Boston, MA, 2008.
- Stanley, R.A.; Gombocz, E.A. System, method, software architecture, and business model for intellegent object based informationplatform. US Patent 7,702,639, 2010.
- Ma, X.; Pietsch, J.; Wehland, M.; Schulz, H.; Saar, K.; Hübner, N.; Bauer, J.; Braun, M.; Schwarzwälder, A.; Segerer, J., et al. Differential gene expression profile and altered cytokine secretion of thyroid cancer cells in space. FASEB J 2014, 28, 813-835.
- Bauer, T.J.; Gombocz, E.; Wehland, M.; Infanger, M.; Grimm, D. Insights in adhesion protein sialylation and microgravity dependent cell adhesion – an omics network approach. Int J Mol Sci 2020, 21, art. no. 1749,.
4. Conclusion
Taking all the information provided in [14] together it was concluded that microgravity influences sialylation dependent activities of adhesion proteins on three levels: i) expression and accumulation of adhesion proteins, ii) expression and accumulation of enzymes sialylating or de-sialylation glycan systems bound to adhesion proteins and iii) expression, accumulation and localization of scaffold proteins supporting the activity of the enzymes. The gained knowledge suggests that the process of linking cells to each other or to the ECM under microgravity includes sialylation of extracellular domains of adhesion proteins.
The conclusion was achieved using an omics network approach to gain advantage from combining the results of different experiments with each other and with the knowledge described in literature while focusing on those parts of data, which are related to the topic of cell adhesion. Data experimentally obtained about the accumulation of adhesion proteins in three different types of cells by mass spectrometry [8][9][10] and by microarray studies on mRNA expression [13] were incorporated in a common network with current knowledge about sialylation [11][12]. This semantic network generation was made possible with the help of KE, an advanced information retrieval tool to generate and interrogate complex knowledge graphs.