Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Eliza Romanczuk-Ruszuk and Version 2 by Catherine Yang.
Hot-melt adhesives (HMAs) are thermoplastic materials that can bond various substrates by solidifying rapidly upon cooling from the molten state, and their modification with organosilicon compounds can result in crosslinking behavior, characteristic of gels. Organosilicon compounds are hybrid molecules that have both inorganic and organic components and can enhance the properties and performance of HMAs.
- organosilicon
- silanes
- hot melt
- HMA
- adhesive
1. Primary Resins and Ingredients of Hot Melts
The main constituents of hot-melt materials for glass sealants, corrosion protection, and water insulation consist of a base polymer, tackifier, and plasticizer. Among other usual materials are viscosity modifiers, extenders, UV stabilizers, antioxidants, and pigments. The composition and proportion of these ingredients significantly influence the performance and properties of the adhesive, such as bond strength, temperature resistance, open time, and adhesion to various substrates. The choice of base polymer for hot-melt formulation is often dictated by its end use and desired properties. The formulator must consider the desired processing method, working conditions, and price. In general, base polymers have a huge impact on viscosity and rheology, cohesive and adhesive strength, creep and tack, as well as processing and working temperature [1][2][3][4][5][39,40,51,52,53]. Common primary resins used in HMAs include ethylene–vinyl acetate (EVA) [6][7][8][9][10][11][38,43,45,49,50,54] polyolefins [11][12][54,55], styrenic block copolymers (SBC) [13][14][56,57], butyl rubber [15][16][58,59], polyamides [6][17][38,60], and reactive resins [18][19][48,61]. The modification of base polymer properties is obtained by the addition of tackifiers. The tackifiers are usually hydrocarbon resins, pure monomer resins, or rosin esters. They are typically selected to be miscible with the base polymer and their main role is the Tg modification. The aim of pure monomer resins with high Tg is mainly reinforcement of the formulation. Low Tg resins function typically as tackifiers by increasing the Tg of the elastomeric polymer (increasing the loss modulus simultaneously) and reducing its elastic modulus. In contrast, plasticizers function mainly as diluents, by reducing both the elastic and loss moduli, softening the formulation, and increasing creep.
The formulations based on SBCs utilize the phase separation of soft and hard blocks. The typical base polymers are of A-B-A triblock structure, with A being typically styrene end-block and B an elastomeric mid-block with Tg below room temperature. The end-block and mid-block should be thermodynamically immiscible, to provide a microphase-separated structure. The styrenic end-block, exhibiting Tg above the typical application temperature, provides physical crosslinks and prevents creep, whereas the soft elastomeric mid-block may be readily modified to meet the demands of the formulator. Owing to the different solubility parameters of both phases, each phase may be modified independently using additives with selective miscibility [20][62]. A notable example is styrene–ethylene–butene copolymer (SEBS), which exhibits excellent affinity for paraffinic oil, and no bleed. Hot-melt oil–gel-type adhesives for dermal applications, comprising more than 600 PHR (per hundred rubber/per hundred resin) may be successfully formulated [21][63]. Such adhesives serve usually as carriers for physiologically active agents. Notably, the liquid nature of the formulation improves the drug release rate. It has been shown by Gennari et al. [22][64], that, in particular, low molecular weight SEBS is the polymer worthy of consideration because of its favorable viscoelastic behavior. Not only are SEBS gels attractive in medical applications, but also the inherent tackiness and the elasticity of such gels allow a compromise between minimizing modulus (to allow the polymer to be stretched with ease) and maximizing interfacial adhesion strength at the laminated polymer–polymer interface. This allows potential applications in wearable electronics such as soft-actuated materials and transducers [23][65]. In combination with the deformability of liquid metals, this allowed the fabrication of a stretchable thermoplastic electric conductor [24][66].
Typical additives for butyl sealants are alkylphenol–formaldehyde resins (APFRs); gum rosin; hydrocarbon resins; low-molecular-weight polyisobutylene [25][67], paraffinic oil [26][68], C5 resin [27][69], high-molecular-weight polyisobutylene, silane-modified poly-alfa-olefin [28][70], butyl rubber, butadiene-styrene, and butadiene–nitrile rubbers, acrylic polymers, among other oligomers, polymers, and mixtures thereof, as well as natural and precipitated calcium carbonate silica [29][71], montmorillonites [29][71], sepiolite [30][72], carbon black [26][68], titanium dioxide [27][69], fumed silica, and halloysite [31][73]. Therefore, there is a large base of additives for HMA formulations, including organic and inorganic materials; however, usually, each one has a specific role, such as rheology or tackiness modifier, extender, pigment, etc. On the other hand, organosilicon compounds may serve multiple roles depending on their chemical structure, site, and method of application within either HMA itself or the adhesive bond formed with it (Figure 14). As mentioned earlier, they may be used to obtain silane-modified resins used for the bulk of HMA, such as silane-modified poly-alfa-olefin [28][70], or as various modifiers of fillers and adherents.
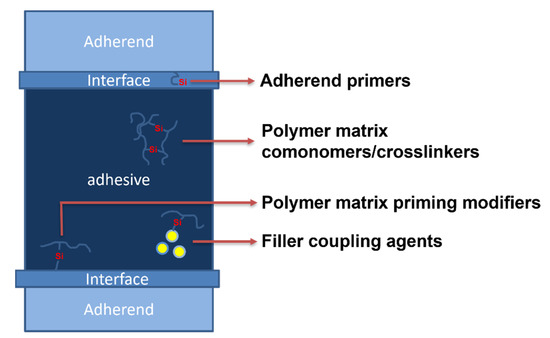
Figure 14.
The application of organosilicon compounds within hot-melt adhesives and adhesive bonds.
Curable hot-melt sealants based on butyl rubber (BR) and containing thermoplastic resins like polyethylene (PE) and ethylene–vinyl acetate copolymer (EVA) exhibit better mechanical properties. It has been shown that an increase in the properties of sealants is due to the chemical interaction between EVA and vinyltrimethoxysilane (VTMOS, Figure 25B1) via transesterification reaction between the ethylene acetate monomers and alkoxysilane moiety [15][58].
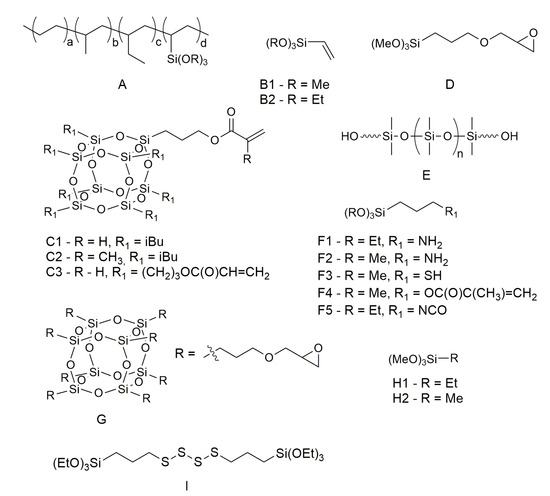
Figure 25. Structures of organosilicon agents.
Structures of organosilicon agents discussed in the review.
2. Organosilicon Compounds as Co-Monomers of Polymer Matrix
The field of organosilicon polymer chemistry has achieved significant advancements over the past century, establishing a fundamental foundation for their utilization in materials science. Due to their unique inorganic–organic chemical composition, organosilicon polymers serve as a crucial link between inorganic and organic polymers, exhibiting a fascinating combination of properties [32][33][34][74,75,76]. Based on variations in their backbone structure, organosilicon polymers can be primarily classified into polysiloxanes (Si-O), polysilsesquioxanes (Si-O), polysilanes (Si-Si), polycarbosilanes (Si-C), and polysilazanes (Si-N) [35][77]. Kowalczyk et al. reported the synthesis and characterization of novel organic–inorganic hybrid copolymers based on acryloxypropyl-heptaisobutyl-POSS (A-POSS, Figure 25(C1)) and various (meth)acrylates, and their application in thermally curable structural self-adhesive tapes (SATs). The authors claim that the incorporation of A-POSS into the epoxyacrylate copolymers (EA-POSS) improves the self-adhesive, mechanical, and thermal properties of the SATs and the properties of the resulting aluminum–SAT–aluminum overlap joints. The results showed that the SATs with EA-POSS copolymers had higher adhesion, tack, and cohesion than the neat SAT-0, especially for lower A-POSS content (0.25–1 mol%). The best mechanical performance was observed for the joints with EA-POSS-0.5-based SAT, which had an increment range of 50–294% in shear strength compared to the EA-0-based joints. The results showed that the SATs with EA-POSS-type copolymers exhibited significantly higher values of adhesion, cohesion, and tack. The study concludes that A-POSS is able to improve the mechanical and thermal properties of SAT-based joints [36][78]. Ma et al. synthesized a series of POSS-containing linear and star multi-arm block copolymers (BCPs) with different architectures by the core-first atom transfer radical polymerization (ATRP) method and using methacryloxypropylheptaisobutylsilsesquioxane (Figure 25(C2)). They then crosslinked the BCPs by reacting the glycidyl groups in the poly(glycidyl methacrylate) (PGMA) block with trimethylamine to form three-dimensional networks (L/S-(PGMA-b-PMAPOSS)1,2,4,6) with various architectures. They found that the surface roughness and hydrophobicity increased with the number of arms of the BCPs, due to the higher aggregation and migration of PMAPOSS chains to the surface. The authors suggested that the surface properties could be tuned by adjusting the architectures of the BCPs. The authors measured the adhesive strength of the BCPs to glass substrates by a mechanics test system (MTS). They found that the adhesive strength increased with the number of arms of the BCPs, from 237 N for linear monobrachial BCPs to 431 N for star six-arm BCPs. They attributed this improvement to two factors: (1) the lower viscosity of the BCP solution with more arms, which enhanced its wetting and diffusion ability on glass surfaces; and (2) the higher density of branches of the BCPs with more arms, which increased their intermolecular forces and cohesion [37][79].
Hanifpour et al. [38][80] described a photo-crosslinkable adhesive by grafting methacrylic groups onto a co-oligomer of 1-decene and 9-decene-1-ol, which was prepared by using a Ti amine bis-phenolate catalyst. The adhesive was then blended with different weight fractions (0.2, 0.4, 0.7, and 1.0 wt.%) of a silsesquioxane referred to as methacrylate-functionalized silsesquioxane (meth-acryloxypropylsilsesquioxane, MA-POSS), although all the figures suggest that the additive was in fact acryloqypropylsilsesquioxane (Figure 25(C3)). The additive was of non-specified average molecular weight, and the obtained mixtures were cured by blue light irradiation. The results showed that the addition of MA-POSS increased the degree of monomer conversion, storage modulus, glass transition temperature, flexural modulus, flexural strength, microhardness, thermal stability, and adhesion properties of the nanocomposites. The authors attributed these improvements to the good dispersion and interfacial adhesion of MA-POSS in the adhesive matrix, as well as the increased crosslinking density and rigidity of the nanocomposites. However, they also observed that excessive MA-POSS content (1.0 wt.%) led to a reduction in mechanical and thermal properties due to the formation of aggregates and incomplete curing. The authors concluded that MA-POSS is an efficient adhesion promoter for olefin-based adhesives and can enhance their mechanical and thermal performance. They suggested that MA-POSS can be used for various industrial applications that require high-performance adhesives [38][80].
Bilgin et al. [39][81] reported the synthesis and characterization of 2-ethylhexyl acrylate (2-EHA)-based latexes via mini-emulsion polymerization for pressure-sensitive adhesive (PSA) applications. The authors investigated the effects of two types of silanes, vinyltrimethoxysilane (VTMOS) and 3-glycidyloxypropyltrimethoxysilane (GPTMOS, Figure 25(D)), on the adhesive performance of the latexes on polar and nonpolar surfaces. They also used n-dodecyl mercaptan (NDM) as both a cosurfactant and a chain transfer agent to control the particle size and molecular weight of the latexes. The authors found that mini-emulsion polymerization was an effective technique for incorporating silanes into 2-EHA-based copolymers without causing coagulation or instability. They also found that the type of silane had a considerable influence on the PSA properties. VTMOS, as a polymerizable silane, increased the shear strength of the latexes due to chemical crosslinking between chains, but decreased the peel adhesion and loop tack values on both polar and nonpolar surfaces. GPTMOS, as a non-polymerizable silane oligomer, increased the peel adhesion and loop tack values on both types of surfaces due to its epoxy functionality and polar interactions with substrates, but decreased the shear strength of the latexes. The work contributes to the understanding of the role of silanes in enhancing the adhesion and cohesion mechanisms of PSAs and offers an approach for tailoring the properties of PSAs according to specific requirements [39][81].
In the studies discussed above, organosilicon compounds were incorporated into the polymer matrix in different ways, such as through the synthesis of novel organic–inorganic hybrid copolymers, the creation of block copolymers (BCPs) with different architectures, and the grafting of methacrylic groups onto a co-oligomer. The use of organosilicon compounds as co-monomers resulted in polymers with improved properties, such as increased adhesion strength, thermal stability, and mechanical performance. In conclusion, organosilicon compounds have proven to be effective co-monomers in the synthesis of polymers, enhancing their properties and expanding their potential applications.
3. Organosilicon Compounds as a Polymer Matrix Additive
Park et al. (2020) [40][82] investigated the adhesion improvement of acrylic pressure-sensitive adhesive (PSA) to low-surface-energy substrates using silicone urethane dimethacrylates (SiUDMAs). By controlling the ratio of diisocyanate to carbinol-terminated PDMS of the terminating unit undisclosed by the supplier (Figure 25E), different types of SiUDMAs were obtained and introduced as additives to the acrylic PSA. The modifiers had urethane moieties imparting the miscibility of the oligomer with acrylic PSA and also have acrylate groups that can crosslink with UV irradiation. The results showed that SiUDMAs significantly improved the loop tack and peel strength of PSAs on low-surface-energy substrates without compromising their thermal stability. The improvement was more pronounced for SiUDMA2.0 (with IPDI:PDMS 2:0 ratio), which had a similar molecular weight to SiDMA (silicone dimethacrylate) but higher miscibility with acrylic PSA. UV irradiation decreased the loop tack and peel strength but increased the shear adhesion failure test (SAFT) of the modified PSAs due to the formation of a semi-interpenetrating polymer network (semi-IPN) structure by SiUDMA. The authors concluded that SiUDMAs are effective adhesion promoters for acrylic PSAs on low-surface-energy substrates and can overcome the limitations of SiDMA. They also suggested that SiUDMAs can be used to tailor the properties of PSAs by adjusting their molecular weight, viscosity, and surface energy.
Wu et al. [41][83] reported on the effects of silane coupling agents on the properties of ethylene/vinyl acetate (EVA) composite hot-melt adhesive. The authors prepared a binary EVA resin blend with suitable viscosity and tensile shear strength as the base resin, and then added dicumyl peroxide (DCP) as the crosslinking agent and three types of silane coupling agents with different functional groups (KH550, KH560, and KH570, which are APTES, Figure 25(F1), GPTMOS, Figure 25(D), and MATMOS, Figure 25(F4), respectively) to improve the bonding performance of the adhesive. The optimal temperature and dosage of DCP for crosslinking EVA resin were 140 °C and 2 phr, respectively. After treatment under these conditions, the tensile shear strength of the adhesive increased from 0.247 MPa to 0.726 MPa when 5 phr KH570 was also added. The addition of the silane coupling agent reduced the degree of crosslinking of EVA resin by reacting with DCP preferentially, which resulted in a decrease in tensile strength, and elongation at the break and tensile modulus of the adhesive. However, KH570 had the lowest reactivity with DCP and improved the fluidity and wettability of the adhesive, as well as enhancing the polarity and bonding effect of the adhesive due to its methacryloxy functional group. The study found that when 2 phr of DCP and 5 phr of the KH570 silane coupling agent were added at the same time, the tensile shear strength of hot-melt adhesive increased from 0.247 MPa to 0.726 MPa. However, it was also found that an excessive silane coupling agent would significantly reduce the tensile strength and shear peel strength of the material. This may have contributed to the loss of entanglement [42][25]. Therefore, the addition of an appropriate amount of silicone coupling agent can improve the performance of EVA hot-melt adhesive.
Yazıcı et al. (2021) [43][84] proposed a novel and environmentally friendly method to enhance the adhesion between natural rubber (NR) and textile cords, which are widely used in tire applications. The authors used acryloxypropyl-functional polyhedral oligomeric silsesquioxane (A-POSS), a reactive silsesquioxane species, as an additive in NR composites, and compared its performance with the conventional resorcinol formaldehyde latex (RFL) dipping system. A-POSS significantly increased the adhesion strength between NR composites and polyamide cords. The H-adhesion force between NR/A-POSS (8 phr) and virgin Nylon 6.6 cord was 123.0 N, while it was only 95.8 N for NR/RFL-coated Nylon 6.6 cord. The work of adhesion between NR/A-POSS (8 phr) and virgin Aramid was also higher than that of NR/virgin Aramid. The authors attributed these findings to the chemical reactions between A-POSS and sulfur during vulcanization, as well as the physical interactions between A-POSS and polyamide cords. They also observed a thin layer of NR/A-POSS on the surface of Nylon 6.6 cords by SEM images, indicating good interfacial adhesion.
The research paper by Murtazina et al. (2020) [44][85] presents a study of the effect of silane-terminated prepolymers (STP) based on oligotetraoxymethylene glycol (polyfurite) and oligooxypropylene glycol (laprol) on the properties of hot-melt sealants based on ethylene propylene diene rubber (EPDM). As a silane agent, 3-aminopropyltrimethoxysilane (Figure 25F2) was used. The results showed that the addition of STP increased the tensile strength and adhesion of the sealants to various substrates (duraluminum, steel, and glass), while reducing their elongation at break. The authors attributed this effect to the formation of a semi-interpenetrating network of cured STP in the uncured EPDM phase. They also found that the viscosity of the sealants decreased with increasing STP content, which indicated a temporary plasticization effect. The authors suggested that this could allow processing the sealants at lower temperatures (130 °C) than conventional hot-melt sealants (180–200 °C).
Lai et al. [45][86] investigated the thermal, mechanical, and shape memory behavior of physical blends of OBC (olefin block copolymer) and EVA (ethylene–vinyl acetate copolymer), with and without modification of one or both of the components via peroxide-initiated comonomer grafting reaction. The modification of EVA with vinyltriethoxysilane (VTEOS, Figure 25(B2)) improved the compatibility, tensile strength, and shape fixity ratio of the OBC/EVA blend. The OBC-g-MA/EVA-g-VTEOS blend, which had numerous interactions between maleic anhydride and silane, showed the highest storage modulus within the 60–80 °C range, thermal stability, and shape memory performance of all investigated blend systems. This modified blend could be reprocessed like a thermoplastic vulcanizate and, thus, could be considered a green shape memory blend in terms of environmental concerns. In summary, the use of silane as a modifying agent improved the adhesion and mechanical properties of OBC/EVA blends.
4. Organosilicon Compounds as Filler Coupling Agents
Dognaci [46][87] investigated the use of glycidyl polyhedral oligomeric silsesquioxane (GPOSS, a glycidoxypropyl-functional silsesquioxane of non-specified molecular weight, Figure 25(G)) as an adhesion promoter to improve the adhesion between the polyester cord and rubber. The study found that the addition of GPOSS to poly(ethylene terephthalate) (PET) cord improved the adhesion of the cord to rubber when compared with other treated PET cords via H-adhesion and strip peel adhesion tests. The authors observed that GPOSS improved the adhesion of the cord to rubber significantly when compared with other treatments, especially at higher concentrations (0.5–1.0 wt.%). The authors attributed this improvement to the strong crosslinking and molecular reinforcement effects of GPOSS. In particular, the adhesion values were better than those obtained using commercially used epoxies, suggesting that GPOSS could be recommended as an adhesion promoter in the rubber industry. The study also found that GPOSS-coated PET yarns increased stiffness and did not change the tensile strength of the PET yarns.
Yang et al. [47][11] investigated the interfacial adhesion between aramid fiber (AF) and rubber matrix by grafting mercapto hyperbranched polysiloxane (HPSi) onto the AFs via a novel in situ growth strategy. The HPSi was grafted via a combination of the formation of a polydopamine (PDA) precursor layer and the co-dehydration condensation between 3-aminopropyltrimethoxysilane (APTMOS) and 3-mercaptopropyltrimethoxysilane (MPTMOS, Figure 25(F3)). This modification strategy can increase the interfacial adhesion by up to approximately 96.5%, with the key factor being the covalent interaction between mercapto groups and double bonds. The study suggests that this surface modification strategy has the potential for application to other high-performance fibers and can expand the application range of fiber/rubber composites.
Ahmed and Mushtaq (2022) [48][88] studied the effects of silane-modified aluminum oxide (m-Al2O3) and ethylene–vinyl acetate-grafted maleic anhydride (EVA-g-MA)/m-Al2O3 hybrid fillers on the thermal stability and mechanical properties of ethylene–vinyl acetate copolymer (EVA)/ternary polyamide (tPA) composites. The authors used 3-aminopropyltriethoxysilane (APTES) as a coupling agent to modify the surface of Al2O3 particles and improve their dispersion and compatibility with the EVA/tPA matrix. They prepared the composites with different filler loadings (20–40 wt.%). Hybrid fillers modified with a silane coupling agent and EVA-g-MA were more effective in terms of improving both tensile and tear strength of such obtained materials. The tensile strength of the EVA/tPA/(m-Al2O3/EVA-g-MA) composite with a ratio of 49/21/30 wt.% increased by up to 66% compared to the neat EVA/tPA. The findings suggested that the silane modification and the EVA-g-MA compatibilizer approach for Al2O3 filler could be considered in future research work to develop reinforced composites of improved thermal stability.
The work of Bi et al. (2019) [49][89] investigates the effects of four different silane coupling agents on the filler–filler and filler–rubber interactions and mechanical properties of the ethylene–vinyl acetate copolymer (EVM)/aluminum trihydrate (ATH) composites. The silane coupling agents were vinyltrimethoxysilane (VTMOS), vinyltriethoxysilane (VTEOS), ethyltrimethoxysilane (ETMOS, Figure 25H1), and aminopropyltrimethoxysilane (APTMOS). The dispersion and adhesion of ATH in the EVM matrix, and the tensile and abrasion properties of the obtained composites, are described. The main findings are that the addition of VTMOS, VTEOS, and APTMOS significantly reduces the Payne effect, which indicates the collapse of the filler network under shear and improves the tensile strength and abrasion resistance of the composites. The authors attribute this to the enhanced filler–rubber interaction mediated by the silane coupling agents, which have functional groups that can react with both ATH and EVM. On the other hand, ETMOS does not show any positive effect on the composites and even lowers the bound rubber content and mechanical properties. The authors suggest that ETMOS shields the ATH surface from wetting by EVM and does not participate in the peroxide curing reaction.
Jo et al. (2022) [50][90] reported on the development of a green and sustainable hot-melt adhesive (HMA) based on polyhydroxyalkanoate (PHA) and silanized cellulose nanofibers (SCNFs). The authors used PHA of a high chain length and high poly (4-hydroxybutyric acid) (P4HB) ratio as a biodegradable and flexible base polymer and modified the surface of cellulose nanofibers (CNFs) with tetraethyl orthosilicate (TEOS) and methyltrimethoxysilane (MTMOS, Figure 25H2) to enhance their hydrophobicity and dispersibility in PHA. The authors found that the double silanization of CNFs using TEOS and MTMOS increased their water contact angle from 18° to 177°, indicating a successful hydrophobization. They found that adding 10% SCNFs increased the tensile strength of PHA from 5.8 MPa to 7.2 MPa, while reducing its viscosity from 11,000 Pa·s to 9000 Pa·s. The authors attributed this to the thixotropic behavior of SCNFs, which enhanced the flowability and infiltration ability of PHA-SCNFs adhesive at low stress. The lap shear test showed that PHA-SCNFs adhesive had a comparable failure load to commercial HMA (around 1 kN), indicating a good adhesion performance. The authors concluded that PHA-SCNFs composite is a promising candidate for green and sustainable HMA applications, as it combines biodegradability, flexibility, processability, and adhesive strength.
In conclusion, the exploration of organosilicon compounds as filler coupling agents in hot-melt adhesives has demonstrated significant potential across various applications. These compounds, characterized by their unique ability to form robust crosslinks and provide molecular reinforcement, have been shown to enhance the adhesion, mechanical properties, and thermal stability of diverse composite materials.
